Abstract
Objective
To evaluate cognitive control in children with attention-deficit/hyperactivity disorder (ADHD) using oculomotor tests of executive function.
Method
Cross-sectional study of children aged 8 to 13 years with ADHD (n = 26) and controls (n = 33) used oculomotor tasks to assess sensorimotor function (visually guided saccades), resistance to peripheral distractors (fixation), response inhibition (antisaccades), and spatial working memory (memory-guided saccades).
Results
All children had intact sensorimotor function and working memory. Children with ADHD showed susceptibility to peripheral distractors and deficits in response inhibition. Increased interstimulus (IS) fixation periods on the antisaccade task were associated with improved performance and decreased reaction times on correct trials for controls but not for children with ADHD. Attention-deficit/hyperactivity disorder–combined and inattentive subtypes showed different patterns of reaction time as a function of IS periods.
Conclusions
Response inhibition deficits in ADHD on oculomotor tasks are consistent with other studies. The failure of children with ADHD to use IS time to decrease response inhibition errors and reaction time suggests that IS time is not used to prepare a response. These findings highlight the importance of considering cognitive processing components affected by ADHD in addition to core behavioral symptoms, particularly in designing new treatment strategies.
Keywords: ADHD, oculomotor, antisaccade, response inhibition, response preparation
Attention-deficit/hyperactivity disorder (ADHD) is characterized by behavioral symptoms of inattention and may include hyperactivity and impulsivity. Studies have demonstrated that children with ADHD often have difficulty on a wide range of tasks requiring complex abilities classified under the umbrella term of executive function or cognitive control.1,2 These abilities include filtering out distractors, inhibiting automatic responses, keeping important information active, and planning to carry out goal-directed behavior. One widely recognized theory of ADHD identifies response inhibition as the core deficit.2
The oculomotor system provides several advantages for investigating executive function and therefore has the potential to reveal specific behavior limitations and, by inference, associated brain regions that function distinctly in ADHD. The neurophysiology and neuroanatomy of the oculomotor pathways have been well characterized in single-cell neuron studies in nonhuman primates.3,4 The oculomotor system consists of a widely distributed network, including the frontal eye fields, posterior parietal cortex, supplementary eye fields, presupplementary motor area, dorsolateral prefrontal cortex, superior colliculus, basal ganglia, thalamus, and cerebellum.3 Volumetric and functional imaging studies implicate frontal-striatal and cerebellar brain regions in executive function deficits observed with ADHD.5-8 Given the overlap between oculomotor and attentional networks,9,10 oculomotor tasks are a means of investigating the integrity of these regions.
Oculomotor tasks are objective nonverbal tests that have been used to study cognitive and brain systems in typically developing populations11,12 and those with neurodevelopmental disorders.3 The tasks are simple, easily performed by children, unlikely to be affected by verbal or other learning strategies, and use the visual domain for both encoding and responding. The visually guided saccade (VGS) task requires the subject to fixate a suddenly appearing stimulus13 and serves as a control task to assess underlying impairments in basic sensorimotor function. The fixation (FIX) task requires the subject to look straight ahead despite the appearance of peripheral distractors, which assesses the ability to inhibit a response toward the distractors. The antisaccade (AS) task requires the subject to inhibit a reflexive response to a suddenly appearing stimulus and instead, to look to the mirror location.14 The AS is used to assess voluntary response suppression or response inhibition, a core component of executive function. In addition, performance and reaction time as a function of the interstimulus fixation period, or the duration of delay between fixation and the stimulus, were also assessed. The memory-guided saccade (MGS) task requires the subject to look to the location of a previously presented visual target15 and assesses maintenance of spatial working memory.
Studies of ADHD using oculomotor tasks have found greater frequency of intrusive saccades during fixation periods16-18 and response inhibition deficits on AS tasks16,19-21 and MGS tasks.17,20 Studies of children with Tourette’s syndrome identify different deficits on oculomotor tasks than those found in ADHD.22,23 These studies have not specifically investigated performance and reaction time as function of varied interstimulus fixation time. An advantage of the AS task is the ability to assess reaction time on correct response inhibition trials. Other response inhibition paradigms, such as the continuous performance test and go/no-go task, do not generate a reaction time on correct response inhibition trials. On the AS task, performance and reaction time are evaluated as a function of the duration of time between fixation and the stimulus. In our AS task, the instructional cue (a red cross) is present throughout the interstimulus period and serves as the fixation point before the appearance of the stimulus.
The primary goal of this study was to evaluate differences in cognitive control in children with ADHD compared with controls using oculomotor tests of executive function. A secondary goal was to conduct post hoc analyses of potential differences between ADHD-combined (ADHD-C) and ADHD-predominantly inattentive (ADHD-I) subtypes. Attempts to differentiate ADHD-C and ADHD-I subtypes using neuropsychological tests yield mixed results.24-26 The lack of differences between behaviorally defined subtypes has been attributed to heterogeneity in the ADHD phenotype and the recognition of multiple causal pathways leading to ADHD.1
We expand and improve on previous studies of AS performance in ADHD by assessing performance and reaction time as a function of interstimulus fixation period. We chose interstimulus fixation periods of 0.5, 2, 4, and 6 seconds based on past single-cell and neuroimaging studies that tested a wide range of interstimulus fixation periods and found that the level of activation in specific brain regions during the interstimulus period is critical to AS performance.27-29 For example, single neuron recordings show a reduction in the level of preparatory saccade-related activity in the superior colliculus before stimulus presentation on AS trials.3 Long periods (>6 seconds) were avoided because these may lead to fatigue and the initiation of additional non–task-related processes. In addition, in our study, participants were free of comorbid disorders and group-matched for age, IQ, and socioeconomic status.
We hypothesized that, compared with controls, children with ADHD would show intact sensorimotor processing, susceptibility to peripheral distractors, and impairments in response inhibition, the use of interstimulus fixation time, and working memory.
