Abstract
There is evidence that visual stimuli used to signal drug delivery in self-administration procedures have primary reinforcing properties, and that drugs of abuse enhance the reinforcing properties of such stimuli. Here, we explored the relationships between locomotor activity, responding for a visual stimulus, and self-administration of methamphetamine (METH). Rats were classified as high or low responders based on activity levels in a novel locomotor chamber and were subsequently tested for responding to produce a visual stimulus followed by self-administration of a low dose of METH (0.025 mg/kg/infusion) paired with the visual stimulus. High responder rats responded more for the visual stimulus than low responder rats indicating that the visual stimulus was reinforcing and that operant responding for a visual stimulus has commonalities with locomotor activity in a novel environment. Similarly, high responder rats responded more for METH paired with a visual stimulus than low responder rats. Because of the reinforcing properties of the visual stimulus, it was not possible to determine if the rats were responding to produce the visual stimulus, METH or the combination. We speculate that responding to produce sensory reinforcers may be a measure of sensation seeking. These results indicate that visual stimuli have unconditioned reinforcing effects which may have a significant role in acquisition of drug self-administration, a role that is not yet well understood.
Keywords: Sensory reinforcement, Self-administration, Visual stimuli, Operant conditioning, Rats
1.1 Sensory reinforcement & Self-administration
Visual stimuli and other sensory events that do not reduce tissue needs can be primary reinforcers (For reviews see, Berlyne 1969; Eisenberger 1972; Kish 1966; Tapp 1969), however the potential primary reinforcing effects of VS are often not taken into account in drug self-administration (SA) procedures where the onset (or offset) of a visual stimulus (VS) is frequently paired with drug delivery. While VS are often considered not to have reinforcing properties of their own, and instead simply indicate a time out period when drug is unavailable, it is possible that VS plays a more direct role in mediating SA. The widespread use of VS in SA procedures may be an important but largely unexamined aspect of many SA studies.
We conducted a limited literature search in the journals (I) Psychopharmacology, (II) Physiology & Behavior, and (III) Pharmacology, Biochemistry and Behavior using the search terms (i) rat, (ii) self-administration, and (iii) cocaine or amphetamine for the years 2007– 2010. Of the 101 articles surveyed, 35 paired a combination of VS and lever retraction with drug delivery, 31 paired a VS alone with drug delivery and 2 paired lever retraction alone with drug delivery. A total of 88 (or 87%) used a visual stimulus as a cue of drug availability/unavailability in the self-administration procedure. Only 15 (or 15%) of the articles had no cue associated with administration of the drug. The exact nature of the VS varied largely across SA procedures. Many studies use the onset of a house light, cue light, or both to signal drug delivery. Other studies use flashing lights, colored lights (i.e., red or green) or even the combination of visual and auditory stimuli (e.g., light and tone paired, lever retraction). Of all 44 articles identified, only one recognized use of a visual stimulus as a possible confound in interpretation of results (Keiflin, Vouillac, and Cador 2008).
There is also strong evidence that systemic administration of methamphetamine, d-amphetamine (Glow and Russell 1973, 1973, 1974; Gomer and Jakubczak 1974; Winterbauer and Balleine 2007), and nicotine (Palmatier et al. 2007; Raiff and Dallery 2009) enhance the primary reinforcing efficacy of visual stimuli, and that visual stimuli play an important role in drug self-administration. Deroche-Gamonet et al. (2002) demonstrated that the presence of a VS enhanced acquisition of self-administration of cocaine. Furthermore, a series of experiments by Donny & Caggiula et al. (Caggiula, Donny, Chaudhri et al. 2002; Caggiula et al. 2009; Caggiula et al. 2001; Caggiula, Donny, White et al. 2002; Chaudhri et al. 2007; Chaudhri et al. 2006; Donny et al. 1998; Donny et al. 2003; Palmatier et al. 2006) have shown that self-administration of nicotine is greatly enhanced by the response contingent presentations of a VS. Many of these studies have also demonstrated that animals will respond to produce a VS that is not paired with any other primary reinforcer, indicating that the VS used in these experiments have primary reinforcing properties of their own. This is of particular interest given the high percentage of studies using a VS as a cue in SA procedures.
Self-administration acquisition studies offer important information regarding sensitivity to the reinforcing properties of drugs of abuse. However, inclusion of a visual stimulus contingent on drug delivery dilutes any strong conclusions that can be drawn from the results of these experiments without proper control conditions.
1.2 Individual differences in locomotor response to novelty and self-administration
Individual differences in the initial locomotor response to an novel environment is predictive of self-administration of cocaine (Piazza et al. 2000; Davis et al. 2008), amphetamine (Pierre and Vezina 1997; Cain, Dotson, and Bardo 2006), ethanol (Nadal, Armario, and Janak 2002), morphine (Ambrosio, Goldberg, and Elmer 1995) and nicotine (Suto, Austin, and Vezina 2001). In these experiments, rats with high locomotor activity in a novel environment are identified as high responders (HR) compared to rats with low locomotor activity, identified as low responders (LR).
There are a number of salient features common to both operant responding to produce a VS and locomotor activity in a novel environment. Both behaviors involve investigating and interacting with environmental stimuli in the absence of homeostatic reinforcers. We have previously found operant responding is greatest for a novel VS (Ashrafioun et al. 2008). Similarly, locomotor activity is also greatest in novel environments (Miller, Sethna, and Young 1970; Feigley and Hamilton 1971). Furthermore, both responding to produce novel VS and locomotor activity in novel environments show between- and within-session declines in activity with continued exposure. Finally, both responding for VS and locomotor activity are increased by administration of stimulant drugs. Thus, it is of interest to investigate the relationship between responding for VS and locomotor activity in a novel environment.
Materials and Methods
2.1 Subjects
Thirty male Long-Evans rats were used in the current study. Subjects were bred at the University of Buffalo then relocated to a colony room at the Research Institute on Addictions. Rats weighed between 300 and 400 g and were housed in pairs in plastic cages (42.5 X 22.5 X 19.25 cm) at the start of the experiment. Rats were singly housed following surgery and for the duration of the self-administration phase of the experiment in order to protect the catheter/harness assembly. Lights were on in the colony room from 0700 to 1900. All behavioral testing occurred during the light phase of the light/dark cycle and subjects were acclimated to this cycle for at least 7 days prior to behavioral testing. Food (Harlan Teklad Laboratory Diet #8604, Harlan Inc., Indianapolis, IN) and water was continuously available. This study was conducted in accordance with the guidelines set up by the Institutional Animal Care and Use Committee of The University at Buffalo, The State University of New York.
2.2 Apparatus
2.2.1 Locomotor Chamber Apparatus
Locomotor activity was recorded by an infrared motion-sensor system (Hamilton-Kinder) fitted outside a standard cage tub (42.5 X 22.5 X 19.25 cm) in the absence of any bedding. The tubs used for locomotor testing did not have shavings in them and clean tubs were used for each test session. Each locomotor chamber was located inside a drawer of a file cabinet. Each drawer was equipped with a wall mounted fan that provided masking noise and was illuminated by an 8 watt light bulb (light output 450 lumens) centrally located inside the drawer. This arrangement provided a novel environment for locomotor testing. Two levels of infrared motion sensors were set at 5.5 (for recording horizontal movement), and 15.5 cm above the cage floor (for recording vertical movements). The sensors at the lower level consist of eight pairs along the long axis and five pairs along the short axis each spaced 5.5 cm apart and were used to determine the position of the animal. The sensors at the upper level were spaced 5.5 cm apart along the short axis and recorded vertical rearing movements. The activity-monitoring system monitored each of the beams at a frequency of 0.01 sec to determine whether the beams are interrupted. The interruption of any beam not interrupted during the previous sample was interpreted as an activity score.
