Abstract
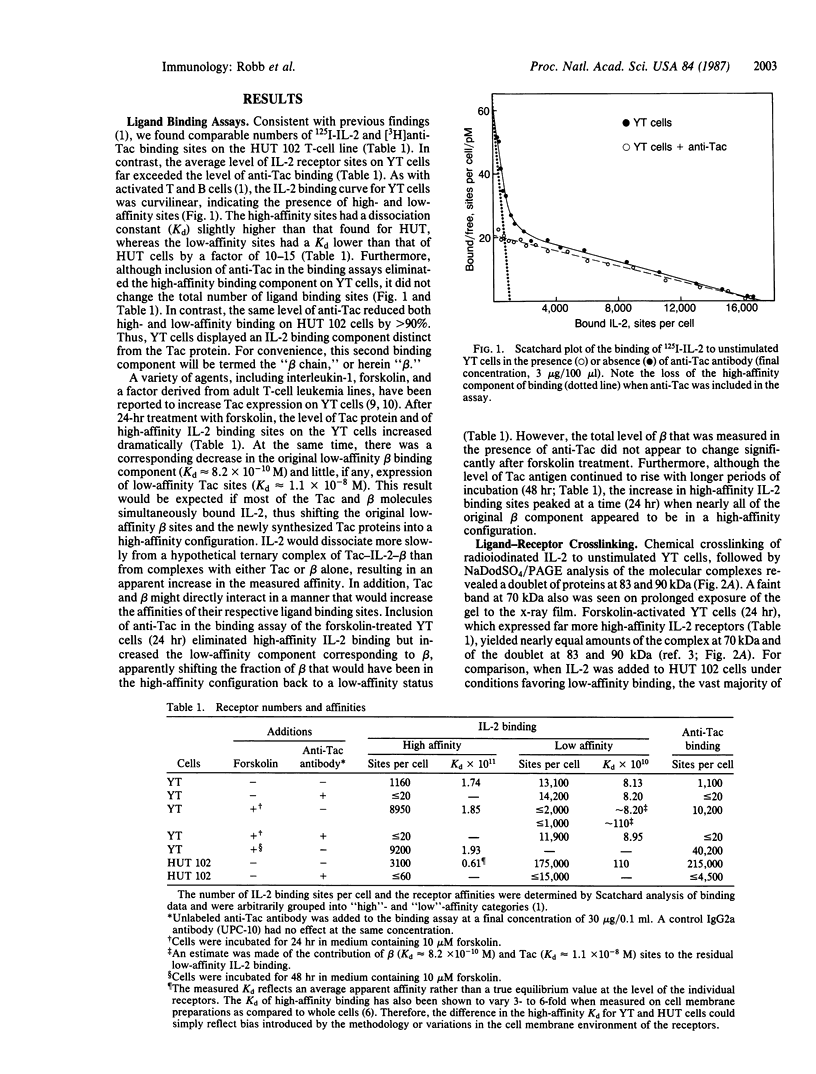
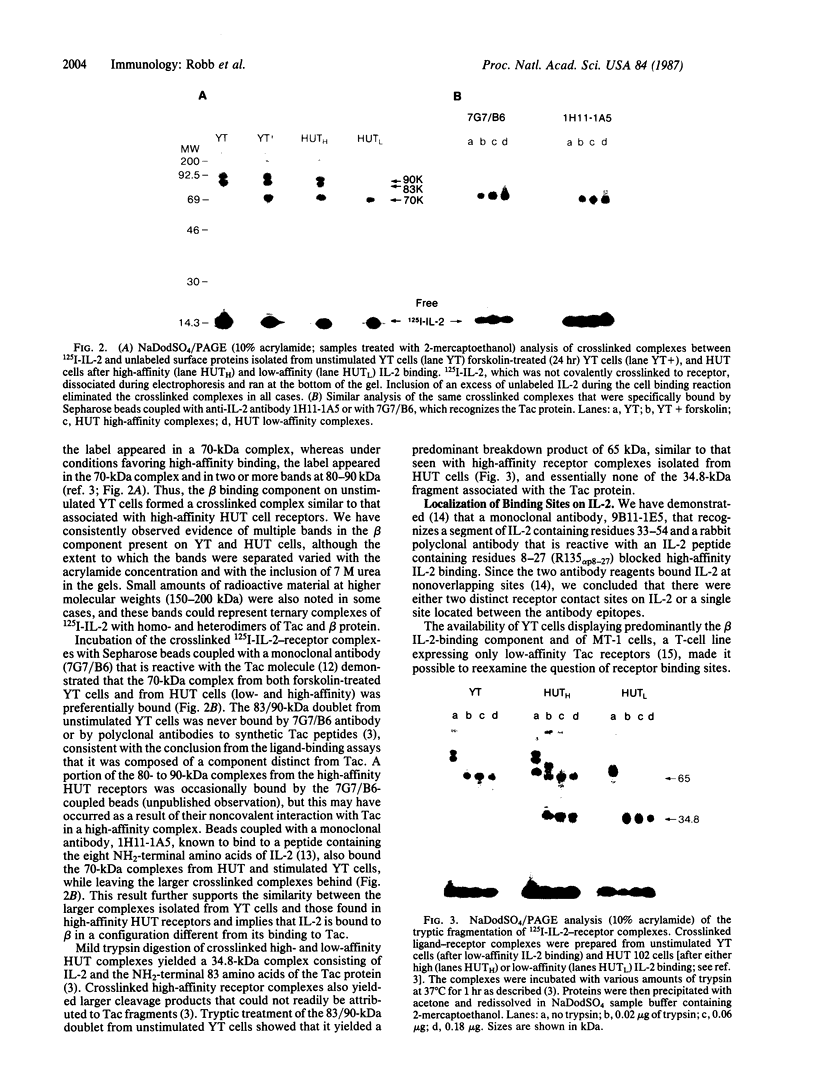
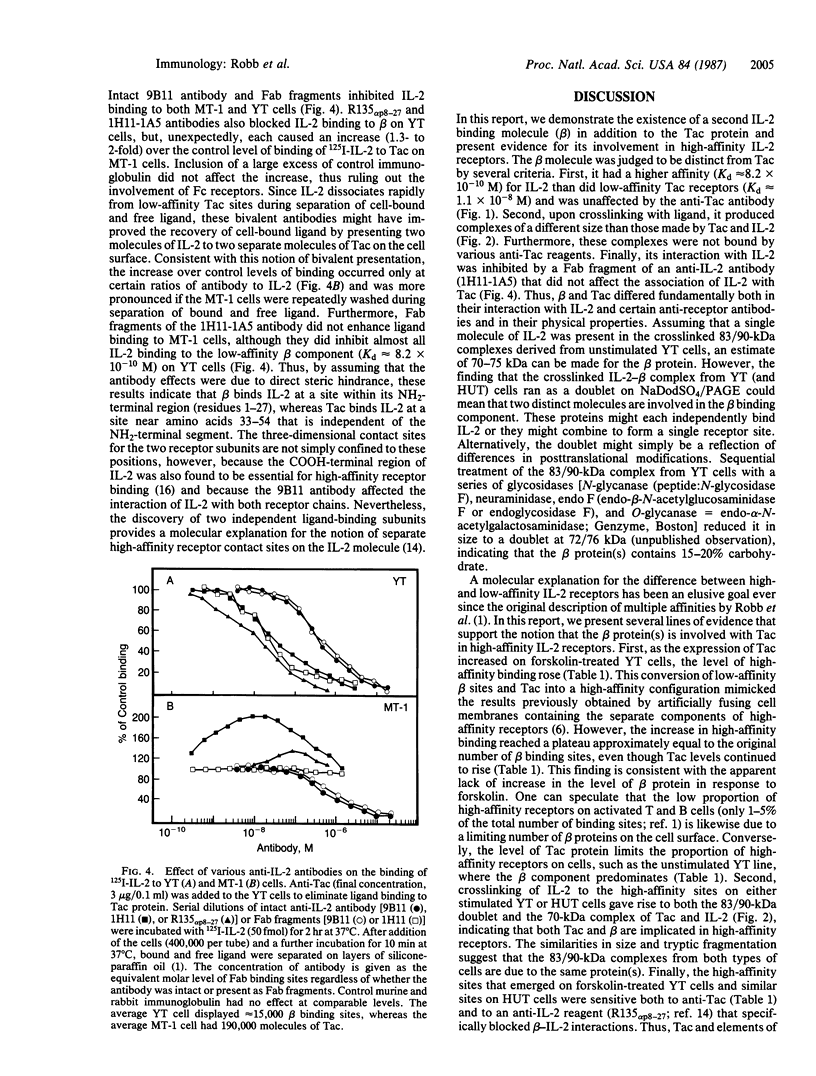
Interleukin 2 (IL-2) receptors on activated T cells exist in high- and low-affinity configurations, both of which share a ligand-binding component known as the Tac protein. Although almost all binding of IL-2 to such cells was inhibited by an antibody to Tac, the predominant component of binding on the natural killer (NK)-like cell line YT was resistant to this reagent. The ligand-binding component on YT cells also differed from Tac in its affinity constant (Kd approximately 8.2 X 10(-10) M vs. Kd approximately equal to 1.1 X 10(-8) M for low-affinity Tac sites) and in its susceptibility to inhibition by certain antibodies to IL-2. When the YT cells were stimulated in a manner that induced expression of the Tac protein, the IL-2 binding sites were converted to a high-affinity configuration (Kd approximately 1.8 X 10(-11) M). Thus, the original binding component on unstimulated YT cells appeared to combine with Tac and IL-2 to produce a high-affinity receptor complex. Use of bifunctional crosslinking agents following ligand binding to unstimulated YT cells yielded covalent IL-2-receptor complexes of 83 and 90 kDa. These complexes were similar in size to those derived from high-affinity receptors on activated T cells and shared a similar fragmentation pattern upon proteolysis. These results demonstrate the existence of a second IL-2 binding component in addition to the Tac protein and suggest that this component combines with Tac and IL-2 to form high-affinity receptor sites.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell J. D., Robb R. J., Malek T. R. Proliferation of T lymphocytes in response to interleukin 2 varies with their state of activation. J Immunol. 1986 Oct 15;137(8):2572–2578. [PubMed] [Google Scholar]
- Bost K. L., Smith E. M., Blalock J. E. Regions of complementarity between the messenger RNAs for epidermal growth factor, transferrin, interleukin-2 and their respective receptors. Biochem Biophys Res Commun. 1985 May 16;128(3):1373–1380. doi: 10.1016/0006-291x(85)91092-7. [DOI] [PubMed] [Google Scholar]
