Abstract
Purpose
To perform a prospective analysis of the clinical outcomes of prophylactic antibiotic treatment before the standard surgical modality of living donor nephrectomy (LDN) without postoperative antibiotic treatment.
Materials and Methods
From November 2005 to June 2010, a total of 470 patients underwent LDN at our medical institution, and 280 of these patients were injected with 1 g cephalosporin 30 minutes before the operation. The group receiving prophylactic antibiotics was compared with a control group composed of 190 patients who received injections of 2 g cephalosporin per day for 5 days after the operation. The presence of fever, incidence of blood transfusion, and period of drainage use were compared between the two groups.
Results
There were no significant differences in gender, age, body mass index, incidence of blood transfusion after the operation, fever over 38℃ 3 days after the operation, or period of drain insertion between the single-dose group and the control group. The follow-up was conducted for 1 month after the operation, and 1 case of surgical site infection (SSI) was observed in each group (p=0.783).
Conclusions
Of 280 patients in the single-dose group, 1 contracted SSI. In comparison with the control group, which was dosed with prophylactic antibiotics for 5 days after the operation, the single-dose group did not have a significantly different occurrence of SSI. We found that the incidence rate of SSI did not increase, even though prophylactic antibiotics were not used after standard and conventional open surgeries, such as video-assisted minilaparotomy surgery.
Keywords: Antibiotic prophylaxis, Living donors, Nephrectomy, Surgical wound infection, Video-assisted surgery
INTRODUCTION
Surgical site infection (SSI) is the third most frequently reported nosocomial infection [1]. SSI is defined as an infection that occurs in the skin and soft tissue of a surgical site within 30 days after an operation. Any purulent discharge from a closed surgical incision, together with signs of inflammation of the surrounding tissue, should be considered to be a wound infection, irrespective of whether microorganisms can be cultured [2].
SSIs are a significant source of postoperative morbidity; they result in longer hospital stays and increased cost. Kirkland et al found that surgical patients (of all specialties) with SSI were twice as likely to die, 60% more apt to be admitted to an intensive care unit, and greater than five times as likely to require hospital readmission [3].
In recent times, antibiotics have been advanced, thus improving the treatment options for various bacterial diseases. Nevertheless, SSI is still an important problem, despite the prophylactic use of antibiotics; in particular, the overuse of antibiotics is a serious problem. Staphylococcus aureus, i.e., the major pathogen of SSI, easily develops antibiotic resistance. In particular, methicillin-resistant S. aureus infection is an important social problem, owing to the difficulty of treatment and patients' mental and physical anguish and financial burden [4,5].
Many recent studies have suggested that the postoperative administration of antibiotics is not necessary to prevent SSI if a standardized surgical treatment has been performed [6]. Standardized techniques are widely used in urologic surgeries, but the prolonged administration of antibiotics after an operation has not been fully studied [7].
At our institute, live donor nephrectomy (LDN) has been performed in accordance with the standardized video-assisted minilaparotomy surgery (VAMS) technique. This study was prospectively performed to ascertain whether a single dose of prophylactic antibiotics is sufficient to prevent SSI in patients who undergo LDN.
MATERIALS AND METHODS
This study was conducted on 470 patients who underwent VAMS-LDN at our institution between November 2005 and June 2010. The patients had not received any antibiotics before the operation and had no problems with immune function. The experimental group included 280 patients who were injected with 1 g cephalosporin 30 minutes before the operation, and these patients were compared with a control group composed of 190 patients who were injected with 2 g cephalosporin per day for 5 days after the operation.
Exclusion criteria included a history of allergic reaction to cephalosporin, age younger than 18 years or older than 80 years, concurrent use of systemic antibiotics or prophylactic antibiotics for a medical condition, extended anesthetic time due to cooperation with other departments, and inability to return for follow-up evaluation.
We collected information about the patient's characteristics, including their past history of hypertension, diabetes mellitus, and blood transfusion, and a calculation was made to determine the length of catheterization (the drain [Hemovac®; Zimmer, Dover, OH, USA] and Foley catheter). Body temperature was checked every day during hospitalization to identify cases in which it exceeded 38℃ even 3 days after the operation. During hospitalization (5 days) and 1 month after the operation, physical examination was used to determine whether the surgical site was infected.
