Abstract
Purpose
The effects of leptin on female sexual behaviors are controversial, and studies on this topic are limited. The objectives of this study were to evaluate the direct effects of leptin on clitoral vasoreactivity in vitro and to determine the mechanism of action.
Materials and Methods
Isometric tension studies were conducted to determine the effects of pretreatment with leptin (10-8 M) on the contractile responses of rabbit clitoral corpus cavernosal smooth muscle strips. The effects of leptin were assessed on precontraction induced by phenylephrine (PE; 10-9-10-4 M) and KCl (35-140 mM). We also examined the effect of leptin on relaxation induced by acetylcholine (ACh; 10-9-10-4 M), verapamil (10-10-10-6 M), and sodium nitroprusside (10-9-10-4 M) in PE-precontracted (10-5 M) strips.
Results
Leptin enhanced ACh-induced relaxation in PE-precontracted strips. L-NAME pretreatment significantly reduced the effect of leptin on ACh-induced relaxation, whereas L-arginine potentiated the effect of leptin. Leptin decreased the KCl-induced contractile responses. Leptin increased verapamil-induced relaxation responses. The relaxation effects of leptin on KCl-induced contraction were inhibited by 10-5 M methylene blue and L-NAME pretreatment.
Conclusions
A high concentration of leptin enhances ACh-dependent relaxation in clitoral cavernosal smooth muscles. These relaxation effects of leptin may occur through an NO-dependent mechanism and voltage-dependent calcium channel blockade.
Keywords: Calcium channels, Clitoris, Leptin, Nitric oxide, Relaxation
INTRODUCTION
Leptin, an adipocyte-derived hormone, is known to modulate body weight by regulating food intake and energy expenditure [1]. Apart from its previously envisaged function as an adipostatin, leptin has been shown to participate in a wide range of biological functions, including the suppression of vascular smooth muscle proliferation and induction of vasodilation [2,3]. Nitric oxide (NO) is the key molecule for the depressor response induced by leptin. Leptin acts on the endothelium by inducing the synthesis of NO via the activation of endothelial NO synthase (NOS), thereby evoking an endothelium-dependent vasodilation [1]. In contrast, leptin also secretes vasoconstrictive factors in human umbilical veins and the endothelium [4]. Longitudinal and cross-sectional studies have shown an association between serum leptin concentrations and various cardiovascular risks including stroke [5], chronic heart failure [6,7], acute myocardial infarction [5], coronary heart disease [8], and left cardiac hypertrophy [9]. Thus, leptin seems to exert contradictory effects on cardiovascular pathophysiology.
Leptin is thought to play important roles in pubertal development and reproduction in females. Low plasma leptin levels in females with anorexia nervosa have been associated with decreased sexual behaviors. An animal study showed that leptin administration reverses this reduction in sexual activity [10]. That study focused on the role of the central nervous system, and the underlying biological processes in humans have not yet been investigated.
In contrast with the study mentioned above, centrally administered leptin did not stimulate sexual behaviors in lean and obese Zucker female rats [11]. Thus, the effects of leptin on female sexual behaviors are controversial, and to date, few studies have addressed this issue. The effect of leptin on sexual desire, motivation, and genital arousal is still under debate, especially in terms of direct effects on clitoral hemodynamics.
The clitoris is an important component of female sexual response. It is highly vascular, and increased blood flow during sexual arousal results in clitoral erection [12]. Clitoral smooth muscle tone appears to regulate the changes in clitoral hemodynamics and genital arousal [13]. Therefore, evaluating the effects of a drug on clitoral smooth muscle tone may be a useful method for assessing changes in female sexual function. The objectives of this study were to evaluate the direct effect of leptin on clitoral vasoreactivity in vitro and to determine the mechanism of action.