METHOD
Subjects
Participants were children aged 8 to 13 years who met DSM-IV criteria for ADHD (n = 26). Participants with ADHD were recruited by flyers and referrals from general pediatric, child development, and psychiatry clinics in Pittsburgh and the surrounding area. The diagnosis of ADHD was established by a qualified professional before study entry and confirmed by the primary author (board-certified developmental-behavioral pediatrician) using a telephone interview and questionnaires reviewing diagnostic criteria for ADHD and comorbid disorders, including the National Initiative for Children’s Healthcare Quality Vanderbilt Assessment Scale for Parents and Teachers (Vanderbilt),30,31 a questionnaire based on DSM-IV behavioral criteria and functional outcomes, and the Achenbach Child Behavior Checklist and the Teacher Report Form.32 In some cases, previous diagnostic assessment, medical, and school records were also reviewed. The diagnosis of ADHD was confirmed if, on the Vanderbilt, children had six of nine symptoms of inattention rated at a level of 2 or 3 on a four-point Likert scale (0 = never, 1 = occasionally, 2 = often, and 3 = very often) and had evidence of impairment in at least two settings as rated by two independent raters (parent and teacher). Children with ADHD were classified as ADHD-C if they also had 6 or more of 9 items on a hyperactivity and impulsivity scale scored as positive (level of 2 or 3; see Table 1 for average symptom scores) and as ADHD-I if the sum of scores on the hyperactivity and impulsivity scales were less than 12. For two children, there was a discrepancy between parent and teacher scores in determining ADHD subtype; teacher information was used to make the final classification. The ADHD-C group was slightly younger in age and had more boys than girls compared with the ADHD-I group.
TABLE 1.
Demographic Characteristics
| Characteristic | Controls (n = 33) | ADHD-all (n = 26) | ADHD-C (n = 14) | ADHD-I (n = 12) | p |
|---|---|---|---|---|---|
| Age, y | 10.4 ± 1.7 | 10.2 ± 1.6 | — | — | .579 |
| 10.4 ± 1.7 | — | 9.5 ± 1.4 | 10.9 ± 1.5 | .072 | |
| IQ | 106.3 ± 11.6 | 107 ± 10.8 | — | — | .806 |
| 106.3 ± 11.6 | — | 107.6 ± 8.9 | 106.3 ± 13 | .934 | |
| Inattentiona symptoms | 19 ± 4.6 | 19.6 ± 5.2 | .765 | ||
| Hyperactive-impulsivea symptoms | 16.2 ± 4.9 | 6.1 ± 3.1 | <.001 | ||
| Sex | 17:16 | 16:10 | — | — | .307 |
| (male:female) | 17:16 | — | 11:3 | 5:7 | .125 |
| Raceb | 25:8 | 15:11 | — | — | .116 |
| (white:nonwhite) | 25:8 | — | 7:7 | 8:4 | .224 |
| SESc | 16:17 | 11:15 | — | — | .417 |
| (high school:college) | 16:17 | — | 6:8 | 5:7 | .893 |
| Stimulant naïve (no vs. yes) | NA | — | 11:3 | 6:6 | .133 |
| Current stimulant (yes vs. no) | NA | — | 9:5 | 6:6 | .368 |
Note: For age, IQ, inattention symptoms, and hyperactive-impulsive symptom, data are presented as mean ± SD and were analyzed by one-way analysis of variance or t test. For sex, race, SES, stimulant naïve, and current stimulant, data are presented as ratios and were analyzed by χ2 —exact significance: one-sided for two-group comparisons, two-sided for three-group comparisons. ADHD = attention-deficit/hyperactivity disorder; ADHD-all = all children with ADHD; ADHD-C = ADHD-combined type; ADHD-I = ADHD-predominantly inattentive type; NA = not applicable; SES = socioeconomic status.
Inattention and hyperactive-impulsive symptom scores are presented from the Vanderbilt scales and range from 12 to 27 when consistent with DSM-IV criteria (6 or more symptoms at a level of 2 [often] or 3 [very often]).
U.S. Census Bureau race/ethnicity categories were collapsed into white versus nonwhite because the only nonwhite group represented was African American.
Socioeconomic status as indicated by maternal education.
Children with ADHD were excluded for learning problems, because of the impact of this common comorbidity on executive function and potential overlap in underlying neural regions associated with learning problems such as dyslexia.33,34 Learning problems were defined as history of grade retention, enrollment in special education, or presence of an individualized education plan for specific learning disability. A total of 130 children with ADHD were screened; most of those excluded had learning problems. Oppositional defiant disorder, a common comorbid condition, was not excluded. Four children with ADHD-C and five with ADHD-I had positive screens for oppositional defiant disorder on the parent Vanderbilt.
Children with ADHD on stimulants were not excluded but withheld medication on the day of testing. Withholding medication on weekends is common in children with ADHD and typically done in research studies to examine performance while unmedicated. Although more children with ADHD-C had current or previous history of stimulant use, there were no statistical differences between the two ADHD subtypes (Table 1).
Children in the control group (n = 33) had no evidence of ADHD or any Axis I psychiatric disorder as determined by questionnaire. Controls were part of an ongoing study of typically developing children undergoing the same procedures and conducted in the same laboratory. The controls were recruited by flyers, magazine advertisements, and word of mouth. The controls were group-matched to children with ADHD for age, sex, IQ, race (white versus nonwhite), and maternal education (high school, trade school, some college but no degree versus 4-year college degree or more) as a measure of socioeconomic status (Table 1).
The children in all groups were excluded for IQ lower than 80; history on screening questionnaires of tics, Tourette’s syndrome, conduct disorder, autism spectrum disorders, psychosis, bipolar disorder, and major depression; major neurological disorder (i.e., seizures, history of meningitis or encephalitis, head injury); eye movement abnormalities (i.e., strabismus); hearing impairments; genetic syndromes; non-English speaker; and use of oral steroids, atomoxetine, or psychotropic medication for anxiety, depression, or aggression. The controls were also excluded for personal and family history of any Axis I psychiatric disorder in a first-degree relative as determined by questionnaire.
Experimental procedures complied with the Code of Ethics of the World Medical Association (1964 Declaration of Helsinki) and the standards of the Children’s Hospital of Pittsburgh and the University of Pittsburgh institutional review board. A parent or legal guardian provided informed consent. Children provided assent. The subjects were compensated for participation.
