Abstract
Bach1 is a transcriptional repressor of heme oxygenase-1 (HO-1, a.k.a. HSP-32), which is an inducible enzyme and has anti-oxidation/anti-inflammatory properties shown in various models of organ injuries. Since oxidative stress plays a pivotal role in the pathogenesis of nonalcoholic steatohepatitis (NASH), HO-1 induction would be expected to prevent the development of NASH. In this study, we investigated the influence of Bach1 ablation in mice on the progression of NASH in methionine-choline deficient (MCD) diet model. Bach1 ablation resulted in significant induction of HO-1 mRNA and its activity in the liver. When fed MCD diet, Bach1−/− mice exhibited negligible hepatic steatosis compared to pronounced steatohepatitis in wild type mice with 6-fold increase in hepatic triglyceride content. Whereas feeding of MCD diet decreased mRNA expressions of peroxisome proliferator-activated receptor (PPAR) α and microsomal triglyceride transfer protein (MTP) in wild type mice, there were no change in Bach1−/− mice. In addition, hepatic concentration of malondialdehyde (MDA), a biomarker for oxidative stress as well as plasma alanine aminotransferase (ALT) was significantly lower in Bach1−/− mice. These findings suggest that Bach1 ablation exerts hepatoprotective effect against steatohepatitis presumably via HO-1 induction and may be a potential therapeutic target.
Keywords: oxidative stress, steatohepatitis, nonalcoholic fatty liver disease, heme oxygenase-1, peroxisome proliferator-activated receptor α
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most major liver dysfunction in the world and its prevalence is increasing with epidemics of obesity, diabetes, and metabolic syndrome. NAFLD includes wide-ranging liver disorder from simple steatosis through steatohepatitis to cirrhosis and possibly hepatocellular carcinoma.(1,2) About one-third of NAFLD is regarded as nonalcoholic steatohepatitis (NASH) which shows activity of steatohepatitis with the potential for a lethal outcome. In the development of NASH, “two hit theory” is assumed to be required, with the first hit being lipid accumulation in hepatocytes increasing the sensitivity of the liver to the second hit such as oxidative stress.(3) In this setting, emerging animal studies have demonstrated the protective effects of antioxidant (e.g., vitamin E, 1-aminobenzotriazole, curcumin, N-acetylcysteine, angiotensin II type I receptor blockers) in experimental models of NASH.(4–7) In human, small clinical trials have revealed potential usefulness of vitamin E in the treatment of NASH.(8–10) Interestingly, administration of fermented green tea extract which exerts antioxidant activity reduced hepatic triglyceride content in addition to necro/inflammation and fibrosis in rat NASH model.(11) These suggest that oxidative stress plays a pivotal role in the pathogenesis of NASH and expected to be one of the promising targets in the treatment of this disease.
Heme oxygenase (HO) is the rate limiting enzyme in the catabolism of heme, to yield biliverdin, carbon monoxide (CO), and free iron. HO exhibits antioxidant properties derived from the elimination of prooxidant heme as well as from biological activities of its reaction products, CO, biliverdin, bilirubin, and iron.(12) In mammals three isoforms, designated as HO-1, HO-2 and HO-3 have been identified in the liver. Among these HO isoforms, HO-3 is considered as processed pseudogenes derived from HO-2 transcripts.(13) While HO-2 is constitutively expressed mainly in parenchymal cells, HO-1 is prominent in Kupffer cells and is markedly induced in hepatocytes in the response to various stimuli. Interestingly, it has been reported that HO-1 expression was significantly increased in the liver from NASH patients and the increase reflected the severity of the disease.(14) These suggest that HO-1 functions as a defense system against oxidative stress in the liver. In fact, accumulating evidences have demonstrated hepatoprotective effects of HO-1. The induction HO-1 by cobalt protoporrphyrin (CoPP) protects human hepatocytes from ethanol-induced cytotoxicity.(15) Ginkgo biloba extract, a naturally occurring HO-1 inducer has been proved to ameliorate ethanol-induced sustained damage and redox imbalance on rat liver.(16) In addition, curcumin which has a beneficial effect on NASH has been recently proved to be an HO-1 inducer.(5,17)
In addition to cytoprotective properties of HO-1, the regulatory mechanisms of this gene have been revealed. A heme-binding factor Bach1 functions as a transcriptional repressor of the gene encoding HO-1 (Hmox1). Bach1 forms complexes with small Maf proteins and competes against NF-E2 related factor (Nrf2), the major transcriptional activator of Hmox1, for binding at the Maf recognition elements (MAREs) of Hmox1 enhancer. Consistently, mice lacking Bach1 exhibited constitutively high level of HO-1 expression in many tissues including the liver.(18) Further studies of Bach1−/− mice have revealed a hepatoprotective role of Bach1 ablation in LPS-induced liver injury.(19)
In the current study, we investigated the influence of Bach1 disruption in animal NASH model. Accompanied with marked up-regulation of HO-1 in the liver, we observed reduced hepatic steatosis in Bach1−/− mice with altered expressions of key genes that regulate hepatic lipid metabolism. Consistent with these findings, the absence of Bach1 also exhibited hepatoprotection confirmed by serum liver enzyme and hepatic malondialdehyde (MDA) concentration. Collectively, these data suggest Bach1 as a potential target in the treatment of NASH presumably via induction of HO-1.