MATERIALS AND METHODS
1. Tissue preparation
With approval from the Ethics Committee for the Protection of Persons and Animals in Biochemical Research at the Institute of Medical Science of Chung-Ang University (Seoul, Korea), 30 New Zealand white female rabbits (2.5-3 kg) were anesthetized with ether and killed by rapid exsanguination. The clitoris was removed and the cavernosal tissues were excised and dissected from the tunica albuginea and surrounding connective tissue in a 100% O2-saturated physiologic solution. The cavernosal tissues were dissected in 2 strip preparations, each measuring approximately 2×2×6 mm. Sixty strips were prepared. To record isometric tension, the strips were placed in a 20 ml organ bath containing bicarbonate-buffered physiologic salt solution (116 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 24 mM NaHCO3, and 11 mM glucose) and continuously bubbled with a mixture of 95% O2 and 5% CO2 at 37℃. The strips were then attached by a silk tie to a force transducer (Harvard, Edenbridge, UK). The signals from this were recorded on a computer (IBM-PC, Pentium 4) with the use of a digital-analog signal converter and amplifier (ADInstruments, Sydney, Australia). The organ chamber was maintained at 37℃ (pH 7.4). The strips were maintained at 2 g of resting tension and were equilibrated for 120 min with several changes of physiologic salt solution to avoid the accumulation of metabolites resulting from surgical injury.
2. Evaluation of tissue status
After resting, the basal tension of each strip was adjusted to the optimal isometric tension at which contraction with 10-5 M phenylephrine (PE; a selective alpha 1 agonist) treatment was maximal. After the maximal contractile response to PE (10-5 M) had been achieved, the optimal length of contraction by PE was regarded as the length maintained for 30 minutes at >90% basal contractile tension. After contraction, the relaxation responses induced by 10-5 M acetylcholine (ACh; endothelium-dependent vasodilator) were checked. The strips that did not show optimal contraction and relaxation were excluded. With each pharmacologic agent, pretreatment was done over a 20-min time period. In these studies, 90 strips were obtained from 45 rabbits and 76 strips with an optimal length were used. At <50% of basal tension, the strips were regarded as damaged tissues and the experiments were completed. The results of the contraction and relaxation responses are expressed as a percentage.
3. Vasomotor responses of the strips
1) Contractile and relaxation responses of the strips
To evaluate the effect of leptin on PE-induced contraction, concentration response curves were determined by adding successive logarithmic increments of PE (10-9-10-4 M) to the strips in the organ bath after they had been pretreated with 10-8 M leptin.
To evaluate the effect of leptin on ACh-induced relaxation, concentration response curves were determined by adding successive logarithmic increments of the endothelium-dependent vasodilator ACh (10-9-10-4 M) to the strips in the organ bath after they had been pretreated with 10-8 M leptin. The results of the relaxation responses are expressed as the percentage relaxation of the contraction that was induced by PE (10-5 M).
2) Mechanism of the relaxation response for leptin
To analyze the mechanism involved in the responses induced by pretreatment with 10-8 M leptin, the effects of leptin were assessed on contraction induced by KCl (a voltage-dependent calcium channel activator, 35-140 mM). The relaxation of PE-precontracted strips induced by verapamil (a voltage-dependent calcium channel blocker, 10-10-10-6 M) and sodium nitroprusside (an endothelium-independent vasodilator, SNP; 10-9-10-4 M) was also assessed.
To determine the relationship between the NO pathway and the effect of leptin, strips were pretreated with N(w)-nitro-L-arginine methyl ester (L-NAME; a nonspecific NO synthetic inhibitor, 10-5 M) and L-arginine (an NO donor, 0.1 mM) with and without leptin pretreatment (10-8 M), and the effects of ACh (10-9-10-4 M) were observed in the PE-induced precontracted strips.
In addition, the effects of 1 mM and 10 mM tetraethyl ammonium (TEA; a nonspecific K+ channel blocker), glibenclamide (an ATP-sensitive K+ channel blocker, 10-5 M), indomethacin (a nonselective cyclooxygenase inhibitor, 10-5 M), and 4-aminopyridine (4-AP; a voltage-dependent K+ channel inhibitor, 10-5 M) on the enhancing effect of ACh-induced relaxation were verified. The effects of L-NAME (10-5 M) and methylene blue (a selective inhibitor of guanylyl cyclase inhibitor, 10-5 M) pretreatment on contraction induced by 70 mM KCl solution was examined in strips treated with increasing leptin doses (10-11-10-7 M).
4. Solutions and reagents
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
5. Statistical analysis
The results were obtained after more than 5 replicates of the experiments, and representative data are described. Results are expressed as the mean±standard error. Statistical analysis of the data was performed by Student's t-test and ANOVA. A p-value<0.05 was considered significant.