Design and Procedures
All children completed testing procedures in fixed order, starting with eye movement testing. IQ was assessed using the Wechsler Intelligence Scale for Children III-R dyad consisting of vocabulary and block design that correlates highly with full-scale IQ scores (r = 0.91).35
Eye Movement Test Procedures
Participants were tested in a darkened room, seated comfortably, and positioned 56 cm from a 17-in. PC monitor where stimuli were displayed. Movement was minimized with a table-mounted chin rest and head restraint. Eye movement measurements were acquired with an Applied Science Laboratories model 504 (ASL, Bedford, MA) table-mounted near-infrared eye tracker with a sampling rate of 60 Hz. A technician monitored eye movements during task performance in real time using E5WIN software (ASL) and provided instructions when necessary. Task stimuli were presented using E-Prime software (Psychology Software Tools, Pittsburgh, PA).
Eye Movement Tasks
Participants were given standardized instructions before each task and allowed to practice the tasks while monitored by a technician, who answered any questions and confirmed that participants understood the instructions. Participants were required to complete at least five trials correctly before testing began to ensure that lack of comprehension of task instructions was not the reason for poor performance. Correct performance of the task was emphasized rather than speed of responding during the instructions. The VGS task was presented first to avoid any possible impact of previous performance of more complicated tasks on this simple sensorimotor task. Then, the FIX, AS, and MGS were presented.
In all four tasks, peripheral targets were presented in the horizontal plane at randomized locations 4 or 8 degrees of visual angle right or left from central fixation. We varied the time during fixation (500 milliseconds and 2, 4, or 6 seconds) on VGS, FIX, and AS tasks to assess group differences in the use of interstimulus fixation time. Interstimulus fixation periods were used in equal proportions and randomized within each task. During VGS, FIX, and AS tasks, there was a 200-millisecond gap between the fixation cross and target presentation. For VGS, FIX, and AS tasks, 48 trials were presented. For MGS task, 32 trials were presented.
VGS Task
The participants were instructed to fixate a green central fixation cross and then look toward the peripheral light (target) that appeared for 1 second. Dependent measures were proportion of errors, latency (saccadic reaction time [SRT], measured as the interval from fixation offset to the initiation of the saccade to the target), and accuracy of saccades to peripheral locations (error measured in degrees of visual angle).
FIX Task
The participants were instructed to fixate a blue central fixation cross for a variable delay and to hold gaze at the central fixation area after the cross was extinguished for a 200-millisecond gap period. The gap was followed by the appearance of a small circular target that appeared at a randomized location for 1 second. Any trial containing a break from central fixation to saccade toward a peripheral target (movement greater than 50 pixels) was considered a failed trial. The dependent measure was proportion of errors or the proportion of trials with breaks from fixation.
AS Task
The participants were instructed to fixate a red central fixation cross for a variable delay. Fixation was extinguished for a 200-millisecond gap, after which a peripheral target appeared for 1 second. The subjects were to direct their gaze to the mirror location of the target. Dependent measures were proportion of errors, latency of primary saccades to peripheral targets on correct trials, and accuracy for spatial location of saccades. The impact of interstimulus fixation period on latency and performance of the task (proportion of errors) was also evaluated.
MGS Task
The participants were instructed to fixate a yellow central fixation cross. After 1.925 seconds, a small target appeared at a randomized location for 75 milliseconds. The participants were instructed not to gaze at the peripheral target but to remember its location during the ensuing working memory delay period of 2.5 or 7.5 seconds. After the delay, the central fixation cross was extinguished, and the participants had 2 seconds to saccade to the remembered location. After 2 seconds, a feedback cross appeared at the correct target location for 2 seconds before starting the next trial. Dependent measures were latency and accuracy of initial and final resting saccade to the remembered location on correct trials. Trials in which the subject made a saccade toward the peripheral target before the end of the delay period were considered failed trials (failures of response inhibition), which were analyzed separately from the correct trials and reported as proportion of errors.
Eye Movement Analysis
Eye movement recordings were analyzed offline using a combination of ILAB36 and in-house programswritten in MATLAB (MathWorks, Natick, MA). Results of algorithm-based measurements were presented graphically and numerically online to a technician for inspection of measurements from each saccade of each trial of every task. Saccades were identified using a velocity algorithm using a 30-degree/s criterion, which reliably detects 0.25-degree saccades. Trials with saccade latencies of less than 80 milliseconds were omitted from the analysis to exclude any predictive responses that were not guided by task stimuli.37 Rare blink artifacts, which occurred on less than 10% of trials and occasionally resulted in failure of the software to identify primary saccades, were also identified and excluded.
Data Analysis
Trials within each experimental condition were averaged for each subject. Data were missing for one control subject on the VGS task and for two controls and one ADHD-C subject on the FIX task because of technical difficulty. Appropriate adjustments in the degrees of freedom are reported for these tasks. Repeated-measures analysis of variance with visual field (right versus left), eccentricity (target location: near [4 degrees] versus far [8 degrees]), and interstimulus fixation period as repeated factors were applied to the data. No differences were found for right versus left visual fields; hence, they were collapsed across visual fields. Differences due to target location occurred for the AS task and are discussed only for that task. Group was considered a between-subjects factor, and planned comparisons were used. Interstimulus fixation period was analyzed for group-by-factor interactions on latency and proportion of errors. The main group analysis compared controls with all the children with ADHD (ADHD-all). Post hoc analyses of ADHD subtypes compared children with ADHD-C with those with ADHD-I. For post hoc analyses, age was used as a covariate because there was a difference in age between the groups (p = .02). Linear interpolation was used for the few missing data points as has been used before.38 All tests were two tailed.
RESULTS
Two-Group Comparisons on Each Task
VGS Task
There were no group differences in proportion of errors, F1,57 = 0.013, p = .910; latency, F1,57 = 0.334, p = .566; or accuracy, F1,57 = 0.534, p = .468 (Table 2).
TABLE 2.