2.2.2 Light Reinforcement Apparatus
Sixteen locally constructed experimental chambers were used. These chambers have been described in detail previously (Richards et al. 1997). Briefly, the chambers have stainless-steel grid floors, aluminum front and back walls, and Plexiglas sides and top. The test panel has two snout poke holes located on either side of a centrally located stimulus light. A second stimulus light was located in the middle of the back wall of the test chamber. Snout pokes and head entries were monitored with infrared detectors. The entire apparatus is computer controlled through a MED Associates interface with MED-PC (version 4). The temporal resolution of the system is 0.01 seconds.
2.2.3 Self-administration Apparatus
Eight experimental chambers were used for the self-administration phase of the experiment. These chambers were similar to those described above for light reinforcement phase, but were located on a different floor of the research facility in a different experimental test room than those used in the light reinforcement phase. Before each session, Vascular Access harnesses (VAH95AB, Instech Solomon, Plymouth Meeting, PA) were connected to a flexible polyethylene tubing enclosed in a spring tether (PS95, Instech Solomon, Plymouth Meeting, PA) attached to a swivel (375,22PS Instech, Plymouth Meeting, PA) mounted by a single axis balance arm on top of the chamber allowing the animal to move freely around the operant chamber.
2.3 Drugs
(+) Methamphetamine (d-N, α-Dimethylphenethlyamine; d-Desoxyephedrine) hydrocholride was obtained from Sigma (Lot 054K0842); solutions were made weekly and dissolved in sterile saline. During the sensitization phase of the study, the rats were injected intraperitoneally with METH (1.5 mg/kg) immediately prior to being placed into the locomotor chamber. In the self-administration phase of the study, METH was delivered via a syringe pump (Model # PHM-100). The concentration of the METH solution for IV self administration was 0.1mg/ml. Each rat was delivered a dose of 0.025mg/kg/infusion with the pump duration adjusted according to body weight in order to deliver the correct dose of drug. Infusion durations ranged between 4.22 s and 5.62 s.
2.4 Procedure
2.4.1 Overall timeline
As is detailed in Table 1 (and discussed below), the study had 3 phases. In phase 1 of the study, rats were tested for locomotor activity in a novel locomotor chamber to determine classification as high or low responders. Following the initial locomotor activity test, the rats were pretreated with either METH or saline in the locomotor chamber. In phase 2 of the study, the rats were habituated to dark operant test chambers and then tested for light reinforced responding. During habituation, snout pokes into two separate holes were recorded but had no programmed consequences. During light reinforcement testing, responses to the active snout poke hole produced a VS and responses to the inactive snout poke hole has no programmed consequences. In phase 3 of the study, the rats were fitted with I.V. catheters and again habituated to the operant test chambers. Following habituation, the rats were tested for responding to produce a combination of the VS and METH. The active and inactive snout poke holes were reversed between phases 2 and 3.
Table 1.
Overall timeline of behavioral testing.
| Phase | Days |
| Phase 1. Locomotor Activity | |
| Response to novelty | 1 |
| Methamphetamine Pre-exposure | 2–12 |
| Phase 2. Light Reinforcement | |
| Habituation to operant chamber | 13–19 |
| Response Contingent VS | 20–30 |
| Phase 3. Self-Administration | |
| Surgery & Recovery | 31–38 |
| Habituation to operant chamber | 39–45 |
| Response contingent METH&VS | 46–56 |
2.4.2 Locomotor activity in a novel environment: Classification of High and Low Responders
Prior to operant testing, subjects were placed into activity monitors for 30 min while locomotor activity was recorded. Basic movements, defined as the total number of horizontal and vertical beam breaks during the activity monitor exposure, were used as the dependent measure of locomotor activity and operationally defined as locomotor counts. Following the initial locomotor activity test, animals’ locomotor counts were ranked according to the sum of horizontal and vertical photobeam breaks (i.e., basic movements). Locomotor scores were then divided into thirds; the middle third of animals (n=10) were removed from the study in order to ensure distinct subject populations. Animals with highest locomotor scores were classified as high responders (HR, n=10) and animals with lowest locomotor scores were classifies as low responders (LR, n=10).
2.4.3 Methamphetamine pre-exposure
Half of both HR (n=5) and LR (n=5) received 10 days pre-exposure to methamphetamine (1.5 mg/kg i.p.); the other half of both groups (HR, n=5; LR, n=5) received saline injections for a total of 10 days. Animals were tested 5 days/week (Monday through Friday, with weekends off) during which rats were injected immediately prior to being placed into the locomotor activity monitors. All animals were exposed to the locomotor chambers for ten 30-min sessions and activity was monitored during each session.
2.4.4 Light Reinforcement habituation phase
Animals were placed in dark operant chambers for a six-day habituation period. Daily sessions lasted 30 min during which snout pokes to either side were recorded, but resulted in no programmed consequences.
2.4.5 Light reinforcement
Animals were placed in operant chambers for ten 30 min test sessions. The test chambers were dark during testing except when a response contingent visual stimulus (VS) was presented. The VS consisted of the onset of two stimulus lights. One of the lights was located above and midway between the two snout poke apertures and the other light was a house light located in the middle of the back wall of the test chamber. Each VS consisted of a Dialight indicator lamp (Dialight, Farmingdale, NJ), light bulb and lens cap. The front center light was equipped with a 28 volt light bulb (SPC Technology, Model # 1819) and white lens cap (Dialight, Model # 081-0135-303). The back houselight was equipped with a 28 volt light bulb (SPC Technology, Model # 1864) covered by a clear lens cap (Dialight, Model # 081-0135-303). Onset of the VS combination produced a total of 120 lux light as measured in the center of the test chamber. Snout pokes into the active alternative resulted in illumination of the house and center stimulus light (5 s) according to a VI 2 min schedule of reinforcement. Snout pokes to the inactive alternative had no programmed consequence. The active alternative was counterbalanced so that for half of the rats the snout poke hole on the left side of the chamber was the active alternative.
The VI 2 min schedule was reproduced from a list of 20 intervals with a mean of 2 minutes generated using Fleshler-Hoffman progressions (Fleshler and Hoffman 1962). During the test session, the computer selected intervals from this list without replacement. The first response after the interval elapsed result in presentation of the VS and another VI value was then selected from the list. Animals were tested 5 days/week (Monday through Friday, weekends off) for ten sessions during the light reinforcement phase of the experiment. Responses to the active and inactive sides were monitored.
2.4.6 Intravenous Catheterization Surgical Procedure
The rats were anesthetized using ketamine/xylazine (60.0 and 5.0 mg/kg, i.p., respectively). Isoflurane was administered if supplemental anesthesia was necessary during surgery. Two incisions were made at the beginning of the surgery. The right external jugular vein was carefully isolated through blunt dissection of the surrounding tissue and the catheters were inserted approximately 3cm into the vein.