RESULTS
1. Contractile and relaxation responses of the strips
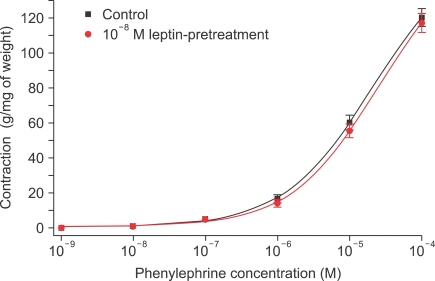
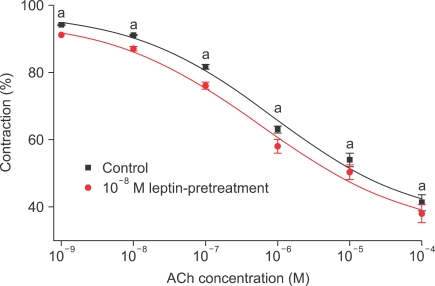
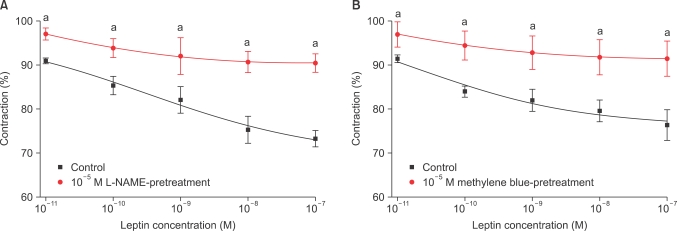
The strips were pretreated with 10-8 M leptin and then administered PE (10-9-10-4 M) in the organ bath. No change in contractile responses was observed (n=8) (Fig. 1). The strips were pretreated with 10-8 M leptin and then ACh (10-9-10-4 M) was administered to PE-induced precontracted strips. Leptin significantly enhanced the ACh-induced endothelium-dependent relaxation (n=12) (Fig. 2).
FIG. 1.
Concentration-response curves for the effect of leptin pretreatment on contraction in rabbit clitoral corpus cavernosal strips in a phenylephrine dose-dependent manner (n=8).
FIG. 2.
Concentration-response curves for the effect of leptin pretreatment on relaxation in rabbit clitoral corpus cavernosal strips in an acetylcholine dose-dependent manner. The preparation was preconstricted with 10-5 M PE. n=12; a: p<0.05.
2. Mechanism of the relaxation response for leptin
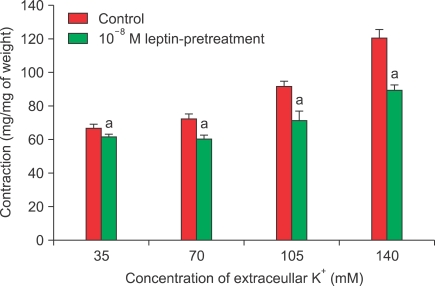
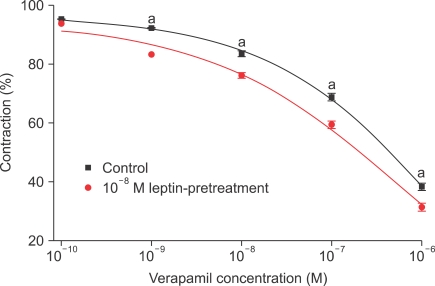
To determine the mechanism involved in the responses induced by pretreatment with 10-8 M leptin, contraction induced by high-concentration KCl solution (35, 70, 105, or 140 mM) was assessed with or without leptin pretreatment. The KCl-induced contractile responses were significantly inhibited by leptin pretreatment (n=8) (Fig. 3). Verapamilinduced relaxation responses were enhanced by leptin with statistical significance (n=12) (Fig. 4).
FIG. 3.
Concentration-response curves for the effect of leptin pretreatment of rabbit clitoral corpus cavernosal strips in high-concentration K solution. n=8; a: p<0.05.
FIG. 4.
Concentration-response curves for the effect of leptin pretreatment on verapamil responses in rabbit clitoral corpus cavernosal strips. The preparation was preconstricted with 10-5 M PE. n=12; a: p<0.05.