- Fujii M., Sugamura K., Sano K., Nakai M., Sugita K., Hinuma Y. High-affinity receptor-mediated internalization and degradation of interleukin 2 in human T cells. J Exp Med. 1986 Mar 1;163(3):550–562. doi: 10.1084/jem.163.3.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Minamoto S., Uchiyama T., Hardy R. R., Yamada G., Taniguchi T. Reconstitution of functional receptor for human interleukin-2 in mouse cells. Nature. 1985 Dec 5;318(6045):467–470. doi: 10.1038/318467a0. [DOI] [PubMed] [Google Scholar]
- Kondo S., Shimizu A., Maeda M., Tagaya Y., Yodoi J., Honjo T. Expression of functional human interleukin-2 receptor in mouse T cells by cDNA transfection. Nature. 1986 Mar 6;320(6057):75–77. doi: 10.1038/320075a0. [DOI] [PubMed] [Google Scholar]
- Kuo L. M., Robb R. J. Structure-function relationships for the IL 2-receptor system. I. Localization of a receptor binding site on IL 2. J Immunol. 1986 Sep 1;137(5):1538–1543. [PubMed] [Google Scholar]
- Kuo L. M., Rusk C. M., Robb R. J. Structure-function relationships for the IL 2-receptor system. II. Localization of an IL 2 binding site on high and low affinity receptors. J Immunol. 1986 Sep 1;137(5):1544–1551. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Liang S. M., Thatcher D. R., Liang C. M., Allet B. Studies of structure-activity relationships of human interleukin-2. J Biol Chem. 1986 Jan 5;261(1):334–337. [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A. T., Gerard J. P., Henderson L. E., Farrar W., Hopkins R. F., 3rd, Herberman R. B., Rabin H. Effects of natural and recombinant IL 2 on regulation of IFN gamma production and natural killer activity: lack of involvement of the Tac antigen for these immunoregulatory effects. J Immunol. 1984 Aug;133(2):779–783. [PubMed] [Google Scholar]
- Ralph P., Jeong G., Welte K., Mertelsmann R., Rabin H., Henderson L. E., Souza L. M., Boone T. C., Robb R. J. Stimulation of immunoglobulin secretion in human B lymphocytes as a direct effect of high concentrations of IL 2. J Immunol. 1984 Nov;133(5):2442–2445. [PubMed] [Google Scholar]