The catheter was tunneled subcutaneously and exited out the back and was connected to a metal cannula located in the center of a vascular access harnesses (VAH95AB, Instech Solomon, Plymouth Meeting, PA). The catheters were flushed daily with 0.1 to 0.2 ml solution of enrofloxacin (4.0 mg/ml) mixed in a heparinized saline solution (50 IU/ml in 0.9% sterile saline) during recovery to preserve catheter patency. At the end of behavioral testing, each animal received an i.v. infusion of ketamine hydrochloride (0.5 mg/kg, IV, in 0.05 ml) and the behavioral response was observed to verify catheter patency. Only rats with patent catheters were used in data analysis.
2.4.7 Methamphetamine Self-administration procedure
The test chambers used for the self-administration testing were a different set of chambers identical to those described and used for the light reinforcement testing, but were located in a different test room than the chambers used for light reinforcement testing. Testing occurred during the animals’ light cycle and was conducted 5 days per week. Following testing, catheters were flushed with 0.1 ml of 10 U heparin sulfate enrofloxacin saline and rats were returned to the colony room.
2.4.8 Self-administration habituation
After recovery from surgery, the animals were tethered and placed into the operant chambers for six 30 min habituation/extinction sessions during which the operant chambers were dark and responses made to either side resulted in no programmed consequences. The infusion lines were filled with saline during this phase. This phase was conducted in order to (i) extinguish responding to the side associated with reinforcement during previous light reinforcement training, (ii) habituate the rats to being tethered, and (iii) re-determine the baseline level of operant responding.
2.4.9 Self-administration
Following the 6 day habituation/extinction period, the responses to the active alternative resulted in 0.025mg/kg/infusion METH and VS according to a VI 2 min schedule of reinforcement. For all rats, the active snout poke hole during the self-administration testing was the inactive alternative during the previous light reinforcement phase of the experiment. For example, a rat assigned to the right snout poke hole in the light reinforcement phase was assigned to the left snout poke hole in the self-administration phase. This reversal was conducted in order to rule out differences in self-administration acquisition due to previous history with the response contingent VS in the light reinforcement phase. Each infusion was accompanied by the 5 s illumination of the VS (same stimulus as used in light reinforcement phase). Responses on the inactive lever were recorded but had no scheduled consequences. The self-administration phase consisted of ten 30 min test sessions.
2.5 Data Analysis
2.5.1 Locomotor Scores
Locomotor scores during each of the 10 sessions of METH exposure were analyzed using a three-factor mixed analysis of variance (ANOVA) with sessions as the within-subject factor and locomotor activity in a novel environment (LR, HR) and drug treatment (METH, SAL) as the between-subject factors. The sources of significant interactions or main effects were determined with post-hoc statistical tests. Because pre-exposure to METH did not produce a significant sensitization effect and because including pre-exposure to METH in the analysis of the light reinforcement and self-administration phases did not produce any unique significant effects, pre-exposure to METH was removed as an independent variable in the final analysis of the light reinforcement and self-administration phases.
2.5.2 Light reinforcement & Methamphetamine Self-Administration
The dependent variables were: (i) active responding, (ii) inactive responding, and (iii) relative frequency of active responding. The active alternative was the snout poke hole that produced the response contingent VS or VS&METH on a VI 2 min schedule. Inactive responses were responses to the alternative snout poke hole that produced no programmed consequences. Responding to the inactive and active snout poke holes were quantified as totals during each 30 minute test session. The relative frequency of active responding provided a measure of preference that was independent of the absolute rates of responding. Relative frequency of active responding = active / (active + inactive responding).
Performance during the habituation periods was analyzed using a two-factor mixed analysis of variance (ANOVA) with two-day session blocks as the within-subject factor and locomotor classification (HR, LR) as the between-subject factor. If significant differences were found, selected statistical tests were performed in order to determine the source of significance. The primary purpose of the analysis of habituation responding was to determine if there were differences between the HR and LR groups during habituation (baseline responding). During the habituation period, active and inactive responding refers to the alternatives that would later be designated as active or inactive during the contingent test period. Each of the three dependent variables (i) active responding, (ii) responding to the inactive alternative, and (iii) relative frequency of active responding were analyzed separately.
Performance during the light reinforcement and SA phases was analyzed using a two-factor mixed ANOVA with session as the within-subject factor and locomotor classification (HR, LR) as the between-subject factor. The session factor was defined for both the light and drug reinforcement phases as average performance during the 10 day phases and the average performance during the last two days of the 6 day habituation phase (Session block 3). If significant interactions or main effects were found, selected statistical tests were performed to determine the source of significance. Each of the three dependent variables (i) active responding, (ii) responding to the inactive snout poke hole, and (iii) relative frequency of active responding were analyzed separately. Two animals died during surgery, and one animal pulled out the catheter following surgery. Therefore, during the SA phase, 8 rats were in the LR group, and 9 rats were in the HR group.
Because large within-session decreases in responding were observed in light reinforced behavior, the within-session pattern of responding was examined in all three phases of the experiment (i.e. locomotor activity, light reinforcement and SA) to further characterize this aspect of light reinforced behavior. In the locomotor phase, the locomotor activity during day 10 of METH treatment was divided into three 10 minute epochs. These data were than analyzed using a three factor mixed repeated measures ANOVA with locomotor classification and drug treatment (METH or saline) as two between-subject factors and time and as the within-subject factor. In the light reinforcement and SA phases, active and inactive responding was collapsed across drug pre-treatment and analyzed using a two-factor mixed repeated measures ANOVA with locomotor classification as the between-subject factor and time as the within-subject factor.
3.1 Results
3.1.1 Locomotor Activity
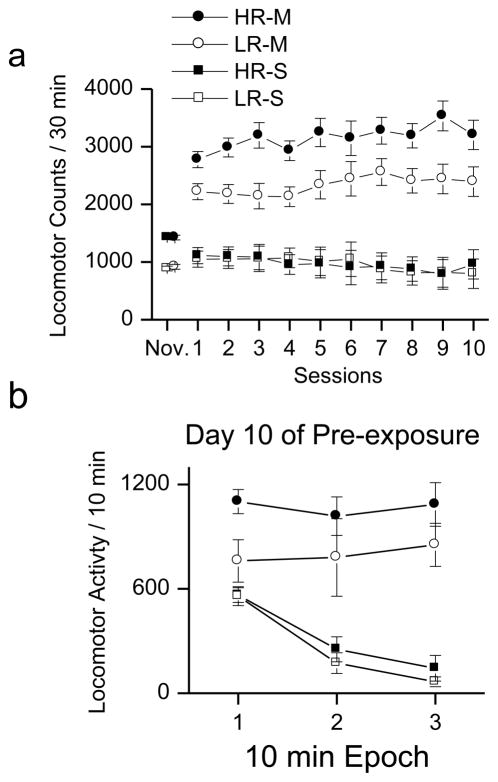
METH increased locomotor activity in both the HR and LR groups compared to SAL (Fig. 1a). The average locomotor activity in the HR rats treated with METH was greater than the average locomotor activity of LR rats treated with METH, although this difference was not significant (F(1,16) = 4.392, p = 0.052). There was also a non-significant trend for locomotion to increase with METH exposure [F(1,8)= 1.907, p < 0.204]. For HR group, locomotion increased from 2776.4 ± 384.596 on day 1 of METH exposure to 3204.6 ± 582.209 on day 10 of METH exposure. Of the HR rats treated with METH, 4 out of 5 rats increased locomotor activity. For the LR group, locomotion increased from 2219.2 ± 317.662 on day 1 of METH exposure to 2394.6 ± 913.528 on day 10 of exposure Of the LR rats treated with METH, 2 out of 5 rats increased locomotor activity. Average locomotor activity of HR and LR animals treated with SAL was very similar following the first day of exposure to the novel activity chambers.