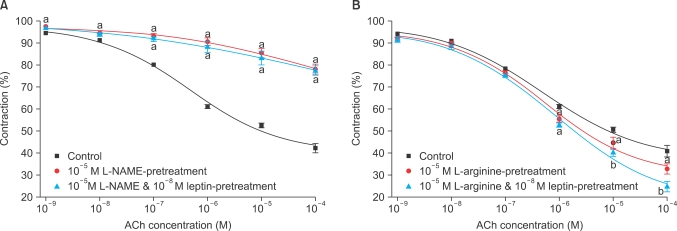
L-NAME reversed the effect of leptin on enhancing ACh-induced relaxation, but there was no statistical difference between L-NAME treatment and L-NAME plus leptin (n=12) (Fig. 5A). L-arginine (0.1 mM) enhanced the AChinduced relaxation responses of leptin, and more potent relaxation responses appeared with combined arginine plus leptin treatment than with arginine single treatment (n=12) (Fig. 5B).
FIG. 5.
Concentration-response curves for the effect of leptin pretreatment on ACh responses in cavernosal strips treated with 10-5 M L-NAME (A) or 0.1 mM L-arginine (B). The preparation was preconstricted with 10-5 M PE. n=12 in each. a: p<0.05 compared with control, b: p<0.01 compared with control.
To determine whether the relationship of inhibiting effects of leptin on KCl-induced contraction was an NO-mediated mechanism, the effects of two inhibitors of the NOS-acting mechanism (L-NAME [10-5 M] and the soluble guanylate cyclase inhibitor methylene blue [10-5 M]) were assessed with respect to dose-dependent effects of leptin (10-11-10-7 M) on KCl-induced contraction. Following treatment with L-NAME and methylene blue, the inhibiting effects of KCl-induced contraction were reversed (n=8 in each) (Fig. 6).
FIG. 6.
Concentration-response curves for the effects of pretreatment with L-NAME (A) or methylene blue (B) on rabbit clitoral corpus cavernosal strips with increasing leptin doses. The preparation was preconstricted with 70 mM KCl solution. n=8 in each; a: p<0.05.
Leptin did not affect the dose-dependent SNP-induced (10-9-10-4 M) relaxation responses. The enhancing effect of leptin on ACh-induced relaxation was not changed by treatment with indomethacin (a COX inhibitor, 10-5 M) or the potassium channel blockers (n=8 in each) TEA (1 and 10 mM), glibenclamide (10-5 M), and 4-AP (10-5 M) (data not shown).
DISCUSSION
Contemporary research suggests that the adipocyte-derived hormone leptin may be an important factor linking obesity, metabolic syndrome, and cardiovascular disorders [14]. Leptin also acts on several peripheral tissues, including the vascular endothelium [15,16]. The leptin receptor has been identified in endothelial cells [17] and the action of leptin on the endothelium modulates several physiologic processes, with potential implications in pathophysiological diseases associated with obesity [18]. In particular, leptin is involved in lipid metabolism and the functioning of vascular smooth muscle. It is known that leptin exerts vasopressor effects by activating the sympathetic nervous system. In contrast to its central neuronal sympathoexcitatory action, leptin has been shown to directly induce vasorelaxation via both NO-dependent and NO-independent mechanisms. Considering the pressor (sympathetic activation) and depressor (increased NO) actions, the integrated mechanism of actions of leptin on vascular tone and arterial blood pressure regulation is rather complex. Whereas the enhanced sympathetic nervous activation is expected to increase vascular tone and blood pressure, leptin has been shown to elicit peripheral vascular relaxation mediated by NO-dependent or NO-independent mechanisms in the absence of sympathetic control. Therefore, Ren suggested that leptin-induced peripheral NO release and vasorelaxation may serve to counterbalance the enhanced sympathetic activity in response to leptin [19].
In the present study, ACh-induced relaxation responses were enhanced by leptin. This relaxation by leptin was reversed by L-NAME administration and was enhanced by a low dose of L-arginine (0.1 mM). Because ACh-induced relaxation is mediated in an endothelium-dependent manner, these results suggest that endothelial integrity is critical for the vascular-relaxing action of leptin on the clitoral corpus cavernosum and is mediated via NOS activation. In addition, verapamil-induced relaxation responses were increased and high-concentration KCl solution-induced contractions were decreased by leptin. This inhibitory effect on KCl-induced contraction was reversed by methylene blue and L-NAME. Our findings suggest that leptin inhibits the voltage-dependent calcium channels indirectly by activating the NO-cGMP pathway.