Oculomotor Task Results
| Task | Controls | ADHD-all | p | Effect Size (Cohen d) |
|---|---|---|---|---|
| Visually guided saccade | (n = 32) | (n = 26) | ||
| Proportion of errors | 0.0103 ± 0.015 | 0.0107 ± 0.014 | .910 | 0.03 |
| Latency, ms | 232.88 ± 38.73 | 226.99 ± 38.32 | .566 | −0.16 |
| Accuracy (degrees of visual angle) | 1.28 ± .73 | 1.16 ± 0.50 | .468 | −0.19 |
| Fixation | (n = 31) | (n = 25) | ||
| Proportion of errors | 0.11 ± 0.14 | 0.26 ±0.19 | .001 | 0.90 |
| Antisaccade | (n = 33) | (n = 26) | ||
| Proportion of errors | 0.46 ± 0.21 | 0.61 ± 0.22 | .010 | 0.70 |
| Accuracy (degrees of visual angle) | 4.39 ± 1.23 | 4.72 ± 0.94 | .260 | 0.30 |
| Memory-guided saccade | (n = 33) | (n = 26) | ||
| Proportion of errors | 0.26 ± 0.19 | 0.41 ± 0.22 | .006 | 0.73 |
| Accuracy | ||||
| Initial saccade (degrees of visual angle) | 2.62 ± 1.14 | 2.52 ± 0.87 | .713 | −0.10 |
| Resting saccade (degrees of visual angle) | 2.25 ± 1.30 | 2.17 ± 0.79 | .780 | −0.07 |
Note: Data (mean values collapsed across all interstimulus fixation or delay periods) were analyzed using t test to determine differences between groups for proportion of errors for all tasks; latency only for visually guided saccade; and accuracy for spatial locations for visually guided saccade, antisaccade, and memory-guided saccade. ADHD = attention-deficit/hyperactivity disorder; ADHD-all = all children with ADHD.
FIX Task
The ADHD group showed more errors than the controls, F1,54 = 11.50, p = .001.
AS Task
Proportion of errors
The ADHD group showed more inhibitory errors than the controls, F1,57 = 7.06, p = .010.
Interstimulus fixation period on AS latency (SRT)
There was an interaction between group and interstimulus fixation period on SRT for correct AS trials, F3,54 = 4.34, p = .008 by Pillai trace, indicating that the control group showed decreased SRT for longer interstimulus fixation periods, whereas the ADHD group did not (Fig. 1). Post hoc analyses of SRT as a function of interstimulus fixation period were conducted within each group. Within the control group, pairwise comparisons (adjusted for multiple comparisons using a Bonferroni correction) showed a significant difference in SRT at 0.5 second compared with the SRTs at 2-, 4-, and 6-second periods, all p < .005. Pairwise comparisons of the SRT at 2-, 4-, and 6-second periods showed differences only with the 0.5-second period but no other differences, all p > .05. Within the ADHD group, pairwise comparisons showed no differences in SRT between any of the interstimulus fixation periods, all p > .05 (Table 3).
Fig. 1.
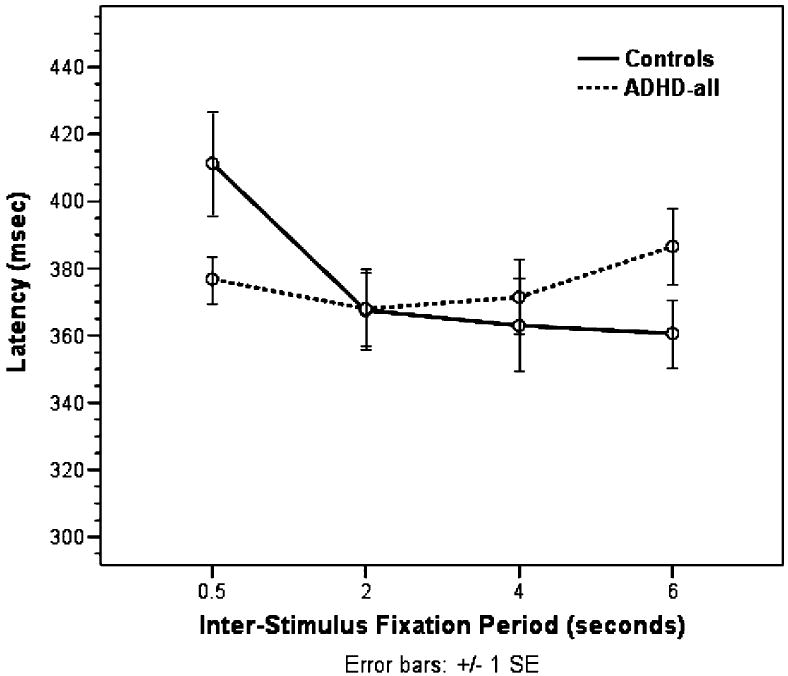
Antisaccade latency: group by interstimulus fixation period interaction.
TABLE 3.
Latency as a Function of Interstimulus Fixation or Delay Period on the AS and MGS Tasks
| Task | IS Fixation Period or Delay Period, s | Groups |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Controlsa (n = 33) |
ADHD-alla (n = 26) |
ADHD-Cb (n = 14) |
ADHD-Ib (n = 12) |
||||||
| SRT ± SD | SE | SRT ± SD | SE | SRT ± SD | SE | SRT ± SD | SE | ||
| AS | 0.5 | 411 ± 86 | 15 | 377 ± 34 | 7 | 381 ± 30 | 8 | 372 ± 40 | 12 |
| 2 | 368 ± 60 | 11 | 368 ± 58 | 12 | 347 ± 45 | 12 | 395 ± 64 | 19 | |
| 4 | 363 ± 80 | 14 | 371 ± 56 | 11 | 380 ± 64 | 17 | 361 ± 44 | 13 | |
| 6 | 361 ± 57 | 10 | 387 ± 54 | 11 | 406 ± 56 | 15 | 362 ± 42 | 13 | |
| MGS | 2.5 | 589 ± 136 | 24 | 638 ± 124 | 24 | 657 ± 136 | 36 | 615 ± 110 | 32 |
| 7.5 | 485 ± 138 | 24 | 603 ± 133 | 26 | 644 ± 139 | 37 | 554 ± 112 | 32 | |
Note: ADHD = attention-deficit/hyperactivity disorder; ADHD-all = all children with ADHD; ADHD-C = ADHD-combined type; ADHD-I = ADHD-predominantly inattentive type; AS = antisaccade; IS = interstimulus; MGS = memory-guided saccade; SRT = saccadic reaction time (in milliseconds).