Fig 1. Locomotor activity and METH pre-exposure.
This plot illustrates the results of the response to novelty as measure in a 30 min locomotor test and the subsequent 10 test sessions of either METH or saline treatment. Closed symbols refer to animals classified as high responders (HR) and open symbols refer to low responders (LR). Circles indicate animals treated with METH, and squares indicate animals treated with saline. a: Animals treated with METH had more locomotor activity than saline treated rats. Although HR rats have a greater average response to METH than LR rats, this difference is not significant. There were no differences between HR and LR rats treated with saline. b: The data for the day 10 of METH or Saline treatment are plotted in 10 minute epochs indicating the within-session pattern of locomotor activity. Both HR and LR rats treated with saline showed significant within-session declines in locomotor activity. In contrast, rats treated with METH showed no within-session decline in locomotor activity. See text for detailed description.
There was a significant interaction between session and drug [F(9,144)= 4.90, p < 0.001] but the interaction between drug and response to novelty was not significant. The source of the interaction between session and drug was significant across-session decline in locomotor activity in animals treated with SAL [F(1,9) = 23.65, p < .001] while the animals treated with METH showed no significant decline. This pattern of results suggests SAL treated groups habituated to the locomotor chamber. We did not detect an effect of drug pre-exposure as measured by a significant increase in locomotor activity across sessions.
Fig. 1b (bottom panel) shows the within-session pattern of locomotor activity for rats in the four different treatment conditions during the last (10th) day of the locomotor drug pretreatment phase. There was a main effect of time [F(2,32)=21.964, p<0.001], and a significant interaction between time and drug pretreatment [F(2,32)=25.737, p<0.001].This figure shows that treatment with METH prevented the within-session decline in locomotor activity. HR and LR animals that were treated with METH showed no decline in locomotor activity during the 30 min test session. In contrast, animals treated with SAL demonstrated within-session declines in locomotor activity.
3.2.1 Light Reinforcement
3.2.2 Habituation
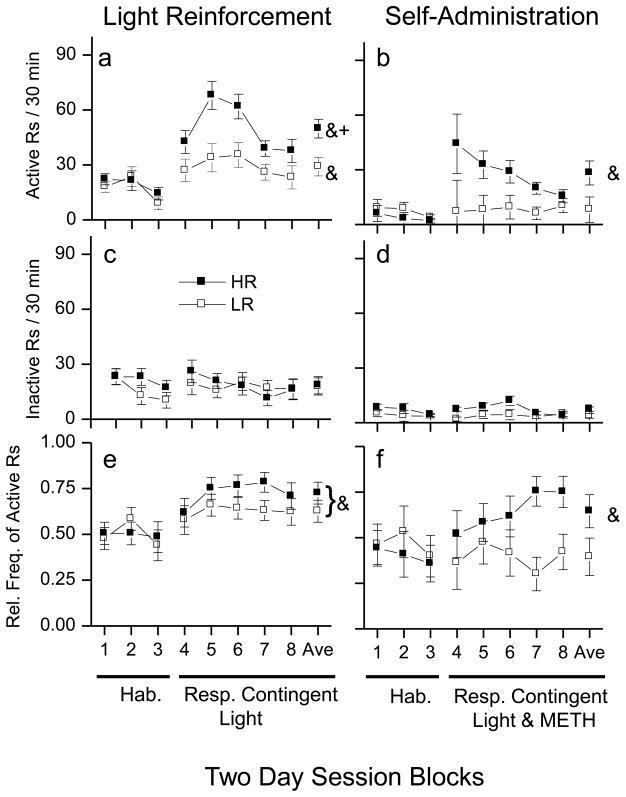
There was a main effect of session [F(2,32)= 5.58, p<0.01] on responding to the snout poke hole that would later become the active side during the light reinforcement phase and also a main effect of session on responding to the side that would later become the inactive snout poke alternative [F(2,32)= 6.57, p < 0.01] (Figs. 2a, c) . No other main effects or interactions were significant. A within-subject t-test found that responding to the alternative that would later be designated as the active response was greater during session block 1 of habituation than active responding during session block 3 of habituation indicating that snout poking decreased as the animals became more familiar with the experimental chamber [t(19)= 3.22, p<0.01]. A second within-subject t-test found that responding to the alternative that would be later designated the inactive response was greater during session block 1 of habituation than inactive responding during session block 3 of habituation, again indicating that snout poking decreased as the animals became more familiar with the chamber [t(19)= 3.79, p < 0.001]. There were no significant effects of session or locomotor classification on the relative frequency of active responding (Fig. 2e). In summary, there were no differences between the HR and LR groups and responding to both the active and inactive snout poke holes decreased during habituation as the animals became familiar with the experimental chamber.
Fig 2. Light and METH reinforced responding.
These plots show the effects of a response contingent VS and VS&METH on snout poking following 6 days of habituation to a dark test chamber. The left panels show the data for response contingent VS phase. The right panels show the data for VS&METH combination phase. Closed square symbols refer to animals identified as high responders (HR) and open square symbols refer to low responders (LR). a&b: Data are illustrated as the average (±SEM) number of responses to the active alternative during each 30 minute session. (a) The response contingent VS increased active responding in both the HR and LR groups. The increase in active responding was significantly greater in the HR group compared to the LR group. (b) The response contingent VS&METH combination increased active responding in HR but not the LR rats. c&d: Data are the average (±SEM) number of responses to the inactive alternative during each 30 min test session. Neither the response contingent VS (c) or VS&METH combination (d) significantly effected responding to the inactive side. e&f: Data are the average (±SEM) relative frequency of active responding (active/(active + inactive)) during each 30 minute test session. (e)The response contingent VS increased the relative frequencies of active responding in both the HR and LR groups. (f) The response contingent VS&METH combination increased the relative frequency of active responding in HR rats but not LR rats. Ampersand (&) indicates a within group difference (p > 0.05) between the last two days of the habituation phase and average responding during response contingent VS testing. Plus (+) indicates a difference between the HR an LR groups (p > 0.05). See text for detailed description.
3.2.3 Response contingent VS
There was a significant interaction between session and locomotor classification [F(1,16)= 7.22, p < 0.05]. A between-subject t-test found that the average of active responding of the HR group was greater than that of the LR group [t(18)= 2.88, p ≤ 0.01]. Within-subject comparisons of average responding during the contingent light period with the responding during the habituation period showed that both the HR [t(9)= 9.27, p < 0.001] and LR [t(9)= 4.69, p ≤ 0.01] groups increased active nose pokes during contingent VS period. Inactive responding was not significantly affected by presentation of the response contingent VS (Fig. 2c). There were no significant effects of session or locomotor classification on responding to the inactive snout poke hole. The relative frequency of active responding was increased in both the HR and LR groups (Fig. 2e, bottom panel). A significant effect of session in the absence of any other interaction or main effects [F(1,16)= 32.48, p < 0.001] indicated that the average relative frequency of active responding was increased in both groups during the contingent VS period in comparison to the habituation period. In summary, the response contingent VS increased active responding and the absolute rate of active responding was greatest in HR animals.