Materials and Methods
Animals and experimental protocol
Male C57BL/6 mice were obtained from Charles River Laboratories (Yokohama, Japan). The generation of Bach1−/− mice on C57BL/6 background was described previously.(18) These mice at 10 weeks of age (5 mice per each group) were fed either methionine-choline deficient (MCD) diet (Oriental Yeast Co., Tokyo, Japan) or standard chow for 4 or 8 weeks with free access to drinking water. All animal protocols and studies were performed according to the guidelines of Institute of Laboratory Animal Science, Hiroshima University.
Histological studies
Liver samples were fixed in 10% formalin, embedded in paraffin, and sliced into 4 µm thick. These were subjected to standard procedure of hematoxylin and eosin (HE) staining or Azan-Mallory staining.
Biochemical assays
Serum alanine aminotransferase (ALT) levels were measured enzymatically. Hepatic triglyceride content was determined using Wako E test triglyceride kit (Wako chemical, Osaka, Japan) following lipid extraction.(20) Hepatic MDA levels were quantified using Lipid Peroxidation Assay Kit (Calbiochem, Gibbstown, NJ) with standardization of tissue protein concentrations.
HO activity
HO activity in the liver was determined as previously described.(21,22) In brief, microsome fraction isolated by ultracentrifugation was reacted with hemin as substrate, and bilirubin formation was measured using Beckman DU640 spectrophotometer (450 nm; Beckman Coulter, Fullerton, CA). The activity was expressed as nmol bilirubin formed per hour per mg of protein. To inhibit HO activity for a negative control, zinc protoporphyrin (ZnPP) in phosphate buffer was injected intraperitoneally at a dose of 7.5 mg/kg once a week for 8 weeks.
Quantitative real-time PCR
RNA was extracted from frozen liver tissues using RNeasy Mini Kit (Qiagen, Hilden, Germany). Complementary DNA was synthesized from 1 µg of total RNA with GeneAmp RNA PCR Core Kit (Applied Biosystems, Foster City, CA). Specific primers (Table 1) were designed using Primer 3 (http://frodo.wi.mit.edu/primer3/) on the basis of nucleotide sequences from Genbank. Quantitative real-time PCR was performed on Light Cycler system using Light Cycler FastStart DNA Master Plus SYBR Green I (Roche Applied Science, Basel, Switzerland). The mRNA expressions were normalized to the housekeeping gene, GAPDH as an internal control.
Table 1.
Primer used for quantitative real-time PCR
| Gene | Forward | Reverse |
|---|---|---|
| HO-1 | acatcgacagccccaccaagttcaa | ctgacgaagtgacgccatctgtgag |
| PPARα | tgcaaacttggacttgaacg | tgatgtcacagaacggcttc |
| MTP | catgtcagccatcctgtttg | ctcgcgataccacagactga |
| αSMA | tcctccctggagaagagctac | tataggtggtttcgtggatgc |
| TGF-β | tgcgcttgcagagattaaaa | ctgccgtacaactccagtga |
| GAPDH | agaacatcatccctgcatcc | ttgtcattgagagcaatgcc |
HO-1, heme oxygenase-1; PPARα, peroxisome proliferator-activated receptor alpha; MTP, microsomal triglyceride transfer protein; αSMA, alpha smooth muscle actin; TGF-β, transforming growth factor beta; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
The results are expressed as the means ± SE. The statistical analysis was performed using Student’s t test and differences were considered statistically significant when p was less than 0.05.