AS task: Repeated-measures analysis of variance with IS period as repeated factor and group (controls versus ADHD-all) as between-subjects variable showed a significant interaction between group and IS period on SRT for correct AS trials (p = .008). Pairwise comparisons of SRT as a function of IS period within the control group showed significant difference in SRT at 0.5 second (in bold) compared with SRT at 2-, 4-, and 6-second periods (p < .005, adjusted for multiple comparisons); SRT at 2-, 4-, and 6-second periods differed with SRT at 0.5 second but not others (all p > .05). Within the ADHD-all group, pairwise comparisons showed no differences in SRT between any IS periods (all p > .05). MGS task: Repeated-measures analysis of variance with delay as repeated factor and group (controls versus ADHD-all) as between-subjects variable showed main effects of delay (p < .001) and group (p = .007) and trend for group-by-delay interaction (p = .063) on SRTs (bold) on correct MGS trials.
AS task: Repeated-measures analysis of variance with IS period as repeated factor and group (ADHD-C versus ADHD-I) as between-subjects factor showed significant interaction between subtype and IS period on SRT for correct AS trials (p = .001). Pairwise comparisons within subtype showed difference in SRT only between 2- and 6-second periods (in bold) for the ADHD-C group (p = .006); within the ADHD-I group, there were no differences in SRT for any IS comparisons (all p > .05). MGS task: For the subtype comparison, there were no main effects or trend for group-by-delay interaction (all p > .05) on the MGS task.
Interstimulus fixation period on AS performance (proportion of errors)
There were main effects of interstimulus fixation period, F3,55 = 2.87, p = .04 by Pillai trace, and group, F1,57 = 4.39, p = .04, on proportion of errors but no group by interstimulus fixation period interaction, F3,55 = 2.05, p = .118. Children with ADHD made the same proportion of errors on the AS regardless of the duration of fixation, whereas the control children improved performance with increasing fixation times (Fig. 2).
Fig. 2.
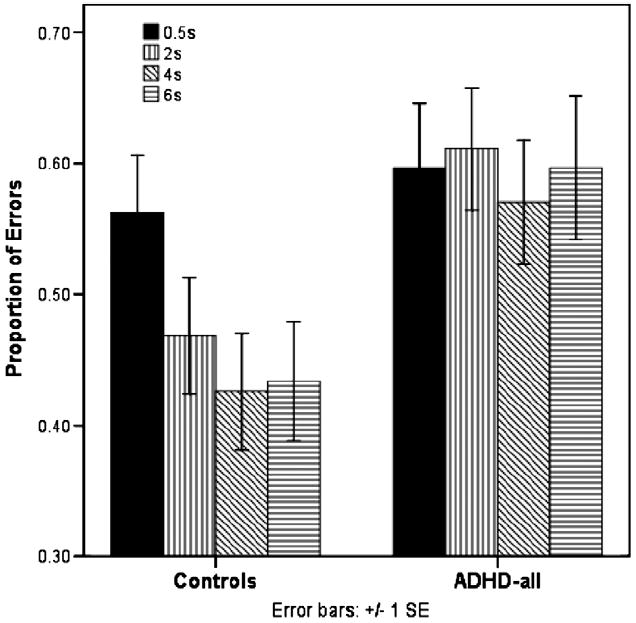
Antisaccade proportion of errors.
Location on AS performance (proportion of errors)
There was a trend for group by location interaction on proportion of errors on the AS, F1,57 = 3.91, p = .053. Although all children showed a higher proportion of errors when targets appeared near as opposed to far, children with ADHD were more susceptible to this effect, showing an even higher proportion of errors on near targets compared with the controls.
Accuracy
There were no group differences in accuracy for saccade locations, F1,57 = .235, p = .630.
MGS Task
Proportion of errors
The ADHD group showed more inhibitory errors (i.e., made a saccade to the peripheral target before the end of the delay period), F1,57 = 8.25, p = .006.
Delay on MGS latency (SRT)
There was a main effect of delay, F1,57 = 14.84, p < .001, and group, F1,57 = 7.72, p = .007, and a trend for group-bydelay interaction by Pillai trace, F1,57 = 3.61, p = .063. The long delay resulted in faster SRTs than the short delay; the control children had faster SRTs than the children with ADHD; and there was a trend for the control children to show shorter SRT at the long delay compared with the children with ADHD, whose SRT was only slightly decreased at the long delay (Table 3).
Accuracy
There were no differences between groups in the accuracy of initial or resting saccades. Both groups improved their accuracy for spatial locations from initial to resting saccades (Table 2).
ADHD Post Hoc Analyses
Proportion of Errors
For the post hoc comparison of ADHD-C (n = 14) versus ADHD-I (n = 12), there were no differences between subtypes in the proportion of errors on the FIX task, F1,22 = 0.045, p = .834; AS task, F1,23 = 0.777, p = .387; or MGS task, F1,23 = 0.625, p = .437. There was a significant effect of age on performance of the FIX task only, F1,22 = 4.71, p = .041.
Interstimulus Fixation Period
For the subtype comparison, the effect of interstimulus fixation period on AS latency remained significant by Pillai trace, F3,20 = 8.51, p = .001. The children with ADHD-C showed a decrease in SRT from the 0.5- to 2-second fixation period, then showed increased SRT with 4- and 6-second periods (Table 3). The children with ADHD-I demonstrated SRT that was essentially unchanged at all four periods. Within the ADHD-C group, pairwise comparisons showed a difference in SRT only between the 2- and 6-second periods, p = .006. Within the ADHD-I group, pairwise comparisons showed no differences in SRT between any of the interstimulus fixation periods, all p > .05. All pairwise comparisons were adjusted for multiple comparisons using a Bonferroni correction.