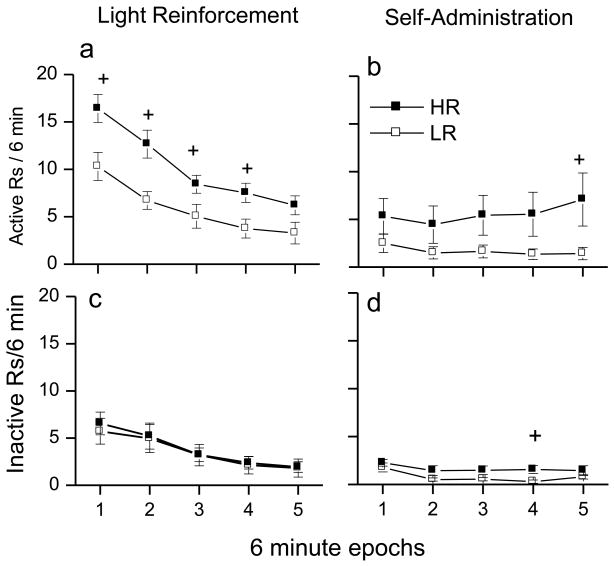
Fig. 3a shows the within-session pattern of active responding (average of the 10 contingent light test sessions). These data were analyzed using a two-factor mixed repeated measures ANOVA with locomotor classification as the between-subject factor and time as the within-subject factor. There was a significant interaction between time and locomotor classification [F(4,72)=91.508, p<0.05]. Follow up t-tests revealed HR rats responded more than LR rats at epoch 1 [t(18)=−3.101], epoch 2 [t(18)= −3.573] epoch 3 [t(18)= −2.253], and epoch 4 [t(18)= −2.579] but not at epoch 5. The greatest difference between HR and LR rats in responding was observed at the beginning of the session, with the difference becoming smaller across the session. In general, light reinforced responding was greatest at the start of the test session and then declined. Fig. 3c shows the within-session pattern of responding to the inactive snout poke hole. There was a significant effect of time [F(4,72)=28.004 p<0.05] due to the within-session decline in inactive responding, but no significant differences were observed between HR and LR.
Fig 3. Within-session light and METH reinforced responding.
These plots show within session changes in snout poking during the 30 min test sessions in 6 min epochs. The left panels show the data for response contingent VS phase. The right panels show the data for VS&METH combination phase. Closed square symbols refer to animals identified as high responders (HR) and open square symbols refer to low responders (LR). a: Data are for active responding that produced the VS. Both HR and LR rats show within session declines in active responding although the HR rats generally respond at a higher rate. b: Data are illustrated for active responding that produced the VS&METH combination. LR rats show within-session declines in active responding. In contrast, HR rats show no within-session decline. c: Both HR and LR rats show within-session declines in inactive responding and there were no differences in the rate of inactive responding between HR and LR groups. d: Both HR and LR rats show within-session declines in inactive responding with the HR rats having a significantly higher rate of inactive responding than the LR rats. Ampersand (&) indicates a within group difference (p > 0.05). See text for detailed description.
3.3 Methamphetamine Self-Administration
3.3.1 Habituation
There were no significant effects of session or locomotor classification on snout poking to the alternatives designated as active and inactive during the 6 day habituation period (Fig. 2b). Furthermore, no significant effects were found for the relative frequency of active responding during the 6 day habituation period (Fig. 2d).
3.3.2 Contingent VS&METH
Response contingent VS&METH increased active snout poking in the HR but not the LR animals. There was a significant interaction between sessions and locomotor classification [F(1,13)= 5.36, p<0.05] (Fig. 2b). Comparison of the active responding during the period between the HR and LR groups with a between-groups t-test indicated no significant difference. However, separate within-group t-tests comparing active responding during the habituation and contingent periods revealed that active responding was increased in the HR group [t(8) = 3.07, p<0.05] but not the LR group. The average number of METH infusions per session across all 10 days of SA testing was 4.94±1.28 in HR rats and 2.65±0.92 in LR rats; however, these means were not significantly different.
Responding to the inactive snout poke hole was not significantly affected by presentation of the response contingent VS/METH (Fig. 2d). There were no significant effects of session or locomotor classification on inactive responding.
There was a significant interaction between sessions and locomotor classification [F(1,12)= 5.98, p<0.05] (Fig. 2f). Comparison of the relative frequency of active responding during the period between the HR and LR groups with a between-groups t-test indicated no significant difference. However, separate within-group t-tests, comparing active responding during the habituation and contingent periods revealed that the relative frequency of active responding was increased in the HR group [t(8) = 4.32, p<0.01] but not the LR group. In summary, response contingent VS&METH increased the relative frequency of active responding in the HR animals but not the LR animals.
The within-session pattern of active responding during the self-administration phase is shown in Fig. 3b. There was no significant effect of time or locomotor classification. However, there was a significant interaction between time and locomotor classification [F(4,60)= 2.904, p<0.05]. There was an increasing trend for HR rats to respond more within the session and LR rats to respond less within the session. The combination of these two trends produced a significant difference at the last six minute epoch [t(15)= −2.289, p<0.05]. For the HR group, there is no indication of a within-session decline in responding. In contrast, there was a decline in within-session responding for the LR group.
There was a significant effect of time [F(4,60)= 6.152, p<0.05] and locomotor classification [F(4,60)=5.643, p<0.05] in within-session responding to the inactive snout poke hole. HR rats responded more than LR rats to the inactive snout poke hole and responding declined for both groups across the session. The within-session pattern of inactive responding (Fig. 3d) shows a different pattern than that observed for active responding. There were within-session decrements in responding for both the HR and LR groups. HR rats had greater within-session responding at epoch 4 [t(15)= 4.444, p<0.05].
4.1 Discussion
4.1.1 Summary of results
Animals were selected based on locomotor activity in a novel environment and divided into HR and LR groups. Half of HR and LR rats were pre-exposed to non-contingent METH for 10 days while the remaining half of each group was pre-exposed to saline. All animals were subsequently tested for responding for a novel VS reinforcer. Animals that were identified as HR responded significantly more to the active alternative that produced the visual stimulus than LR rats. No differences in light reinforced responding were found in rats pre-exposed to METH and saline. Following light reinforcement testing, all rats were tested for acquisition of a low dose of METH paired with a VS. Again, HR rats responded more to the active alternative than LR rats.
4.1.2 Locomotor Activity- Classification of HR/LR
The observation that both HR and LR saline rats were no longer different in activity following the initial exposure to the locomotor chamber indicates that the difference between HR and LR in locomotor activity on the first exposure to the locomotor chamber was due to the novelty of the test environment rather than a difference in basal activity levels. Alternatively, LR rats may have learned about the novel activity chambers more rapidly than HR counterparts. This interpretation, however, is contradictory to the results of the light and drug reinforcement phases, where it was observed that HR rats acquired responding more rapidly than LR counterparts. An interesting observation about the effects of METH during the locomotor phase of the study was that animal’s receiving SAL throughout the 10 days of testing showed within-session declines in locomotor activity (Fig. 1b), while rats receiving METH did not demonstrate within-session declines in activity (Fig. 1b). One interpretation of this pattern of results is that daily injections of METH prevented habituation to the locomotor chamber. As is discussed below, a similar pattern of within- and between-session habituation was observed during the light reinforcement and self-administration phases of this experiment (Fig 3a).