Results
Hepatic HO-1 expression and its activity in the absence of Bach1
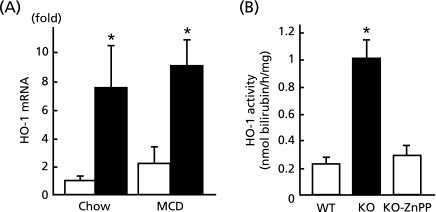
Fig. 1 demonstrates the regulation of HO-1 expression and its activity by Bach1. As shown in Fig. 1A, real-time PCR quantified a 8-fold up-regulation of HO-1 mRNA in Bach1−/− mice on chow diet. Despite not being statistically significant, there was 2-fold up-regulation of mRNA by MCD diet in wild type mice. Consistent with these changes in mRNA expressions, Fig. 1B demonstrates 5-fold increase in hepatic HO-1 activity in the absence of Bach1, which was entirely repressed by intraperitoneal injection of ZnPP, an HO-1 inhibitor.
Fig. 1.
Hepatic HO-1 expression and its activity in the absence of Bach1. (A) Wild type mice (open bar) and Bach1−/− mice (closed bar) were fed either regular chow or MCD diet for 8 w prior to quantification of hepatic expression of HO-1 mRNA by real-time PCR (n = 5/each group). (B) HO-1 activity in the liver from wild type (WT) or Bach1−/− (KO) mice were determined with standardlized of protein concentration (n = 5/each group). ZnPP was utilized as a HO-1 inhibitor for negative control. *p<0.05, wild type vs Bach1−/− mice.
Influence of Bach1 ablation on hepatic lipid metabolism
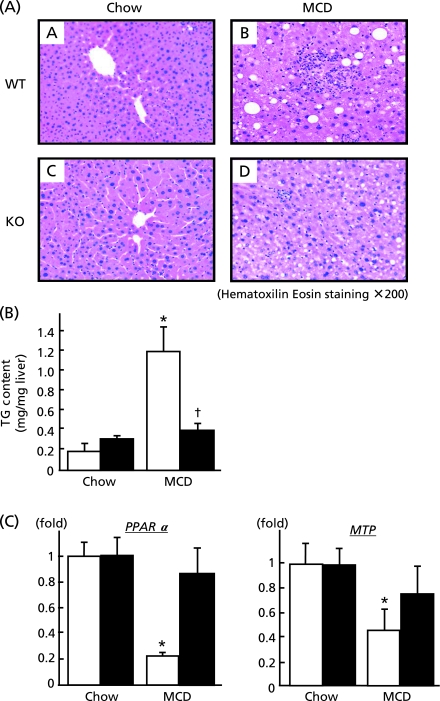
Eight weeks feeding of MCD diet induced moderate infiltration of inflammatory cells with macrovesicular steatosis in the liver of wild type mice as depicted in Fig. 2A (A, B). In contrast to wild type mice, Bach1−/− mice appeared devoid of hepatic steatosis (C, D). In keeping with these morphological changes, hepatic triglyceride content increased by 6-fold in wild type mice following MCD diet feeding whereas values remained unchanged in Bach1−/− mice. To further explore the effect of Bach1 ablation, mRNA expressions of key genes involving in hepatic lipid metabolism were investigated. This revealed marked down-regulation of PPARα and MTP in wild type mice whereas there were no significant changes in Bach1−/− mice, suggesting that fatty acid utilization and excretion were preserved in Bach1−/− mice on MCD diet.
Fig. 2.
Influence of Bach1 ablation on hepatic lipid metabolism. (A) Liver sections from wild type (A and B) and Bach1−/− mice (C and D) fed either regular chow (left panels) or MCD diet (right panels) were processed for haematoxylin & eosin (HE) staining. (original magnification 200×). (B) Hepatic triglyceride concentrations in wild type (open bars) and Bach1−/− (closed bars) mice (n = 5/each group) were determined after feeding either regular chow or MCD diet. (C) Hepatic mRNA expressions of PPARα and MTP were quantified (n = 5/each group) by quantitative real-time PCR. *p<0.05, regular chow vs MCD diet. †p<0.05, wild type vs Bach1−/− mice.
