Abstract
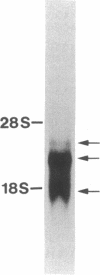
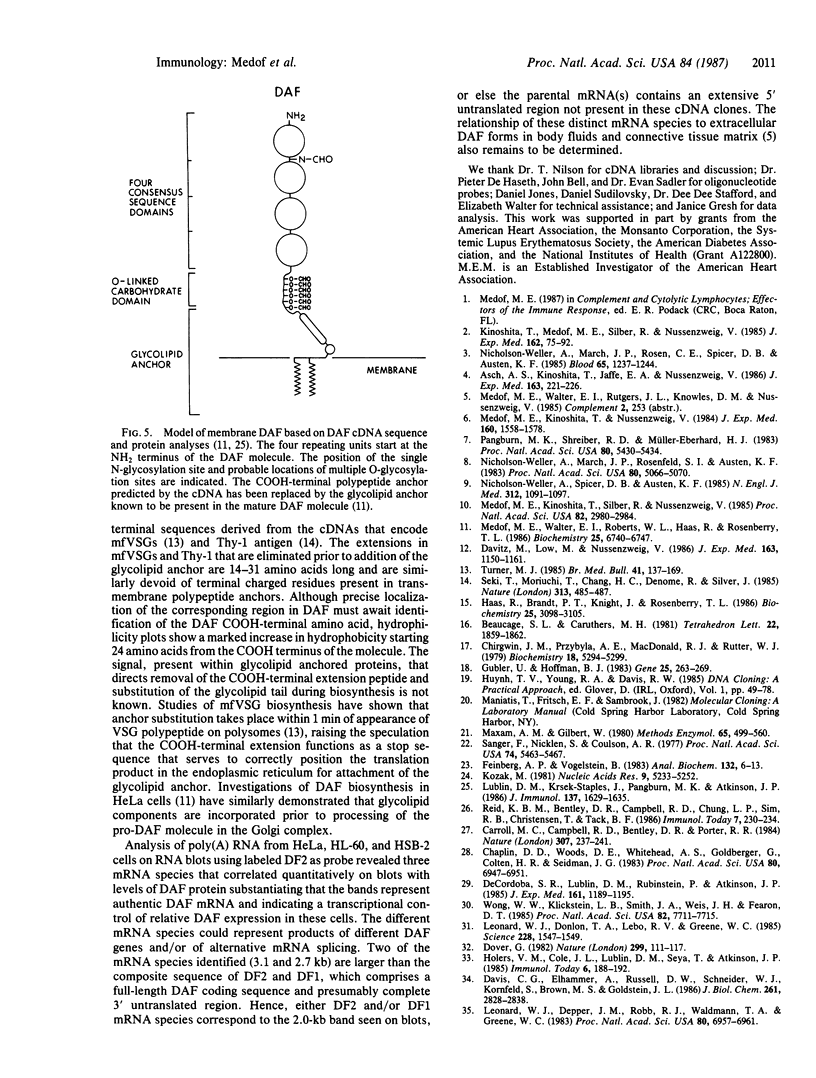
cDNAs encoding the complement decay-accelerating factor (DAF) were isolated from HeLa and differentiated HL-60 lambda gt cDNA libraries by screening with a codon preference oligonucleotide corresponding to DAF NH2-terminal amino acids 3-14. The composite cDNA sequence showed a 347-amino acid protein preceded by an NH2-terminal leader peptide sequence. The translated sequence beginning at the DAF NH2 terminus encodes four contiguous approximately equal to 61-amino acid long repetitive units of internal homology. The repetitive regions contain four conserved cysteines, one proline, one glycine, one glycine/alanine, four leucines/isoleucines/valines, one serine, three tyrosines/phenylalanines, and one tryptophan and show striking homology to similar regions previously identified in factor B, C2, C4 binding protein, factor H, C1r, factor XIII, interleukin 2 receptor, and serum beta 2-glycoprotein I. The consensus repeats are attached to a 70-amino acid long segment rich in serine and threonine (potential O-glycosylation sites), which is in turn followed by a stretch of hydrophobic amino acids. RNA blot analysis of HeLa and HL-60 RNA revealed three DAF mRNA species of 3.1, 2.7, and 2.0 kilobases. The results indicate that portions of the DAF gene may have evolved from a DNA element common to the above proteins, that DAF cDNA predicts a COOH-terminal anchoring polypeptide, and that distinct species of DAF message are elaborated in cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Kinoshita T., Jaffe E. A., Nussenzweig V. Decay-accelerating factor is present on cultured human umbilical vein endothelial cells. J Exp Med. 1986 Jan 1;163(1):221–226. doi: 10.1084/jem.163.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. C., Campbell R. D., Bentley D. R., Porter R. R. A molecular map of the human major histocompatibility complex class III region linking complement genes C4, C2 and factor B. Nature. 1984 Jan 19;307(5948):237–241. doi: 10.1038/307237a0. [DOI] [PubMed] [Google Scholar]
- Chaplin D. D., Woods D. E., Whitehead A. S., Goldberger G., Colten H. R., Seidman J. G. Molecular map of the murine S region. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6947–6951. doi: 10.1073/pnas.80.22.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davis C. G., Elhammer A., Russell D. W., Schneider W. J., Kornfeld S., Brown M. S., Goldstein J. L. Deletion of clustered O-linked carbohydrates does not impair function of low density lipoprotein receptor in transfected fibroblasts. J Biol Chem. 1986 Feb 25;261(6):2828–2838. [PubMed] [Google Scholar]
- Davitz M. A., Low M. G., Nussenzweig V. Release of decay-accelerating factor (DAF) from the cell membrane by phosphatidylinositol-specific phospholipase C (PIPLC). Selective modification of a complement regulatory protein. J Exp Med. 1986 May 1;163(5):1150–1161. doi: 10.1084/jem.163.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover G. Molecular drive: a cohesive mode of species evolution. Nature. 1982 Sep 9;299(5879):111–117. doi: 10.1038/299111a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Haas R., Brandt P. T., Knight J., Rosenberry T. L. Identification of amine components in a glycolipid membrane-binding domain at the C-terminus of human erythrocyte acetylcholinesterase. Biochemistry. 1986 Jun 3;25(11):3098–3105. doi: 10.1021/bi00359a005. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Silber R., Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985 Jul 1;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Possible role of flanking nucleotides in recognition of the AUG initiator codon by eukaryotic ribosomes. Nucleic Acids Res. 1981 Oct 24;9(20):5233–5252. doi: 10.1093/nar/9.20.5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Robb R. J., Waldmann T. A., Greene W. C. Characterization of the human receptor for T-cell growth factor. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6957–6961. doi: 10.1073/pnas.80.22.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Donlon T. A., Lebo R. V., Greene W. C. Localization of the gene encoding the human interleukin-2 receptor on chromosome 10. Science. 1985 Jun 28;228(4707):1547–1549. doi: 10.1126/science.3925551. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Krsek-Staples J., Pangburn M. K., Atkinson J. P. Biosynthesis and glycosylation of the human complement regulatory protein decay-accelerating factor. J Immunol. 1986 Sep 1;137(5):1629–1635. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Silber R., Nussenzweig V. Amelioration of lytic abnormalities of paroxysmal nocturnal hemoglobinuria with decay-accelerating factor. Proc Natl Acad Sci U S A. 1985 May;82(9):2980–2984. doi: 10.1073/pnas.82.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Roberts W. L., Haas R., Rosenberry T. L. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986 Nov 4;25(22):6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosen C. E., Spicer D. B., Austen K. F. Surface membrane expression by human blood leukocytes and platelets of decay-accelerating factor, a regulatory protein of the complement system. Blood. 1985 May;65(5):1237–1244. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosenfeld S. I., Austen K. F. Affected erythrocytes of patients with paroxysmal nocturnal hemoglobinuria are deficient in the complement regulatory protein, decay accelerating factor. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5066–5070. doi: 10.1073/pnas.80.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Spicer D. B., Austen K. F. Deficiency of the complement regulatory protein, "decay-accelerating factor," on membranes of granulocytes, monocytes, and platelets in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1985 Apr 25;312(17):1091–1097. doi: 10.1056/NEJM198504253121704. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Deficiency of an erythrocyte membrane protein with complement regulatory activity in paroxysmal nocturnal hemoglobinuria. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5430–5434. doi: 10.1073/pnas.80.17.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Cordoba S., Lublin D. M., Rubinstein P., Atkinson J. P. Human genes for three complement components that regulate the activation of C3 are tightly linked. J Exp Med. 1985 May 1;161(5):1189–1195. doi: 10.1084/jem.161.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T., Moriuchi T., Chang H. C., Denome R., Silver J. Structural organization of the rat thy-1 gene. Nature. 1985 Feb 7;313(6002):485–487. doi: 10.1038/313485a0. [DOI] [PubMed] [Google Scholar]
- Turner M. J. The biochemistry of the surface antigens of the African trypanosomes. Br Med Bull. 1985 Apr;41(2):137–143. doi: 10.1093/oxfordjournals.bmb.a072040. [DOI] [PubMed] [Google Scholar]
- Wong W. W., Klickstein L. B., Smith J. A., Weis J. H., Fearon D. T. Identification of a partial cDNA clone for the human receptor for complement fragments C3b/C4b. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7711–7715. doi: 10.1073/pnas.82.22.7711. [DOI] [PMC free article] [PubMed] [Google Scholar]