There were no main effects of interstimulus fixation period, F3,21 = 0.466, p = .709; group, F1,23 = 0.494, p =.489; age, F1,23 = 1.25, p = .275; or group-by–interstimulus fixation period interaction, F3,21 = 1.152, p = .351, on AS performance (proportion of errors). The trend for group-by-location interaction on AS performance did not remain, F1,23 = 1.417, p = .246.
Delay on MGS Latency
There was no main effect of delay, F1,23 = 0.668, p = .422; age, F1,23 = 0.403, p = .532; or group, F1,23 = 1.56, p = .224. The trend for group-by-delay interaction on MGS latency did not remain, F1,23 = 0.047, p = .83.
DISCUSSION
Sensorimotor Function
Similar to other studies,39,40 our study found no differences in overall percent correct, latency, or accuracy of saccades on the VGS. Impaired performance on the oculomotor tasks therefore cannot be attributed to sensorimotor impairment but rather is related to cognitive limitations.
Resistance to Peripheral Distractors
The children with ADHD have increased susceptibility to peripheral distractors. Other studies using various tasks found greater frequency of intrusive saccades during fixation periods in children with ADHD.16-18
Response Inhibition
On the AS and MGS tasks, we found response inhibition failures in the children with ADHD compared with the controls. Our data are consistent with other studies showing response inhibition deficits on oculomotor tasks in children with ADHD.16,20,41 A study of ADHD subtype differences in boys using oculomotor tasks found deficits in motor planning and response inhibition for the ADHD-C subtype but not for ADHD-I.39 Our post hoc analyses revealed no differences between ADHD-C and ADHD-I subtypes on measures of response inhibition. Prefrontal regions have been widely implicated in executive function or the cognitive control of behavior.42 Functional magnetic resonance imaging (fMRI) studies using different response inhibition paradigms show differences in the ability of the children with ADHD to activate frontostriatal regions compared with the controls.7,43
Spatial Working Memory
Similar to other studies,20,41,44 our study showed that children with ADHD did not show spatial working memory deficits on the MGS compared with the controls. Our task required maintenance of spatial locations but no manipulation of working memory information. Spatial working memory paradigms that include manipulation of working memory information have demonstrated differences between those with ADHD and the controls.45 These results suggest that processes supporting working memory maintenance are intact in ADHD and are distinct from inhibitory control.
Interstimulus Fixation
Children with ADHD did not improve their performance or reaction time on the AS with increased interstimulus fixation periods. When performing the task correctly, their SRT remained the same, regardless of the amount of time during the fixation period. This pattern of results was in contrast with those of the control children, who showed longer SRT at the shortest (0.5 second) fixation period and shorter SRT when given longer interstimulus fixation time, starting with the 2-second fixation period. Post hoc analyses showed that the children with ADHD-C had shorter SRT at the 2-second period and had longer SRT at 6 seconds, whereas the children with ADHD-I had SRT that remained the same regardless of interstimulus fixation period. These potential subtype differences should be interpreted with caution, given the different proportions of girls and boys in each subtype group. We found a trend for a similar pattern of results for SRT as a function of delay on the MGS task in the controls compared with all the children with ADHD.
The children with ADHD tend to be more slow and variable in their responses on a variety of reaction time tasks,46,47 which supports an alerting deficit or inability to prepare a response. One theoretical model of ADHD attributes cognitive and performance deficits in ADHD to underactivation or an inability to maintain readiness to respond.48 Behavioral activation is influenced by interstimulus interval in the children with ADHD, who show average performance at medium event rates and weakness at slow and fast event rates on response inhibition tasks such as continuous performance tests and go/no-go tasks.49,50 The stop task generates the stop signal reaction time, which provides a measure of capacity for inhibition but is not completely analogous to reaction time after the interstimulus fixation period. The children are instructed to press a key as quickly as possible in response to a particular visual stimulus except for a minority of trials when a stop signal occurs. Stop signal reaction time is generated as a measure of the amount of time needed to stop or interrupt the response. Numerous studies of the stop task indicate a deficit in response inhibition for children with ADHD, although there are questions about whether this relates to low-arousal responding. 1 In our AS task, the interstimulus fixation period is defined as the delay between the instructional cue (a red fixation cross) and the appearance of the stimulus. In the oculomotor literature, this period is referred to as a preparatory delay period or response preparation period. This definition differs from descriptions of response preparation in other paradigms, in which the stimulus appears and is then followed by a response preparation period and subsequent response execution. The exact cognitive processes that are taking place during the interstimulus fixation period are unknown. However, the differences in performance and reaction time as a function of interstimulus fixation period in the children with ADHD compared with the controls suggest that they do not use this time efficiently to prepare a response, consistent with an alerting deficit or behavioral underactivation.
Functional magnetic resonance imaging studies of oculomotor tasks have investigated the neural regions associated with the preparatory delay period as distinct from regions associated with activation from the motor response or eye movement itself.51,52 A study compared complete AS trials with AS “half trials” consisting only of instructions (i.e., provided the cue but no target) to assess the preparatory delay period. Both trial types resulted in activation in the frontal eye field, supplementary eye field, intraparietal sulcus, and precuneus, but the half trials also activated the left dorsolateral prefrontal cortex and anterior cingulate cortex, implicating these regions in response preparation but not response execution.53 An fMRI study of adults showed greater prestimulus preparatory activity in the presupplementary motor area during the AS compared with the VGS; greater presupplementary motor area activity was also critically associated with successful response inhibition.29 These fMRI studies that show differences in prefrontal and premotor circuits associated with response inhibition and response preparation on oculomotor tasks suggest that these regions may also be implicated in ADHD.
Study limitations include small sample size and a small number of correct trials because of poor performance of the subjects with ADHD. The number of trials for each task was not increased because of concerns of subject fatigue and subsequent impact on performance. Another limitation was the predominance of younger male children in our cohort of ADHD-C children, consistent with prevalence studies. Because of the small number of girls, we are unable to evaluate sex-related differences in performance, which may be a factor in studies of motor control. There were no differences in performance of ADHD-C and ADHD-I subtypes, with the exception of group-by-delay interaction on AS latency, which should be interpreted with caution, given the differences in sex in the subgroups. The small sample size may have resulted in an inability to detect subtype differences. Similar to many other studies, the lack of subtype differences suggests that there may not be significant neuropsychological differences between subtypes as they are currently defined.