Protective effect of Bach1 ablation on MCD diet-induced liver injury
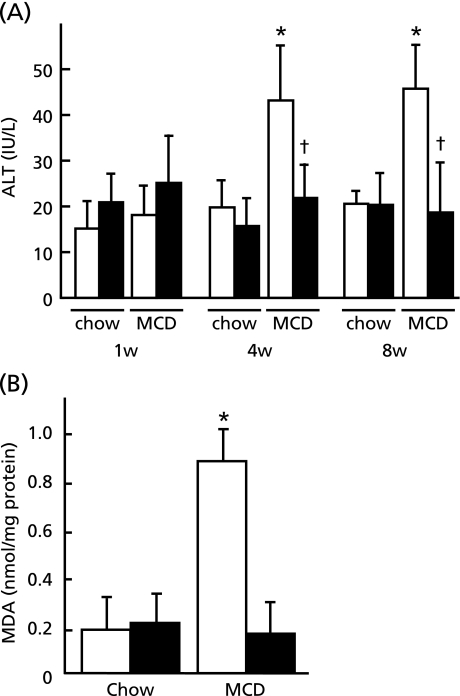
Feeding of MCD diet increased serum ALT levels in wild type mice at 4 and 8 weeks whereas it remained the basal level in Bach1−/− mice, implying protective effect of Bach1 ablation against liver injury (Fig. 3A). Consistent with these findings, hepatic MDA concentration which reflects oxidative damage elicited by lipid peroxidation in the liver remained unchanged in Bach1−/− mice following MCD diet (Fig. 3B). This was in marked contrast to 4-fold increase in MDA level in wild type mice on MCD diet.
Fig. 3.
Protective effect of Bach1 ablation on MCD diet-induced liver injury. (A) Serum ALT levels were determined at indicated time points by 8 w in wild type mice and Bach1−/− mice fed either regular chow or MCD diet (n = 5/each group). (B) Hepatic MDA concentration was assessed with standarlization of protein concentration in wild type (open bar) and Bach1−/− (closed bar) mice (n = 5/each group). *p<0.05, regular chow vs MCD diet. †p<0.05, wild type vs Bach1−/− mice.
Influence of Bach1−/− ablation on hepatic fibrogenesis
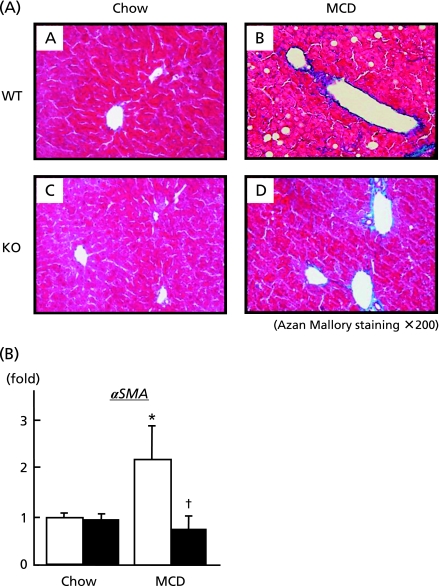
As previously reported,(23) rodents fed MCD diet develops steatohepatitis mimicking human NASH. Fig. 4A depicts mild pericellular fibrosis with fat deposition in the liver from wild type mice fed MCD diet (upper panel). In contrast, Bach1−/− mice displayed no significant change in the liver (lower panel). Consistent with these morphological changes, hepatic mRNA expression of α smooth muscle actin (αSMA) which is predominantly expressed in activated stellate cells was up-regulated in the liver of wild type mice on MCD diet while it remained unchanged in Bach1−/− mice.
Fig. 4.
Influence of Bach1−/− ablation on hepatic fibrogenesis. (A) Liver sections from wild type (A and B) and Bach1−/− mice (C and D) fed either regular chow (left panels) or MCD diet (right panels) were processed for Azan-Mallory staining. (original magnification 200×). (B) Following 4 w of either regular chow or MCD diet, hepatic mRNA expressions of αSMA were quantified (n = 5/each group) by quantitative real-time PCR. *p<0.05, regular chow vs MCD diet. †p<0.05, wild type (open bar) vs Bach1−/− mice (closed bar).