For statistical analysis, SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) was used. To compare intergroup variables, the Student's t-test and the chi-square test were used. The significance level was set at a p-value of less than 0.05.
RESULTS
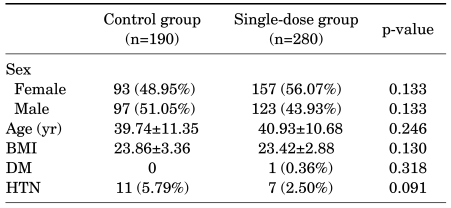
The 280 patients who were given the single dose of antibiotics (the single-dose group) were composed of 123 males (43.93%) and 157 females (56.07%), with an average age of 40.93 years (range, 17-63 years). The control group of 190 patients was composed of 97 males (51.05%) and 93 females (48.95%), with an average age of 39.74 years (range, 16-66 years). The body mass index (BMI) was 23.42 and 23.86 in the single-dose group and the control group, respectively, and no significant inter-group difference was observed (p=0.130). There was only one patient with diabetes mellitus in the single-dose group (0.36%), and there was no significant difference between the groups in the number of patients with hypertension (Table 1).
TABLE 1.
Patient characteristics in living donor nephrectomy
BMI: body mass index, DM: diabetes mellitus, HTN: hypertension
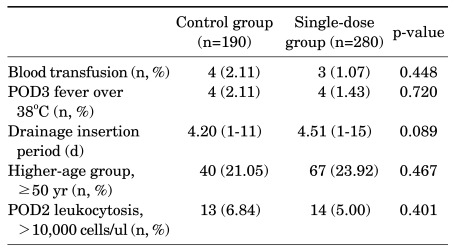
In the control group, 4 patients (2.11%) received a blood transfusion after the operation, and in the single-dose group, 3 patients (1.07%) received a transfusion (p=0.448). Each group had 4 patients who had a fever over 38℃ for 3 days after the operation, but this was not statistically significant (p=0.720). The period of drain insertion was 4.51 days (range, 1-15 days) and 4.20 days (range, 1-11 days) in the single-dose group and the control group, respectively, but there was no significant intergroup difference (p=0.089) (Table 2).
TABLE 2.
Perioperative results comparing the control group and the single-dose group
POD: postoperative day
The follow-up was conducted for 1 month after the operation, during which time 1 case of wound infection was observed at the superficial surgical site in each group (p=0.783).
DISCUSSION
SSI has been reported to account for 38% of all of pathogenic infections. To prevent pathogenic infection, prophylactic antibiotics have been used after operations [2]. There have been many cases in which prophylactic antibiotics were administered before the operation and were used until the drain was removed or the stitches removed. As surgical techniques have advanced, however, the operative time has been shortened, surgeries have become less invasive, and techniques have become standardized. As the case stands now, there is controversy about the postoperative use of prophylactic antibiotics [6,8].
The guidelines established by the American Urological Association stipulate that, in operations for clean-contaminated wounds, prophylactic antibiotics should only be used when risk factors are observed [9]. The European Association of Urology guidelines stipulate that prophylactic antibiotics are not to be used for cases in which laparoscopic surgeries or open surgeries are free from contamination, but, in clean-contaminated wounds, a single dose of prophylactic antibiotics needs to be given [10].
Prophylactic antibiotics should be used for the shortest possible period. Inappropriate antibiotic use increases environmental pressures favoring the emergence of antimicrobial-resistant bacteria that can cause SSI, resulting in an increase in the cost of health care [11]. In Korea, the use of antibiotics is hardly restricted, and antimicrobial-resistant bacteria, such as methicillin-resistant S. aureus, are more prevalent than in other countries. Methicillin-resistant bacteria have increased since 1970, and in 1987, resistant bacteria accounted for 55.6% of infections. In 2005, antimicrobial resistance was reported to have reached 70% [4,5].
