Injury results in increased ROS and insulin resistance. Blocking the increase of ROS normalizes JNK activation, glycemic control, and prevents development of hepatic insulin resistance.
Abstract
Acute insulin resistance is common after injury, infection, and critical illness. To investigate the role of reactive oxygen species (ROS) in critical illness diabetes, we measured hepatic ROS, which rapidly increased in mouse liver. Overexpression of superoxide dismutase 2, which decreased mitochondrial ROS levels, protected mice from the development of acute hepatic insulin resistance. Insulin-induced intracellular signaling was dramatically decreased, and cellular stress signaling was rapidly increased after injury, resulting in the hyperglycemia of critical illness diabetes. Insulin-induced intracellular signaling, activation of stress (c-Jun N-terminal kinase) signaling, and glucose metabolism were all normalized by superoxide dismutase 2 overexpression or by pretreatment with antioxidants. Thus, ROS play an important role in the development of acute hepatic insulin resistance and activation of stress signaling after injury.
Increased levels of reactive oxygen species (ROS) are correlated with chronic insulin-resistant states, such as type 2 diabetes, obesity, hypertension, cardiovascular disease, and the metabolic syndrome (1–5). However, recent work indicates that ROS levels may be necessary for normal insulin sensitivity, possibly due to a ROS-mediated decrease in protein tyrosine phosphatase activity (6, 7). Little is known about the role of ROS in the acute development of hyperglycemia and insulin resistance in the critical illness diabetes that rapidly develops after surgery, injury, or critical illness.
The Leuven study reported that control of hyperglycemia, by intensive insulin therapy in critically ill patients, results in a 34–50% reduction in mortality and other morbidities (8). Since then, achieving euglycemia has become a major therapeutic target in the intensive care unit (ICU) after injury or critical illness (9–11). In addition, intensive insulin therapy can also decrease the incidence of wound infection and increase survival after cardiac surgery (12). Unfortunately, intensive insulin therapy is often associated with an increased frequency of hypoglycemic incidents, which can result in deleterious events in the ICU (13, 14). Thus, it is important to understand the mechanisms resulting in the development of the acute insulin resistance that occurs in critical illness diabetes and to develop additional or alternative therapeutic approaches to control the associated hyperglycemia.
The liver is a main source of glucose production, and insulin is a primary inhibitor of hepatic glucose output. Binding of insulin to its receptor [insulin receptor (IR)] leads to activation of its tyrosine kinase activity, which promotes association and phosphorylation of intracellular IR substrate (IRS) proteins and activation of the phosphatidylinositol 3-kinase/Akt pathway (15, 16). Decreased signaling through this pathway results in insulin resistance and increased hepatic glucose output, contributing to systemic hyperglycemia (17, 18).
A major cellular source of ROS, some of which are damaging free radicals, is from the mitochondria, generated by complex I and complex III of the electron transport chain (19, 20). ROS can also be produced from reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase or xanthine oxidase (21–23). Cellular ROS levels are the result of the balance between ROS production and elimination, and superoxide dismutases (SOD) are important antioxidant enzymes that decrease ROS levels. There are three distinct isoforms of SOD: SOD1 (Cu-Zn-SOD) located in the cytoplasm; SOD2 (Mn-SOD), the predominant antioxidant enzyme in mitochondria; and SOD3 (Ec-SOD) located in the extracellular space (24). A major role of SOD2 is to dismutate superoxide to generate H2O2.
The present study was designed to investigate the role of ROS in the acute development of hepatic insulin resistance after injury, in particular the effect of damaging free radicals, a subset of ROS. We found that in this mouse model of critical illness diabetes, there was a rapid development of hyperglycemia and hyperinsulinemia, and that hepatic insulin resistance occurred as early as 30 min, becoming more severe by 60–90 min after the initiation of hemorrhage, with a concomitant increase in ROS levels. In mice overexpressing SOD2, there was a decrease of the elevated ROS levels, improved hepatic insulin sensitivity, normalization of blood glucose and insulin levels, and less activation of cellular stress signaling pathways. Similarly, pretreatment with the antioxidants, N-acetyl-l-cysteine (NAC) or Manganese (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP), decreased the acute development of insulin resistance after injury. These data suggest that mitochondrial ROS play an important role in the rapid development of insulin resistance after injury and in the dysregulation of glucose metabolism.