Discussion
The aim of this study was to explore the influence of Bach1 ablation in the development of NASH. As demonstrated in Fig. 1, Bach1 ablation in mice leads to significant up-regulation of HO-1 mRNA as well as its activity. This was in keeping with previous data that hepatic expressions of HO-1 mRNA and protein were constitutively high in the absence of Bach1.(18) To date, HO-1, β-globin, ferritin, thioredoxin reductase1 and NADP(H) quinine (oxido) reductase have been identified as a target genes of Bach1, but other downstream genes of Bach1 have not been fully understood.(24) HO-1 has been reported to have cytoprotective, anti-inflammatory and anti-oxidative properties. Emerging reports have suggested protective roles of HO-1 in experimental-induced hepatic damage including lipopolysaccharide-induced injury, immune liver fibrosis model, and hepatic ischemia-reperfusion injury.(12,19,25–27) In addition, pathophysiological importance of HO-1 has also been suggested by previous findings that HO-1 is induced under various etiological conditions including human NASH.(14)
The pathogenesis of NASH is widely recognized as two hit theory, in which the first hit is an initial metabolic disturbance causes steatosis and second pathogenic stimulus such as free radical and cytokines triggers necro/inflammation leading to the progression of steatohepatitis. The current study was undertaken on the basis of our initial hypothesis that HO-1 induction by Bach1 ablation may ameliorate oxidative stress and inflammation, the second hit in NASH pathogenesis and may repress the transition from bland steatosis to steatohepatitis. Surprisingly, in addition to reduction in hepatic damage, Bach1 ablation significantly diminished hepatic steatosis following MCD feeding. This was explained by the finding in this study that hepatic expression of PPARα and MTP, that are involved in hepatic fatty acid oxidation and hepatic secretion of triglyceride-rich VLDL, remained unchanged in MCD diet-fed Bach1−/− mice in comparison to significant reduction in wild type mice. These changes were inversely paralleled with hepatic MDA concentrations, suggesting the possibility that relief from oxidative damage in Bach1−/− mice conserved hepatic lipid metabolism. Along a similar line, the previous study of ob/ob mice fed MCD diet has revealed that YHK, a naturally derived herbal medicine that is reported to reduce reactive oxygen species declined hepatic steatosis via induction of hepatic mRNAs of PPARα and MTP.(28,29) This suggests a potential mechanism of the previous findings that antioxidant agents have beneficial effects not only on hepatic necro/inlflammation but also on hepatic steatosis.(4,28) Since mice assigned to high fat diet or dietary choline restriction exhibited mitochondrial dysfunction which negatively affects the bioenergenetics of liver, antioxidative status observed in Bach−/− mice might preserve the integrity of the mitochondria from oxidative damage and help to maintain cellular functions including hepatic lipid metabolism.(30,31) However, the current study lacks the direct evidence that hepatoprotective property against NASH in Bach1−/− mice was due to reduction in oxidative stress attributed to HO-1 induction, and further investigations are needed.
As shown in Fig. 4, MCD diet caused modest pericellular fibrosis with significant induction of αSMA mRNA in wild type mice whereas Bach1−/− mice exhibited almost normal in liver histology as well as αSMA expression. Reduction in hepatic fibrosis is likely to be elicited by relief of hepatic damage and inflammation in Bach1−/− mice. However, previous report has demonstrated that HO-1 is expressed in human hepatic myofibroblast and induction of HO-1 leads to inhibition of collagen synthesis and to reduction in proliferation of myofibroblast.(32) This suggests direct role for HO-1 induction against the development of hepatic fibrosis and might be a possible explanation for our finding.
In the current study, MCD dietary model was utilized as a NASH animal model. Although this model is widely used on the basis that morphological changes of the liver resembles human NASH with induction of oxidative stress, this does not entirely reflect the etiological background of human NASH such as obesity and insulin resistance. Alternative dietary models for NASH include high fat diet, which develops obesity and insulin resistance but not remarkable steatohepatitis as seen in MCD diet model.(33) Interestingly, recent study has reported that feeding rats with choline-deficient fat-rich diet for 8 w lead to marked steatohepatitis.(34)
In conclusion, Bach1−/− mice are resistant to the development of hepatic steatohepatitis with concomitant induction of HO-1 in MCD diet-induced NASH model. Reduced steatosis in Bach1−/− mice following MCD diet might be attributable to preserved expressions of PPARα and MTP that play an important role in hepatic fatty acid oxidation and triglyceride secretion respectively. Hepatic levels of MDA, a biomarker of oxidative stress, as well as serum ALT, an indicator of liver injury were significantly decreased Bach1−/− mice compared to wild type mice, suggesting anti-oxidative effect of HO-1 induction in the absence of Bach1. Since oxidative stress, the second hit in NASH pathogenesis causes impairment of cellular bioenergetics leading to further disruption of hepatic lipid metabolism, abatement of oxidative stress by Bach1 inhibition might be one of therapeutic strategies for the treatment of NASH/NAFLD.