Antibiotics themselves may cause side effects, such as nephrotoxicity, allergy, and even hemolytic anemia as a result of the production of drug-induced antibodies [12,13]. A rare but important complication of antibiotic use is pseudomembranous enterocolitis, which is induced most commonly by clindamycin, the cephalosporins, and ampicillin [14,15].
Due to these adverse effects, appropriate reduction of the use of antimicrobial prophylaxis should be considered.
In Korea, there have been few well-designed prospective studies on the prophylactic use of antibiotics for urologic surgeries, and as a result, prophylactic antibiotics are often inappropriately used in a variety of urologic surgical procedures [7,16].
SSI occurs when bacteria are resistant to antibiotics or the surgical site is exposed to innumerable bacteria, most of which are introduced by the operators' contaminated hands [17-21]. Thus, laparoscopic surgeries and minimal-incision surgeries lower the possibility of occurrence, because they are performed mostly by the use of instruments instead of surgeons' hands [22,23].
Yoshida et al, who performed minimum-incision endoscopic surgeries for adrenal and renal tumors, reported that superficial SSI occurred in 1 of 31 in a group that received a single dose of prophylactic antibiotics 30 minutes before the operation, but there were no cases of deep surgical sites or distant regions being infected [6].
With regard to this study, SSI occurred in one patient from each group, and no significant intergroup differences were observed (p=0.783). Our results suggest that LDN, which technically is a well-standardized surgery, does not require the use of postoperative prophylactic antibiotics, thus minimizing the risks of adverse effects and the probable development of a new resistant bacterium.
Furthermore, our study could provide basic data for establishing Korean guidelines for antibiotic prophylaxis in urology.
The present study had some limitations. The study was conducted on comparatively healthy patients, who are not at high risk for infection. Therefore, on the basis of our study results, we cannot ascertain whether the prophylactic single dose is sufficient for high-risk groups. Consequently, there is a need to perform further studies on high-risk groups.
CONCLUSIONS
Of 280 patients who received a single dose of prophylactic antibiotics before the operation, 1 patient contracted an SSI. In comparison with the control group, which received prophylactic antibiotics for 5 days after the operation, the single-dose group did not show a significant difference in the occurrence of SSI. In conclusion, we found that the incidence rate of SSI did not increase when prophylactic antibiotics were not used after VAMS, a standard, conventional open surgery.
Footnotes
The authors have nothing to disclose.
References
- 1.Sakong P, Lee JS, Lee EJ, Ko KP, Kim CH, Kim Y, et al. Association between the pattern of prophylactic antibiotic use and surgical site infection rate for major surgeries in Korea. J Prev Med Public Health. 2009;42:12–20. doi: 10.3961/jpmph.2009.42.1.12. [DOI] [PubMed] [Google Scholar]
- 2.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999;20:250–278. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 3.Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20:725–730. doi: 10.1086/501572. [DOI] [PubMed] [Google Scholar]
- 4.Brumfitt W, Hamilton-Miller J. Methicillin-resistant Staphylococcus aureus. N Engl J Med. 1989;320:1188–1196. doi: 10.1056/NEJM198905043201806. [DOI] [PubMed] [Google Scholar]
- 5.Yi J, Kim EC. Microbiological characteristics of methicillin-resistant Staphylococcus aureus. Korean J Clin Microbiol. 2010;13:1–6. [Google Scholar]
- 6.Yoshida S, Masuda H, Yokoyama M, Kobayashi T, Kawakami S, Kihara K. Absence of prophylactic antibiotics in minimum incision endoscopic urological surgery (MEUS) of adrenal and renal tumors. Int J Urol. 2007;14:384–387. doi: 10.1111/j.1442-2042.2006.01728.x. [DOI] [PubMed] [Google Scholar]