Results
Characterization of hepatic insulin resistance after trauma and hemorrhage in mice
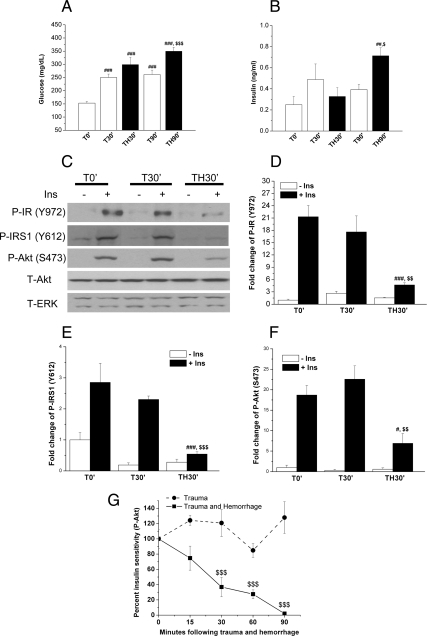
We previously reported that hyperglycemia, hyperinsulinemia, and hepatic insulin resistance are all rapidly induced in our rat model of critical illness diabetes (25–29). However, we do not know whether hepatic insulin resistance occurs in mice after injury/hemorrhage. Thus, we first examined blood glucose and insulin levels in wild-type (WT) mice (C57BL/6). Compared with trauma alone immediately after surgery (T0′; 153 mg/dl), blood glucose levels increased significantly 30 or 90 min after trauma alone (T30′, 250 mg/dl; T90′, 261 mg/dl; Fig. 1A). This suggests that the stress of anesthesia, laporatomy, and catheterization can cause hyperglycemia. Moreover, surgical trauma followed by the further stress of hemorrhage for 30 or 90 min (TH30′ or TH90′) resulted in even greater increases in blood glucose levels (to 298 or 349 mg/dl, respectively; Fig. 1A). Although there was a trend of increased insulin levels by trauma alone (T30′ and T90′), these never reached statistical significance. However, trauma and hemorrhage for 90 min dramatically increased insulin levels compared with the earliest time point of trauma alone (T0′) and the time-matched control, trauma alone for 90 min (T90′; Fig. 1B).
Fig. 1.
Characterization of hepatic insulin resistance after trauma and hemorrhage in mice. Mice were subjected to trauma alone (T) or trauma and hemorrhage (TH) as described in the Materials and Methods. A and B, At 0, 30, and 90 min, fasting glucose (A) and insulin (B) levels were measured from femoral arterial blood. Data are presented as mean ± sem of samples from nine to 10 mice in each group. In this and all the other figures, a single symbol (# or $) represents P < 0.05; a double symbol (**, ##, or $$) represents P < 0.01; a triple symbol (### or $$$) represents P < 0.001, and no symbol or ns represents not significant. #, Statistics vs. T0′; $, statistics vs. time-matched trauma alone. C, At 0 and 30 min, either saline (−Ins) or 1 U insulin (+Ins) was injected into the inferior vena cava of C57BL/6 mice. After 4 min, the liver was removed and frozen in liquid nitrogen. Liver tissue lysates were later prepared and subjected to Western blotting. D–F, Multiple autoradiographs were quantified by scanning densitometry. Data are presented as mean ± sem of samples from three to seven mice in each group. #, Statistics vs. T0′+Ins; $, statistics vs. T30′+Ins. G, Time-dependent decrease in insulin sensitivity in the liver after trauma and hemorrhage as measured by insulin's ability to induce Akt phosphorylation. Data are presented as mean ± sem of samples from three to seven mice in each group. $, Statistics vs. time-matched trauma-only group T30′, T60′, or T90′. # or $, P < 0.05; **, ##, or $$, P < 0.01; ### or $$$, P < 0.001; ns, not significant.
It was then asked in this mouse model of critical illness diabetes whether trauma and hemorrhage could inhibit hepatic insulin signaling. Insulin-induced signaling, such as phosphorylation of tyrosine 972 of IR [phospho- (P-) IR(Y972)], tyrosine 612 of IRS1 [P-IRS1(Y612)], and serine 473 of Akt [P-Akt(S473)], were all significantly reduced after the combination of trauma and hemorrhage for 30 min compared with trauma alone (T0′ or T30′; Fig. 1, C–F). The time course of the decrease in hepatic insulin sensitivity, the inability of insulin to respond to exogenous insulin, after trauma and hemorrhage was also studied, with only a modest decrease of insulin-induced signaling 15 min after trauma and hemorrhage, which was not quite statistically significant (Fig. 1G). There was a further decrease of insulin sensitivity as the blood pressure was maintained at 30–35 mm Hg, with little or no insulin-induced signaling by 90 min after hemorrhage (Fig. 1G). Total levels of Akt or ERK1/2 did not change at any of the time points (Fig. 1C). These data suggest that the decrease of insulin sensitivity after trauma and hemorrhage is not due to the change of total levels of insulin signaling molecules and that hepatic insulin resistance occurred as early as 30 min after hemorrhage and became most severe by 90 min.