The effects of estrogen on increasing genital blood flow and smooth muscle relaxation have been attributed mostly to the regulation of eNOS [20]. It has been reported that the application of NO donor drugs, which are known to increase tissue levels of cyclic GMP, may facilitate female genital vascular and nonvascular smooth muscle relaxation and thus sexual response [21]. Therefore, it is possible that the direct smooth muscle-relaxant effects of leptin may serve to counterbalance the enhanced sympathetic activity as well as in blood vessels, preserving genital arousal in hyperleptinemia.
It has been reported that clitoral engorgement results from NO-initiated activation of BKCa channels (large-conductance, calcium-activated potassium channels) [22]. Earlier studies on the corpus cavernosum of diabetic rabbits have also revealed that prostacyclin is an endothelium-dependent relaxing factor [23]. However, the results of our study show that indomethacin, TEA, glibenclamide, and 4-AP did not affect leptin-induced relaxation effects. Therefore, we concluded that leptin-induced relaxant effects are not mediated by the cyclooxygenase pathway or various potassium channels.
The proportion and effects of adipose tissue are greater in women than in men. This may be the reason for the gender difference in the level and distribution of leptin secreted from adipocytes. Many studies have shown that the plasma leptin level is higher in females than in males, even when leptin is corrected for differences in body composition [24]. Rosenbaum et al suggested that this sexual dimorphism is apparently also due, in part, to a suppressive effect of circulating androgens on leptin [25]. It has been reported that the mean serum leptin concentrations in obese subjects and normal-weight subjects are 10-55 ng/ml and 5-10 ng/ml, respectively, thus showing a strong positive correlation between serum leptin concentrations and the percentage of body fat [26]. In our study, we used a leptin concentration of 10-8 M, which is equivalent to 160 ng/ml. Although this concentration is 3 to 5 times higher than that reported in obese people, in general, severely obese women may have a leptin serum concentration of this range considering that leptin levels are significantly higher in females than in males.
To date, a functional leptin receptor has not been found in the clitoral or corporal cavernosum, but in vascular endothelial cells. Therefore, further studies should investigate the possible presence of leptin receptors in the cavernosal endothelium. In addition, in vivo animal studies and clinical studies on the effect of leptin on female sexuality in hyperleptinemia are required.
CONCLUSIONS
Leptin in high concentrations may have an enhancing effect on rabbit clitoral corpus cavernosal smooth muscle relaxation. These processes may occur via clitoral endothelium-dependent relaxation and voltage-dependent calcium channel blockade. We suggest that endothelium-dependent NO may have potential calcium channel blocking activity. Leptin may modulate the vasomotor action of the clitoris and serve to counterbalance enhanced sympathetic activity, thus preserving genital arousal in hyperleptinemia.
Footnotes
This work was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea (A085138).
The authors have nothing to disclose.
References
- 1.Rodríguez A, Fortuño A, Gómez-Ambrosi J, Zalba G, Díez J, Frühbeck G. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction in vascular smooth muscle cells is mediated via a nitric oxide-dependent mechanism. Endocrinology. 2007;148:324–331. doi: 10.1210/en.2006-0940. [DOI] [PubMed] [Google Scholar]
- 2.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 3.Kimura K, Tsuda K, Baba A, Kawabe T, Boh-oka S, Ibata M, et al. Involvement of nitric oxide in endothelium-dependent arterial relaxation by leptin. Biochem Biophys Res Commun. 2000;273:745–749. doi: 10.1006/bbrc.2000.3005. [DOI] [PubMed] [Google Scholar]
- 4.Quehenberger P, Exner M, Sunder-Plassmann R, Ruzicka K, Bieglmayer C, Endler G, et al. Leptin induces endothelin-1 in endothelial cells in vitro. Circ Res. 2002;90:711–718. doi: 10.1161/01.res.0000014226.74709.90. [DOI] [PubMed] [Google Scholar]