4.2.1 Light Reinforcement
Introduction of the response contingent VS resulted in an increase in the absolute and relative frequency of active responding with HR rats showing a significantly greater increase in active responding than LR rats (Fig 2a). There were no differences in the operant level of snout poking between HR and LR rats during the six-day habituation period prior to introduction of the response contingent VS. It is unclear why the HR rats did not snout poke more during habituation than LR rats, particularly on the first day of habituation when the operant chamber was novel. Perhaps this absence of a greater operant level of responding during habituation in the HR rats was due to the specificity of the response (snout poking) in contrast to the more general activity measure used in the locomotor chamber. All rats showed within-session declines in light reinforced responding (Fig. 3). The pattern of the within-session decline was similar to that seen for the saline rats during locomotor testing (Fig. 1).
The greater absolute rate of responding for the response contingent light onset in the HR rats compared to LR rats, together with the absence of a difference snout poking between the two groups during the habituation indicates that HR rats are differentially affected by response contingent light onset. These results are, to the best of our knowledge, the first demonstration that individual differences in locomotor activity in a novel environment are predictive of responding for a response contingent VS. It is notable that we did not observe a significant difference in the relative frequency of responding between LR and HR rats. The absence of a significant difference in the relative frequency of responding between the LR and HR rats suggests that the response contingent VS may have had a general activating effect. On the other hand, it is difficult to explain enhanced responding for the VS in HR animals exclusively as a non-specific activating effect because inactive responding was not significantly increased. It may be that the response contingent VS had both reinforcing effects which differentially increased active responding and weaker activating effects which increased inactive responding enough to prevent a significant increase in the relative frequency of active responding. Nonetheless, it is clear that the response contingent VS had a significantly greater impact on active responding in HR rats than the LR rats.
4.3.1 Self-administration of METH
Introduction of the response contingent VS&METH combination increased both absolute and relative frequency of active responding in HR but not LR rats (Fig. 2b, f). These data are consistent with previous reports that HR rats self-administer psychostimulants at doses that LR rats do not self-administer (Marinelli and White 2000; Piazza et al. 1989; Piazza et al. 1990; Pierre and Vezina 1997). However, examination of Fig 2b shows a marked decline in active responding across the 10 day SA phase. This decline is not consistent with acquisition of drug reinforced responding but is consistent with a decline in the reinforcing properties of VS across days of testing. It is notable that Pierre and Vezina (1997) observed a similar decline in active responding in their study of HR and LR differences in the SA of d-amphetamine indicating that this pattern of responding during acquisition amphetamine SA is not unique to the present study. In addition, comparison of Fig 2b to Fig 2a indicates there was less active responding during the SA phase than in the light reinforcement phase. There are a few possible explanations to explain the decline in active responding across days and lower levels of responding in the SA phase: (i) animals were fitted with harnesses that may have affected the overall level of operant behavior, (ii) rats were pair housed during light reinforced responding, however following surgery, rats were singly housed in order to protect the harness/catheter assembly, (iii) the decrease in active responding may have occurred in order to regulate drug intake, or (iv) another possible explanation is that the decline in responding across days during the SA was due to a decrease in the novelty of the VS with repeated testing.
The within-session pattern of responding during the self-administration phase of the experiment provided some evidence that response contingent METH affected responding of the HR animals during the self-administration phase of the experiment. Specifically, the within-session pattern of responding of the HR group did not show a decline as was observed in the light reinforcement phase. The absence of a within-session decline in responding in the HR group is consistent with the effects of METH observed in the locomotor phase of the study as well as previous light reinforcement studies where systemic injections of METH differentially prevented within-session declines in light reinforced responding (Ashrafioun et al. 2008).
Based on these results, it is not clear that the HR animals were responding to produce the VS, the drug, or the combination of the two. Systemic injections of both amphetamine (Glow and Russell 1973, 1973, 1974; Gomer and Jakubczak 1974; Winterbauer and Balleine 2007) and nicotine (NIC; Palmatier et al. 2007; Raiff and Dallery 2009) have been reported to increase the primary reinforcing value of visual stimuli. According to these studies, the primary reinforcing effects of visual stimuli are amplified by amphetamine and nicotine. Thus, the increases in responding demonstrated by the HR rats for the VS&METH combination can be explained in a variety of different ways: (i) the reinforcing effects of METH, (ii) primary reinforcing effects of the VS, or (iii) METH induced increase in the primary reinforcing effects of the VS.
It is important to note, however, the procedures used in the current experiment were designed to maximize the effects of the VS as a primary reinforcer. First, the animals were habituated to the operant test chamber for 6 days before the response contingent VS was introduced to ensure that the test chamber was familiar and that the response contingent VS was relatively novel and salient. Previous studies have demonstrated that habituation to the test environment prior to introduction of the response contingent VS increases acquisition of responding for the VS (Kish 1966). Second, VI 2 min schedules of reinforcement were used in both the light reinforcement and self-administration phases of the experiment. This schedule was chosen because it ensures the VS is experienced (Only a single response is required to produce the VS once the required interval has elapsed), while minimizing prolonged exposure to the VS (At the most, the animals were exposed to the VS only once every 2 minutes), allowing the VS to remain relatively novel for a longer period of time. Variable interval schedules of reinforcement are not widely used in self-administration studies; however, robust responding in self-administration has been reported to be maintained by these schedules (Sizemore et al. 1997; Quick and Shahan 2009). Third, the 30 minute session duration used in this experiment is shorter than is often used in SA experiments. This short session duration, although optimal for light reinforcement studies, may have affected acquisition of SA. Another possible procedural problem is that prior to SA testing, the animals were first trained to respond for visual reinforcers in boxes that were very similar to those used for SA testing. However, the active alternative in the SA phase was the inactive alternative during the previous light reinforcement phase, so that increases in active responding during the SA phase at the very least reflected reversal learning for the visual reinforcer.
The results from the SA phase of this experiment cannot be clearly interpreted as self-administration of METH or as light reinforced responding. Future studies including a saline self-administration group as well as other control groups (i.e., a response independent drug injection group), are needed to draw a stronger conclusion about the role of the VS in METH SA. However, this study does provide evidence that the role of VS may be underappreciated in SA experiments using VS as cues of drug availability/unavailability. Other studies have also provided evidence that VS play a role in SA. Deroche-Gamonet et al. (2002) observed an acceleration in the acquisition of cocaine SA in rats under conditions where cocaine was paired with a VS compared to drug without cue. However, the differences between the cue and no cue group disappeared with continued training. This is interesting, as it is in contrast to results of self-administration of nicotine (Caggiula et al. 2001), which showed that the presence or absence of the VS affects of the rate of responding even after the animals have acquired nicotine SA. The results of these studies suggest that cocaine SA maybe less affected than nicotine SA by the presence or absence of VS. However, increases in responding for VS have also been observed in association with the self-administration of cocaine (Chaudhri 2003). In this study, rats that received non-contingent injections of cocaine increased responding for a response contingent VS. Other studies have shown that non-contingent systemic injections of amphetamine (Glow and Russell 1973, 1973, 1974; Gomer and Jakubczak 1974; Winterbauer and Balleine 2007) and nicotine (Palmatier et al. 2007; Raiff and Dallery 2009) increase responding for visual stimuli. Taken together, the results from the present study and other recent studies indicate that potentially reinforcing visual stimuli may play an important role in animal SA studies.