Conclusions
Our study provides unique contributions to our understanding of ADHD beyond deficits in response inhibition by elucidating potential differences in response preparation. The AS task revealed a difference in how the children with ADHD performed the task compared with the controls, and post hoc analyses suggest a difference between ADHD-C and ADHD-I subtypes, which warrants replication. Children with ADHD are frequently described as having off-task behavior and an inability to complete tasks or assignments. Our results suggest that these difficulties not only are apparent in longer daily activities but also occur with much shorter time frames, such as AS task trials that last only a few seconds. These findings highlight the importance of considering the cognitive processing components that are affected by ADHD in addition to core behavioral symptoms, especially in devising new treatment strategies. Oculomotor tasks paired with fMRI hold promise for further investigation of the neural correlates underlying response inhibition and response preparation differences in ADHD.
Acknowledgments
This research was supported by Grant 5 R01 MH067924 from the National Institute of Mental Health and Grant R01 HD46500 from the National Institute of Child and Human Development; Seed Grant from the Research Advisory Council, Children’s Hospital of Pittsburgh; National Institutes of Health Pediatric Research Loan Repayment Program Award support for the first author (I.M.L.); and training grants T32 NS07495-5, T73 MC00036, and T77 MC 00031 from the National Institutes of Health and Maternal and Child Health Bureau, Health Resources and Services Administration, Health and Human Services (support for the first author [I.M.L.]).
The authors thank Lynne Huffman, M.D., for helpful comments and review of the manuscript. The authors also thank the children and families who made this work possible.
Footnotes
Disclosure: The authors report no conflicts of interest.
Contributor Information
IRENE M. LOE, Department of Pediatrics, Stanford University School of Medicine
HEIDI M. FELDMAN, Department of Pediatrics, Stanford University School of Medicine
ENAMI YASUI, Departments of Psychiatry and Psychology, University of Pittsburgh School of Medicine
BEATRIZ LUNA, Departments of Psychiatry and Psychology, University of Pittsburgh School of Medicine
References
- 1.Nigg JT. What Causes ADHD?: Understanding What Goes Wrong and Why. New York: Guilford Press; 2006. [Google Scholar]
- 2.Barkley RA. Attention-Deficit Hyperactivity Disorder, Third Edition: A Handbook for Diagnosis and Treatment. New York: Guilford Press; 2006. [Google Scholar]
- 3.Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- 4.Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- 5.Booth JR, Burman DD, Meyer JR, et al. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) J Child Psychol Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 6.Durston S, Hulshoff Pol HE, Schnack HG, et al. Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. J Am Acad Child Adolesc Psychiatry. 2004;43:332–340. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Durston S, Tottenham NT, Thomas KM, et al. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 8.Castellanos FX, Giedd JN, Marsh WL, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 9.Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. Neuroimage. 2001;14:310–321. doi: 10.1006/nimg.2001.0788. [DOI] [PubMed] [Google Scholar]
- 10.Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: overlapping neural systems. Neuroimage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- 11.Luna B, Thulborn KR, Munoz DP, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- 12.Munoz DP, Broughton JR, Goldring JE, Armstrong IT. Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- 13.Leigh RJ, Zee DS. The Neurology of Eye Movements. 3. New York: Oxford University Press; 1999. [Google Scholar]
- 14.Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- 15.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- 16.Munoz DP, Armstrong IT, Hampton KA, Moore KD. Altered control of visual fixation and saccadic eye movements in attention-deficit hyperactivity disorder. J Neurophysiol. 2003;90:503–514. doi: 10.1152/jn.00192.2003. [DOI] [PubMed] [Google Scholar]
- 17.Castellanos FX, Marvasti FF, Ducharme JL, et al. Executive function oculomotor tasks in girls with ADHD. J Am Acad Child Adolesc Psychiatry. 2000;39:644–650. doi: 10.1097/00004583-200005000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Gould TD, Bastain TM, Israel ME, Hommer DW, Castellanos F. Altered performance on an ocular fixation task in attention-deficit/ hyperactivity disorder. Biol Psychiatry. 2001;50:633–635. doi: 10.1016/s0006-3223(01)01095-2. [DOI] [PubMed] [Google Scholar]
- 19.Klein CH, Raschke A, Brandenbusch A. Development of pro- and antisaccades in children with attention-deficit hyperactivity disorder (ADHD) and healthy controls. Psychophysiology. 2003;40:17–28. doi: 10.1111/1469-8986.00003. [DOI] [PubMed] [Google Scholar]
- 20.Mostofsky SH, Lasker AG, Cutting LE, Denckla MB, Zee DS. Oculomotor abnormalities in attention deficit hyperactivity disorder: a preliminary study. Neurology. 2001;57:423–430. doi: 10.1212/wnl.57.3.423. [DOI] [PubMed] [Google Scholar]
- 21.Feifel D, Farber RH, Clementz BA, Perry W, Anllo-Vento L. Inhibitory deficits in ocular motor behavior in adults with attention-deficit/ hyperactivity disorder. Biol Psychiatry. 2004;56:333–339. doi: 10.1016/j.biopsych.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 22.Mostofsky SH, Lasker AG, Singer HS, Denckla MB, Zee DS. Oculomotor abnormalities in boys with Tourette syndrome with and without ADHD. J Am Acad Child Adolesc Psychiatry. 2001;40:1464–1472. doi: 10.1097/00004583-200112000-00018. [DOI] [PubMed] [Google Scholar]
- 23.LeVasseur AL, Flanagan JR, Riopelle RJ, Munoz DP. Control of volitional and reflexive saccades in Tourette’s syndrome. Brain. 2001;124:2045–2058. doi: 10.1093/brain/124.10.2045. [DOI] [PubMed] [Google Scholar]