- 5.Söderberg S, Ahrén B, Jansson JH, Johnson O, Hallmans G, Asplund K, et al. Leptin is associated with increased risk of myocardial infarction. J Intern Med. 1999;246:409–418. doi: 10.1046/j.1365-2796.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- 6.Leyva F, Anker SD, Egerer K, Stevenson JC, Kox WJ, Coats AJ. Hyperleptinaemia in chronic heart failure. Relationships with insulin. Eur Heart J. 1998;19:1547–1551. doi: 10.1053/euhj.1998.1045. [DOI] [PubMed] [Google Scholar]
- 7.Schulze PC, Kratzsch J, Linke A, Schoene N, Adams V, Gielen S, et al. Elevated serum levels of leptin and soluble leptin receptor in patients with advanced chronic heart failure. Eur J Heart Fail. 2003;5:33–40. doi: 10.1016/s1388-9842(02)00177-0. [DOI] [PubMed] [Google Scholar]
- 8.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104:3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 9.Paolisso G, Tagliamonte MR, Galderisi M, Zito GA, Petrocelli A, Carella C, et al. Plasma leptin level is associated with myocardial wall thickness in hypertensive insulin-resistant men. Hypertension. 1999;34:1047–1052. doi: 10.1161/01.hyp.34.5.1047. [DOI] [PubMed] [Google Scholar]
- 10.Ehrlich S, Burghardt R, Schneider N, Hein J, Weiss D, Pfeiffer E, et al. Leptin and its associations with measures of psychopathology in patients with anorexia nervosa. J Neural Transm. 2009;116:109–115. doi: 10.1007/s00702-008-0151-3. [DOI] [PubMed] [Google Scholar]
- 11.Fox AS, Olster DH. Effects of intracerebroventricular leptin administration on feeding and sexual behaviors in lean and obese female Zucker rats. Horm Behav. 2000;37:377–387. doi: 10.1006/hbeh.2000.1580. [DOI] [PubMed] [Google Scholar]
- 12.Levin LJ. VIP, vagina, clitoral and periurethral glans--an update on human female genital arousal. Exp Clin Endocrinol. 1991;98:61–69. doi: 10.1055/s-0029-1211102. [DOI] [PubMed] [Google Scholar]
- 13.Azadzoi KM, Siroky MB. Neurologic factors in female sexual function and dysfunction. Korean J Urol. 2010;51:443–449. doi: 10.4111/kju.2010.51.7.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel SB, Reams GP, Spear RM, Freeman RH, Villarreal D. Leptin: linking obesity, the metabolic syndrome, and cardiovascular disease. Curr Hypertens Rep. 2008;10:131–137. doi: 10.1007/s11906-008-0025-y. [DOI] [PubMed] [Google Scholar]
- 15.Sierra-Honigmann MR, Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, Sessa WC, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 16.Bouloumié A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 17.Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, et al. Potential role of leptin in angiogenesis: leptin induces endothelial proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001;33:95–102. doi: 10.1038/emm.2001.17. [DOI] [PubMed] [Google Scholar]
- 18.Rahmouni K, Haynes WG. Endothelial effects of leptin: implications in health and diseases. Curr Diab Rep. 2005;5:260–266. doi: 10.1007/s11892-005-0020-5. [DOI] [PubMed] [Google Scholar]
- 19.Ren J. Leptin and hyperleptinemia - from friend to foe for cardiovascular function. J Endocrinol. 2004;181:1–10. doi: 10.1677/joe.0.1810001. [DOI] [PubMed] [Google Scholar]
- 20.Musicki B, Liu T, Lagoda GA, Bivalacqua TJ, Strong TD, Burnett AL. Endothelial nitric oxide synthase regulation in female genital tract structures. J Sex Med. 2009;6(Suppl 3):247–253. doi: 10.1111/j.1743-6109.2008.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uckert S, Mayer ME, Jonas U, Stief CG. Potential future options in the pharmacotherapy of female sexual dysfunction. World J Urol. 2006;24:630–638. doi: 10.1007/s00345-006-0121-z. [DOI] [PubMed] [Google Scholar]
- 22.Gragasin FS, Michelakis ED, Hogan A, Moudgil R, Hashimoto K, Wu X, et al. The neurovascular mechanism of clitoral erection: nitric oxide and cGMP-stimulated activation of BKCa channels. FASEB J. 2004;18:1382–1391. doi: 10.1096/fj.04-1978com. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan M, Thompson CS, Mikhailidis DP, Morgan RJ, Angelini GD, Jeremy JY. Differential alterations of prostacyclin, cyclic AMP and cyclic GMP formation in the corpus cavernosum of the diabetic rabbit. Br J Urol. 1998;82:578–584. [PubMed] [Google Scholar]
- 24.Takizawa H, Ura N, Saitoh S, Wang L, Higashiura K, Takagi S, et al. Gender difference in the relationships among hyperleptinemia, hyperinsulinemia, and hypertension. Clin Exp Hypertens. 2001;23:357–368. doi: 10.1081/ceh-100102673. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81:3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 26.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]