- Robb R. J. Conversion of low-affinity interleukin 2 receptors to a high-affinity state following fusion of cell membranes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3992–3996. doi: 10.1073/pnas.83.11.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Kutny R. M., Panico M., Morris H., DeGrado W. F., Chowdhry V. Posttranslational modification of human T-cell growth factor. Biochem Biophys Res Commun. 1983 Nov 15;116(3):1049–1055. doi: 10.1016/s0006-291x(83)80248-4. [DOI] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Biddison W. E., Goldman N. D., Nelson D. L. A monoclonal antibody 7G7/B6, binds to an epitope on the human interleukin-2 (IL-2) receptor that is distinct from that recognized by IL-2 or anti-Tac. Hybridoma. 1985 Summer;4(2):91–102. doi: 10.1089/hyb.1985.4.91. [DOI] [PubMed] [Google Scholar]
- Sharon M., Klausner R. D., Cullen B. R., Chizzonite R., Leonard W. J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986 Nov 14;234(4778):859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- Shirakawa F., Tanaka Y., Eto S., Suzuki H., Yodoi J., Yamashita U. Effect of interleukin 1 on the expression of interleukin 2 receptor (Tac antigen) on human natural killer cells and natural killer-like cell line (YT cells). J Immunol. 1986 Jul 15;137(2):551–556. [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Kozak R. W., Goldman C. K., Waldmann T. A. Demonstration of a non-Tac peptide that binds interleukin 2: a potential participant in a multichain interleukin 2 receptor complex. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9694–9698. doi: 10.1073/pnas.83.24.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yodoi J., Teshigawara K., Nikaido T., Fukui K., Noma T., Honjo T., Takigawa M., Sasaki M., Minato N., Tsudo M. TCGF (IL 2)-receptor inducing factor(s). I. Regulation of IL 2 receptor on a natural killer-like cell line (YT cells). J Immunol. 1985 Mar;134(3):1623–1630. [PubMed] [Google Scholar]