- 24.Bauermeister JJ, Matos M, Reina G, et al. Comparison of the DSM-IV combined and inattentive types of ADHD in a school-based sample of Latino/Hispanic children. J Child Psychol Psychiatry. 2005;46:166–179. doi: 10.1111/j.1469-7610.2004.00343.x. [DOI] [PubMed] [Google Scholar]
- 25.Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. J Am Acad Child Adolesc Psychiatry. 2002;41:59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Geurts HM, Verte S, Oosterlaan J, et al. ADHD subtypes: do they differ in their executive functioning profile? Arch Clin Neuropsychol. 2005;20:457–477. doi: 10.1016/j.acn.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- 28.Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curtis CE, D’Esposito M. Success and failure suppressing reflexive behavior. J Cogn Neurosci. 2003;15:409–418. doi: 10.1162/089892903321593126. [DOI] [PubMed] [Google Scholar]
- 30.Wolraich ML, Lambert W, Doffing MA, Bickman L, Simmons T, Worley K. Psychometric properties of the Vanderbilt ADHD diagnostic parent rating scale in a referred population. J Pediatr Psychol. 2003;28:559–567. doi: 10.1093/jpepsy/jsg046. [DOI] [PubMed] [Google Scholar]
- 31.Wolraich ML, Feurer ID, Hannah JN, Baumgaertel A, Pinnock TY. Obtaining systematic teacher reports of disruptive behavior disorders utilizing DSM-IV. J Abnorm Child Psychol. 1998;26:141–152. doi: 10.1023/a:1022673906401. [DOI] [PubMed] [Google Scholar]
- 32.Achenbach TM. The Child Behavior Checklist and related instruments. In: Maruish ME, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. 2. Mahwah: Lawrence Erlbaum Associates, Publishers; 1999. pp. 429–466. [Google Scholar]
- 33.Willcutt EG, Pennington BF, Olson RK, et al. Neuropsychological analyses of comorbidity between reading disability and attention deficit hyperactivity disorder: in search of the common deficit. Dev Neuropsychol. 2005;27:35–78. doi: 10.1207/s15326942dn2701_3. [DOI] [PubMed] [Google Scholar]
- 34.Mayes SD, Calhoun SL, Crowell EW. Learning disabilities and ADHD: overlapping spectrum disorders. J Learn Disabil. 2000;33:417–424. doi: 10.1177/002221940003300502. [DOI] [PubMed] [Google Scholar]
- 35.Sattler JM. Assessment of Children’s Intelligence and Special Abilities. Boston: Allyn and Bacon; 1988. [Google Scholar]
- 36.Gitelman DR. ILAB: a program for postexperimental eye movement analysis. Behav Res Methods Instrum Comput. 2002;34:605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- 37.Fischer B, Biscaldi M, Gezeck S, Weber H. On the development of voluntary and reflexive components in human saccade generation. Brain Res. 1997;754:285–297. doi: 10.1016/s0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- 38.Luna B, Doll SK, Hegedus SJ, et al. Maturation of executive function in autism. Biol Psychiatry. 2007;61:474–481. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 39.O’Driscoll GA, Depatie L, Holahan A-LV, et al. Executive functions and methylphenidate response in subtypes of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1452–1460. doi: 10.1016/j.biopsych.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 40.Hanisch C, Radach R, Holtkamp K, Herpertz-Dahlmann B, Konrad K. Oculomotor inhibition in children with and without attention-deficit hyperactivity disorder (ADHD) J Neural Transm. 2006;113:671–684. doi: 10.1007/s00702-005-0344-y. [DOI] [PubMed] [Google Scholar]
- 41.Ross RG, Hommer D, Breiger D, Varley C, Radant A. Eye movement task related to frontal lobe functioning in children with attention deficit disorder. J Am Acad Child Adolesc Psychiatry. 1994;33:869–874. doi: 10.1097/00004583-199407000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey B. A neural basis for the development of inhibitory control. Dev Sci. 2002;5:F9–F16. [Google Scholar]
- 43.Vaidya CJ, Austin G, Kirkorian G, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci U S A. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross RG, Harris JG, Olincy A, Radant A. Eye movement task measures inhibition and spatial working memory in adults with schizophrenia, ADHD, and a normal comparison group. Psychiatry Res. 2000;95:35–42. doi: 10.1016/s0165-1781(00)00153-0. [DOI] [PubMed] [Google Scholar]
- 45.Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- 46.Oosterlaan J, Logan GD, Sergeant JA, Oosterlaan J, Logan GD, Sergeant JA. Response inhibition in AD/HD, CD, comorbid AD/HD + CD, anxious, and control children: a meta-analysis of studies with the stop task. J Child Psychol Psychiatry. 1998;39:411–425. [PubMed] [Google Scholar]
- 47.Huang-Pollock CL, Nigg JT, Huang-Pollock CL, Nigg JT. Searching for the attention deficit in attention deficit hyperactivity disorder: the case of visuospatial orienting. Clin Psychol Rev. 2003;23:801–830. doi: 10.1016/s0272-7358(03)00073-4. [DOI] [PubMed] [Google Scholar]
- 48.Sergeant JA, Oosterlaan J, van der Meere J. Information Processing and Energetic Factors in Attention-Deficit/Hyperactivity Disorder Handbook of Disruptive Behavior Disorders. Dordrecht: Kluwer Academic Publishers; 1999. pp. 75–104. [Google Scholar]
- 49.van der Meere J, Shalev R, Borger N, Gross-Tsur V. Sustained attention, activation and MPH in ADHD: a research note. J Child Psychol Psychiatry. 1995;36:697–703. [PubMed] [Google Scholar]
- 50.van der Meere JJ. The role of attention. In: Sandberg S, editor. Hyperactivity and Attention Disorders of Childhood. 2. Cambridge: Cambridge University Press; 2002. pp. 162–213. [Google Scholar]
- 51.Curtis CE, Connolly JD. Saccade preparation signals in the human frontal and parietal cortices. J Neurophysiol. 2008;99:133–145. doi: 10.1152/jn.00899.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeSouza JF, Menon RS, Everling S. Preparatory set associated with prosaccades and anti-saccades in humans investigated with event-related FMRI. J Neurophysiol. 2003;89:1016–1023. doi: 10.1152/jn.00562.2002. [DOI] [PubMed] [Google Scholar]
- 53.Brown MR, Vilis T, Everling S. Frontoparietal activation with preparation for antisaccades. J Neurophysiol. 2007;98:1751–1762. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]