Acknowlegment
This study was supported by a Grant-in-aid from the Ministry of Health, Labor and Welfare of Japan to S. Tazuma (Grant number: H20-Nanchi-Ippan-025).
Abbreviations
- HO
heme oxygenase
- NASH
nonalcoholic steatohepatitis
- MCD diet
methionine-choline deficient diet
- PPARα
peroxisome proliferator-activated receptor α
- MTP
microsomal triglyceride transfer protein
- MDA
malondialdehyde
- ALT
alanine aminotransferase
- NAFLD
nonalcoholic fatty liver disease
- CO
carbon monoxide
- CoPP
cobalt protoporrphyrin
- Nrf2
NF-E2 related factor
- MAREs
Maf recognition elements
- HE
hematoxylin and eosin
- ZnPP
zinc protoporphyrin
- αSMA
alpha smooth muscle actin
References
- 1.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43:S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 3.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 4.Nan YM, Wu WJ, Fu N, et al. Antioxidants vitamin E and 1-aminobenzotriazole prevent experimental non-alcoholic steatohepatitis in mice. Scand J Gastroenterol. 2009;15:1–11. doi: 10.1080/00365520903114912. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez-Tortosa MC, Ramirez-Tortosa CL, Mesa MD, Granados S, Gil A, Quiles JL. Curcumin ameliorates rabbits’s steatohepatitis via respiratory chain, oxidative stress, and TNF-alpha. Free Radic Biol Med. 2009;47:924–931. doi: 10.1016/j.freeradbiomed.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Baumgardner JN, Shankar K, Hennings L, Albano E, Badger TM, Ronis MJ. N-acetylcysteine attenuates progression of liver pathology in a rat model of nonalcoholic steatohepatitis. J Nutr. 2008;138:1872–1879. doi: 10.1093/jn/138.10.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirose A, Ono M, Saibara T, et al. Angiotensin II type 1 receptor blocker inhibits fibrosis in rat nonalcoholic steatohepatitis. Hepatology. 2007;45:1375–1381. doi: 10.1002/hep.21638. [DOI] [PubMed] [Google Scholar]
- 8.Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: a pilot study. Aliment Pharmacol Ther. 2001;15:1667–1672. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 9.Dufour JF, Oneta CM, Gonvers JJ, et al. Swiss Association for the Study of the Liver: Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–1543. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 10.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–2490. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 11.Nakamoto K, Takayama F, Mankura M, et al. Beneficial effects of fermented green tea extract in a rat model of non-alcoholic steatohepatitis. J Clin Biochem Nutr. 2009;44:239–246. doi: 10.3164/jcbn.08-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi T, Shimizu H, Morimatsu H, et al. Heme oxygenase-1 is an essential cytoprotective component in oxidative tissue injury induced by hemorrhagic shock. J Clin Biochem Nutr. 2009;44:28–40. doi: 10.3164/jcbn.08-210-HO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi S, Omata Y, Sakamoto H, et al. Characterization of rat heme oxygenase-3 gene. Implication of processed pseudogenes derived from heme oxygenase-2 gene. Gene. 2004;336:241–250. doi: 10.1016/j.gene.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Malaguarnera L, Madeddu R, Palio E, Arena N, Malaguarnera M. Heme oxygenase-1 levels and oxidative stress-related parameters in non-alcoholic fatty liver disease patients. J Hepatol. 2005;42:585–591. doi: 10.1016/j.jhep.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 15.Liu LG, Yan H, Zhang W, et al. Induction of heme oxygenase-1 in human hepatocytes to protect them from ethanol-induced cytotoxicity. Biomed Environ Sci. 2004;17:315–326. [PubMed] [Google Scholar]