- 7.Koh JI, Kim YH, Kim ME. Antibiotic prophylaxis practice in urology: a survey of 21 Korean Medical Institutions. Korean J Urogenital Tract Infect Inflamm. 2009;4:72–79. [Google Scholar]
- 8.Takeyama K, Shimizu T, Mutoh M, Nishiyama N, Kunishima Y, Matsukawa M, et al. Prophylactic antimicrobial agents in urologic laparoscopic surgery: 1-day versus 3-day treatments. J Infect Chemother. 2004;10:168–171. doi: 10.1007/s10156-004-0317-3. [DOI] [PubMed] [Google Scholar]
- 9.Wolf JS, Jr, Bennett CJ, Dmochowski RR, Hollenbeck BK, Pearle MS, Schaeffer AJ. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379–1390. doi: 10.1016/j.juro.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 10.Trinchieri A, Paparella S, Cappoli S, Esposito N, Butti A, Vaiani R, et al. Prospective assessment of the efficacy of the EAU guidelines for the prevention of nosocomial acquired infections after genitourinary surgery in a district hospital. Arch Ital Urol Androl. 2009;81:46–50. [PubMed] [Google Scholar]
- 11.Leaper DJ, van Goor H, Reilly J, Petrosillo N, Geiss HK, Torres AJ, et al. Surgical site infection - a European perspective of incidence and economic burden. Int Wound J. 2004;1:247–273. doi: 10.1111/j.1742-4801.2004.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garratty G, Arndt PA. An update on drug-induced immune hemolytic anemia. Immunohematology. 2007;23:105–119. [PubMed] [Google Scholar]
- 13.Martin ME, Laber DA. Cefotetan-induced hemolytic anemia after perioperative prophylaxis. Am J Hematol. 2006;81:186–188. doi: 10.1002/ajh.20459. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser AB, Clayson KR, Mulherin JL, Jr, Roach AC, Allen TR, Edwards WH, et al. Antibiotic prophylaxis in vascular surgery. Ann Surg. 1978;188:283–289. doi: 10.1097/00000658-197809000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.International Infection Control Council. Global consensus conference on infection prevention and control practice for Clostridium difficile associated disease (CDAD) Can J Infect Control. 2008;23:110–111. [PubMed] [Google Scholar]
- 16.Kim CS. Antimicrobial prophylaxis for urologic surgery. Korean J Urogenital Tract Infect Inflamm. 2009;4:20–36. [Google Scholar]
- 17.Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189:395–404. doi: 10.1016/j.amjsurg.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Polk HC, Christmas AB. Prophylactic antibiotics in surgery and surgical wound infections. Am Surg. 2000;66:105–111. [PubMed] [Google Scholar]
- 19.Page CP, Bohnen JM, Fletcher JR, McManus AT, Solomkin JS, Wittmann DH. Antimicrobial prophylaxis for surgical wounds. Guidelines for clinical care. Arch Surg. 1993;128:79–88. doi: 10.1001/archsurg.1993.01420130087014. [DOI] [PubMed] [Google Scholar]
- 20.Kageyama Y, Kihara K, Ishizaka K, Okuno T, Hayashi T, Kawakami S, et al. Endoscopic minilaparotomy radical nephrectomy for chronic dialysis patients. Int J Urol. 2002;9:73–76. doi: 10.1046/j.1442-2042.2002.00422.x. [DOI] [PubMed] [Google Scholar]
- 21.Hamasuna R, Betsunoh H, Sueyoshi T, Yakushiji K, Tsukino H, Nagano M, et al. Bacteria of preoperative urinary tract infections contaminate the surgical fields and develop surgical site infections in urological operations. Int J Urol. 2004;11:941–947. doi: 10.1111/j.1442-2042.2004.00941.x. [DOI] [PubMed] [Google Scholar]
- 22.O'Toole JE, Eichholz KM, Fessler RG. Surgical site infection rates after minimally invasive spinal surgery. J Neurosurg Spine. 2009;11:471–476. doi: 10.3171/2009.5.SPINE08633. [DOI] [PubMed] [Google Scholar]
- 23.Dobson MW, Daniel Geisler DG, Victor Fazio VF, Feza Remzi FR, Tracy Hull TH, Jon Vogel JV. Minimally invasive surgical wound infections: laparoscopic surgery decreases morbidity of surgical site infections and decreases the cost of wound care. Colorectal Dis. 2010 doi: 10.1111/j.1463-1318.2010.02302.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]