4.4.1 Sensory reinforcement, self-administration and sensation seeking
Sensory reinforcement has most often been defined by what it is not rather than what it is. For example, Kish (1966) defined sensory reinforcement as a primary reinforcement process resulting from the response-contingent presentation or removal of stimuli of moderate intensity which are not related to some organic need or removal of aversive stimulation. In a later review, Eisenberger (1972) defined sensory reinforcers as “incentives that have no evident tissue-maintenance or reproductive functions”. According to these definitions the VS used in this study and IV infusions of METH are both sensory reinforcers. There are other commonalities between sensory reinforcers and drug SA as well. In this paper, we have shown that locomotor activity in a novel environment is predictive of both responding for a VS and drug SA. Consistent with the results of this study, isolation reared rats which have been shown to respond more for a VS (Cain, Green, and Bardo 2006) and to have greater responses to novelty (Fuller 1967; Green et al. 2003; Bowling, Rowlett, and Bardo 1993) also have a higher propensity to self-administer drugs of abuse (Bardo et al. 2001; Green, Gehrke, and Bardo 2002) compared to enriched reared counterparts. In addition, it is known that responding for novel stimuli (Richards and Leslie 1962; Fehrer 1956), sensory reinforcers (Smith and Donahoe 1966; Davis 1958) and drug SA (Carroll, France, and Meisch 1979; Carroll and Boe 1982; Oei 1983) are increased by food and water deprivation.
Past research into sensory reinforcement included broad classes of sensory stimuli and behaviors, including visual, auditory, locomotor exploration and manipulation. It has been suggested that operant responding for sensory reinforcers is analogous to locomotor and orienting responses which expose organisms to novel environmental stimuli such as those found in a novel locomotor chamber (Kish 1966). According to this analysis, responding to produce a VS and exploring a novel locomotor chamber are mediated by common underlying behavioral and neural processes. The association reported in this paper between locomotor activity in a novel environment and responding for a visual reinforcer is consistent with this interpretation of sensory reinforcement.
Locomotor activity in a novel environment has been suggested to be an animal model of sensation seeking (Blanchard, Mendelsohn, and Stamp 2009; Dellu et al. 1996). Establishing an animal model of sensation seeking is important because in humans sensation seeking is correlated with drug abuse (Zuckerman 1994). We suggest that visual reinforcement may also share similar features to sensation seeking in humans and that responding to produce a VS has attributes that may make it a better measure of sensation seeking. Locomotor activity in a novel environment most likely involves a combination of both investigatory and stress behaviors. Bardo et al. (1996) characterize the rats reaction to a novel locomotor chamber as an inescapable novelty test, implying that locomotor activity in a novel locomotor chamber is stress-induced, and may in part reflect the animal’s attempts to escape from the locomotor chamber. This conceptualization suggests the animal is not approaching novelty, but rather is trying to escape it. Operant responding to produce a VS may be a better measure of sensation seeking because it is an approach behavior and is unlikely to involve stress or “fearfulness” because of previous habituation to the test chamber.
4.5.1 Conclusion
We have demonstrated that locomotor activity in a novel environment, which is predictive of acquisition of drug self-administration, is also predictive of responding to produce a visual stimulus. This has important practical implications for laboratory models of drug self-administration, in that a visual stimulus is often paired with presentation of the drug. If animals identified as HR are differentially sensitive to the reinforcing effects of a visual stimulus it becomes unclear if the animals are responding to produce the drug, visual stimulus, or combination of the two. We speculate that sensory reinforcement in rodents may be related to the construct of sensation seeking in humans and that it may be a better model of sensation seeking than the locomotor activity in a novel environment because it does not involve fearfulness. These data suggest that further research is needed to understand the impact of potentially reinforcing VS in animal SA studies and to determine the functional relationships between responding to produce sensory stimuli and responding to produce drugs of abuse. Indeed, a better understanding of the role of VS in animal SA studies may also lead to a better understanding of similar cues in human drug taking.
Acknowledgments
We wish to thank Drs. Paul Vezina and Ashley Acheson for critical comments and editorial assistance on earlier versions of this manuscript. This work was conducted in partial fulfillment of the requirements for doctoral degree for Amy M. Gancarz. This work was partly supported by DA10588 to Jerry B. Richards.
This work was partly supported by DA10588.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.1 References
- Ambrosio E, Goldberg SR, Elmer GI. Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav Pharmacol. 1995;6:229–237. [PubMed] [Google Scholar]
- Ashrafioun L, Gancarz AM, San George M, Richards JB. Effects of methamphetamine on the reinforcing value of novel and familiar visual stimuli. Society for Neuroscience Annual Meeting; Washington, D.C: 2008. [Google Scholar]
- Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology (Berl) 2001;155:278–84. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Berlyne DE. The Reward-Value of Indifferent Stimulation. In: Tapp JT, editor. Reinforcement and Behavior. Academic Press; New York: 1969. [Google Scholar]
- Bevins RA, Peterson JL. Individual differences in rats' reactivity to novelty and the unconditioned and conditioned locomotor effects of methamphetamine. Pharmacol Biochem Behav. 2004;79:65–74. doi: 10.1016/j.pbb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Blanchard MM, Mendelsohn D, Stamp JA. The HR/LR model: Further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev. 2009;33:1145–54. doi: 10.1016/j.neubiorev.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Bowling SL, Rowlett JK, Bardo MT. The effect of environmental enrichment on amphetamine-stimulated locomotor activity, dopamine synthesis and dopamine release. Neuropharmacology. 1993;32:885–93. doi: 10.1016/0028-3908(93)90144-r. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF. Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav. 2002;77:683–7. doi: 10.1016/s0031-9384(02)00918-6. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–30. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–7. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Cain ME, Dotson WF, Bardo MT. Individual differences in the effect of novel environmental stimuli prior to amphetamine self-administration in rats (Rattus norvegicus) Exp Clin Psychopharmacol. 2006;14:389–401. doi: 10.1037/1064-1297.14.3.389. [DOI] [PubMed] [Google Scholar]
- Cain ME, Green TA, Bardo MT. Environmental enrichment decreases responding for visual novelty. Behav Processes. 2006;73:360–6. doi: 10.1016/j.beproc.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Boe IN. Increased intravenous drug self-administration during deprivation of other reinforcers. Pharmacol Biochem Behav. 1982;17:563–7. doi: 10.1016/0091-3057(82)90319-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Food deprivation increases oral and intravenous drug intake in rats. Science. 1979;205:319–21. doi: 10.1126/science.36665. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology (Berl) 2007;190:353–62. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–66. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Clements LA, Allen SS, Sved AF. Enhancement of reinforced operant responding by nicotine and cocaine in rats; analysis of dose and drug-contingency. Society for Neuroscience; New Orleans, LA: 2003. [Google Scholar]