- 16.Yao P, Li K, Song F, et al. Heme oxygenase-1 upregulated by Ginkgo biloba extract: potential protection against ethanol-induced oxidative liver damage. Food Chem Toxicol. 2007;45:1333–1342. doi: 10.1016/j.fct.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 17.McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ. Curcumin induces heme oxygenase-1 in hepatocytes and is protective in simulated cold preservation and warm reperfusion injury. Transplantation. 2006;81:623–626. doi: 10.1097/01.tp.0000184635.62570.13. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Hoshino H, Takaku K, et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002;21:5216–5224. doi: 10.1093/emboj/cdf516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iida A, Inagaki K, Miyazaki A, Yonemori F, Ito E, Igarashi K. Bach1 deficiency ameliorates hepatic injury in a mouse model. Tohoku J Exp Med. 2009;217:223–229. doi: 10.1620/tjem.217.223. [DOI] [PubMed] [Google Scholar]
- 20.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 21.Yano Y, Ozono R, Oishi Y, et al. Genetic ablation of the transcription repressor Bach1 leads to myocardial protection against ischemia/reperfusion in mice. Genes Cells. 2006;11:791–803. doi: 10.1111/j.1365-2443.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida T, Kikuchi G. Reaction of the microsomal heme oxygenase with cobaltic protoporphyrin IX, and extremely poor substrate. J Biol Chem. 1978;253:8479–8482. [PubMed] [Google Scholar]
- 23.Nabeshima Y, Tazuma S, Kanno K, Hyogo H, Chayama K. Deletion of angiotensin II type I receptor reduces hepatic steatosis. J Hepatol. 2009;50:1226–1235. doi: 10.1016/j.jhep.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Hintze KJ, Katoh Y, Igarashi K, Theil EC. Bach1 repression of ferritin and thioredoxin reductase1 is heme-sensitive in cells and in vitro and coordinates expression with heme oxygenase1, beta-globin, and NADP(H) quinone (oxido) reductase1. J Biol Chem. 2007;282:34365–34371. doi: 10.1074/jbc.M700254200. [DOI] [PubMed] [Google Scholar]
- 25.Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006;39:479–491. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- 26.Wang F, Duan ZJ, Sun YJ. Influence of heme oxygenase-1 expression on immune liver fibrosis induced by cobalt protoporphyrin in rats. World J Gastroenterol. 2009;15:3009–3014. doi: 10.3748/wjg.15.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt R, Tritschler E, Hoetzel A, et al. Heme oxygenase-1 induction by the clinically used anesthetic isoflurane protects rat livers from ischemia/reperfusion injury. Ann Surg. 2007;245:931–942. doi: 10.1097/01.sla.0000256891.45790.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Lima VM, de Oliveira CP, Sawada LY, et al. Yo jyo hen shi ko, a novel Chinese herbal, prevents nonalcoholic steatohepatitis in ob/ob mice fed a high fat or methionine-choline-deficient diet. Liver Int. 2007;27:227–234. doi: 10.1111/j.1478-3231.2006.01405.x. [DOI] [PubMed] [Google Scholar]
- 29.Stefano JT, de Oliveira CP, Corrêa-Giannella ML, et al. Nonalcoholic steatohepatitis (NASH) in ob/ob mice treated with yo jyo hen shi ko (YHK): effects on peroxisome proliferator-activated receptors (PPARs) and microsomal triglyceride transfer protein (MTP) Dig Dis Sci. 2007;52:3448–3454. doi: 10.1007/s10620-007-9810-8. [DOI] [PubMed] [Google Scholar]
- 30.Mantena SK, Vaughn DP, Andringa KK, et al. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417:183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hensley K, Kotake Y, Sang H, et al. Dietary choline restriction causes complex I dysfunction and increased H(2)O(2) generation in liver mitochondria. Carcinogenesis. 2000;21:983–989. doi: 10.1093/carcin/21.5.983. [DOI] [PubMed] [Google Scholar]
- 32.Li L, Grenard P, Nhieu JT, et al. Heme oxygenase-1 is an antifibrogenic protein in human hepatic myofibroblasts. Gastroenterology. 2003;125:460–469. doi: 10.1016/s0016-5085(03)00906-5. [DOI] [PubMed] [Google Scholar]
- 33.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Takayama F, Egashira T, Kawasaki H, et al. A novel animal model of nonalcoholic steatohepatitis (NASH): hypoxemia enhances the development of NASH. J Clin Biochem Nutr. 2009;45:335–340. doi: 10.3164/jcbn.09-29. [DOI] [PMC free article] [PubMed] [Google Scholar]