- Clifford PS, Hart N, Thompson J, Buckman S, Wellman PJ, Bratton GR, Nation JR. Prenatal lead exposure enhances methamphetamine sensitization in rats. Pharmacol Biochem Behav. 2009;93:165–9. doi: 10.1016/j.pbb.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred High-Responder and Low-Responder rats. Pharmacol Biochem Behav. 2008;90:331–8. doi: 10.1016/j.pbb.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JD. The reinforcing effect of weak-light onset as a function of amount of food deprivation. Journal of Comparative and Physiological Psychology. 1958;51:496–498. doi: 10.1037/h0049158. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–45. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV. Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. Eur J Neurosci. 2002;15:1363–70. doi: 10.1046/j.1460-9568.2002.01974.x. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Eisenberger R. Explanation of rewards that do not reduce tissue needs. Psychol Bull. 1972;77:319–39. doi: 10.1037/h0032483. [DOI] [PubMed] [Google Scholar]
- Fehrer E. The effects of hunger and familiarity of locale on exploration. Journal of Comparative and Physiological Psychology. 1956;49:549–552. doi: 10.1037/h0047540. [DOI] [PubMed] [Google Scholar]
- Feigley DA, Hamilton LW. Response to novel environment following septal lesions or cholinergic blockade in rats. J Comp Physiol Psychol. 1971;76:496–504. doi: 10.1037/h0031368. [DOI] [PubMed] [Google Scholar]
- Fleshler M, Hoffman HS. A progression for generating variable-interval schedules. J Exp Anal Behav. 1962;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller JL. Experiential deprivation and later behavior. Science. 1967;158:1645–52. doi: 10.1126/science.158.3809.1645. [DOI] [PubMed] [Google Scholar]
- Glow PH. Response contingent schedule-control: Control over the environment motivates bar pressing. Australian Journal of Psychology. 1985 Dec;37(3):233–255. [Google Scholar]
- Glow PH, Russell A. Drug enhanced sensory contingent bar pressing: Comparing the effect of contingent and noncontingent sensory change. Psychopharmacologia. 1973;32(3):285–292. doi: 10.1007/BF00422151. [DOI] [PubMed] [Google Scholar]
- Glow PH, Russell A. Effects of dexamphetamine, amylobarbitone sodium and their mixture on sensory contingent bar pressing behaviour in the rat. Psychopharmacologia. 1973;31(3):239–251. doi: 10.1007/BF00422514. [DOI] [PubMed] [Google Scholar]
- Glow PH, Russell A. Sensory-contingent barpressing for familiar and novel change under a dexamphetamine-amylobarbitone mixture. Animal Learning & Behavior. 1974 Feb;2(1):27–30. [Google Scholar]
- Gomer FE, Jakubczak LF. Dose-dependent selective facilitation of response-contingent light-onset behavior by d-amphetamine. Psychopharmacologia. 1974;34(3):199–208. doi: 10.1007/BF00421961. [DOI] [PubMed] [Google Scholar]
- Green TA, Cain ME, Thompson M, Bardo MT. Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology (Berl) 2003;170:235–41. doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology (Berl) 2002;162:373–8. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behav Pharmacol. 1998;9:299–308. [PubMed] [Google Scholar]
- Keiflin R, Vouillac C, Cador M. Level of operant training rather than cocaine intake predicts level of reinstatement. Psychopharmacology (Berl) 2008;197:247–61. doi: 10.1007/s00213-007-1026-2. [DOI] [PubMed] [Google Scholar]
- Kish GB. Studies of Sensory Reinforcement. In: Honig W, editor. Operant Behavior: Areas of research and application. Appletone-Century-Crofts; New York: 1966. [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–85. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AA, Sethna DM, Young PA. Initial suppression of the locomotor stimulant response to dexamphetamine in rats exposed to a novel environment. Br J Pharmacol. 1970;39:230P–232P. [PMC free article] [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162:333–8. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- Nordquist RE, Voorn P, de Mooij-van Malsen JG, Joosten RN, Pennartz CM, Vanderschuren LJ. Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. Eur Neuropsychopharmacol. 2007;17:532–40. doi: 10.1016/j.euroneuro.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Oei TP. Effects of body weight reduction and food deprivation on cocaine self-administration. Pharmacol Biochem Behav. 1983;19:453–5. doi: 10.1016/0091-3057(83)90119-3. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004;173:98–104. doi: 10.1007/s00213-003-1702-9. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend. 2007;89:52–9. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–3. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Maccari S, Mormede P, Le Moal M, Simon H. Individual reactivity to novelty predicts probability of amphetamine self-administration. Behav Pharmacol. 1990;1:339–345. doi: 10.1097/00008877-199000140-00007. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–32. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology (Berl) 1997;129:277–84. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- Quick SL, Shahan TA. Behavioral momentum of cocaine self-administration: effects of frequency of reinforcement on resistance to extinction. Behav Pharmacol. 2009;20:337–45. doi: 10.1097/FBP.0b013e32832f01a8. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Responding maintained by primary reinforcing visual stimuli is increased by nicotine administration in rats. Behav Processes. 2009;82:95–9. doi: 10.1016/j.beproc.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of discount functions in rats with an adjusting-amount procedure. J Exp Anal Behav. 1997;67:353–66. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards WJ, Leslie GR. Food and water deprivation as influences on exploration. Journal of Comparative and Physiological Psychology. 1962;55:834–837. doi: 10.1037/h0046065. [DOI] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Effects of prior amphetamine exposure on approach strategy in appetitive Pavlovian conditioning in rats. Psychopharmacology (Berl) 2009;202:699–709. doi: 10.1007/s00213-008-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore GM, Gaspard TM, Kim SA, Walker LE, Vrana SL, Dworkin SI. Dose-effect functions for cocaine self-administration: effects of schedule and dosing procedure. Pharmacol Biochem Behav. 1997;57:523–31. doi: 10.1016/s0091-3057(96)00437-6. [DOI] [PubMed] [Google Scholar]
- Smith RC, Donahoe JW. The effects of food deprivation on unreinforced and light-reinforced bar pressing. J Genet Psychol. 1966;108:213–9. doi: 10.1080/00221325.1966.10532779. [DOI] [PubMed] [Google Scholar]
- Stewart JH, HMB Studies in light reinforced behavior: III. The effects of continuous zero and fixed ratio reinforcement. The Quarterly Journal of Experimental Psychology. 1958;10:56–61. [Google Scholar]
- Suto N, Austin JD, Vezina P. Locomotor response to novelty predicts a rat's propensity to self-administer nicotine. Psychopharmacology (Berl) 2001;158:175–80. doi: 10.1007/s002130100867. [DOI] [PubMed] [Google Scholar]
- Tapp JT. Activity, Reactivity, and the Behavior-Directing Properties of Stimuli. In: Tapp JT, editor. Reinforcement and Behavior. Academic Press; New York: 1969. [Google Scholar]
- Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4- methylenedioxymethamphetamine ("Ecstasy") Biol Psychiatry. 2001;50:137–43. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- Winterbauer NE, Balleine BW. The influence of amphetamine on sensory and conditioned reinforcement: evidence for the re-selection hypothesis of dopamine function. Front Integr Neurosci. 2007;1:9. doi: 10.3389/neuro.07.009.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered "wanting" for sucrose reward. J Neurosci. 2001;21:7831–40. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Behavioral Expressions and Biosocial Bases of Sensation Seeking. Press Syndicate of the University of Cambridge; 1994. [Google Scholar]