Correlation between levels of ROS and insulin sensitivity after trauma and hemorrhage
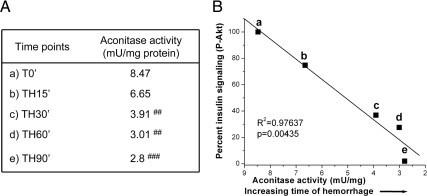
Damage due to a subset of ROS have been reported to cause insulin resistance in multiple diseases associated with chronic insulin resistance (2–4). Thus, we wondered whether a rapid increase in ROS levels was associated with the acute development of hepatic insulin resistance after trauma and hemorrhage. First, we determined the relative changes in hepatic ROS levels by an indirect measurement, aconitase enzyme activity. Superoxide and peroxynitrite (but not H2O2) (30, 31) directly decrease aconitase enzyme activity by causing release of ferrous iron from the holoenzyme (4Fe/4S). There is therefore an inverse correlation, with low aconitase enzyme activity indicating high levels of superoxide and/or peroxynitrite. Aconitase enzyme activity decreased as the time of hemorrhage increased compared with trauma-alone mice (T0′; Fig. 2A). A strong linear correlation was found between decreasing hepatic insulin sensitivity and decreasing aconitase enzyme activity (indicating increasing damaging ROS levels) after trauma and hemorrhage (Fig. 2B). Although not determinant of a cause and effect, the data clearly indicate a strong correlation between increasing damaging ROS levels, such as superoxide, and the rapid development of hepatic insulin resistance after trauma and hemorrhage.
Fig. 2.
Correlation between aconitase activity and insulin sensitivity after trauma and hemorrhage. A, As described in the legend to Fig. 1, mice were subjected to trauma alone (T) or trauma and hemorrhage (TH), and at the T0, TH15, TH30, TH60, and TH90 time points, the liver was removed and frozen in liquid nitrogen. Tissue lysates were prepared, and aconitase activity was assayed (n = 6). #, Statistics vs. T0′. ##, P < 0.01; ###, P < 0.001. B, The regression line comparing insulin-induced phosphorylation of Akt after trauma and hemorrhage and aconitase activity (and also on the x-axis is an indication of the increasing time of hemorrhage) .
Effects of SOD2 overexpression on ROS, glucose, and insulin levels after trauma and hemorrhage
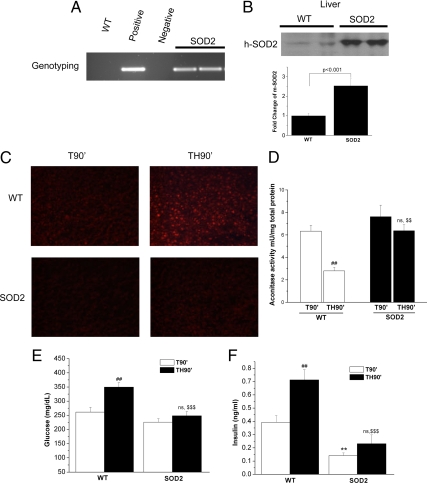
To determine whether damaging ROS play a role in trauma- and hemorrhage-induced hepatic insulin resistance, transgenic mice globally overexpressing human SOD2 were used (32). Because SOD2 is a major antioxidant enzyme in mitochondria, which are a primary source of cellular ROS production, any increase in damaging ROS after the combination of trauma and hemorrhage should be inhibited in SOD2-overexpressing mice. Genotyping and Western blot analysis confirmed that transgenic mice overexpress the human SOD2 gene (Fig. 3, A and B). The increase in total SOD2, human and mouse, equaled 2.5-fold, compared with endogenous mouse SOD2 in the WT mice (Fig. 3B). In addition, SOD2 activity increased approximately 2.7-fold (from 0.063 ± 0.009 in WT mice to 0.171 ± 0.005 U/mg protein in SOD2-overexpressing mice), whereas the sum of SOD1 and SOD3 activity (non-SOD2) did not change significantly (from 0.487 ± 0.095 in WT mice to 0.589 ± 0.011 U/mg protein in SOD2-overexpressing mice). This lack of a change in non-SOD2 activity was similar to what occurred in lung and other tissues in these SOD2-overexpressing mice (32).
Fig. 3.
The effect of overexpression of SOD2 on ROS, aconitase activity, and blood glucose and insulin levels after trauma and hemorrhage. A and B, Genotyping (A) and Western blot analysis (B, top panel) were performed as described to confirm the overexpression SOD2 in transgenic mice as compared with WT mice. Positive and negative controls are presented for the PCR in A. Also in B is a bar graph of the total level of SOD2, using a mouse antibody that cross-reacts with both endogenous mouse SOD2 and expressed human SOD2. As described in Fig. 1, either trauma alone (T90′) or trauma and hemorrhage (TH90′) was performed in C57BL/6 (WT) or mice overexpressing SOD2. C and D, ROS levels were assessed by direct DHE staining in situ (C) (n =3) and by indirect measurement of aconitase activity (D) (n =6). E and F, Fasting blood glucose levels (E) (n = 8–11) and insulin levels (F) (n =5–7) were also measured in these mice. #, Statistics vs. WT-T90′; *, statistics vs. WT-T90′; $, vs. WT-TH90′; ns, not significant vs. SOD2–T90′. # or $, P < 0.05; **, ## or $$, P < 0.01; ### or $$$, P < 0.001.
Next, it was determined whether overexpressing SOD2 could decrease ROS after trauma and hemorrhage using a dye, dihydroethidium (DHE), which fluoresces strongly in the presence of superoxide in situ. After trauma and hemorrhage in WT mice, there is an increase in DHE staining in liver (Fig. 3C). Hepatic overexpression of SOD2 decreased liver ROS levels after trauma and hemorrhage as measured by decreased DHE staining (Fig. 3C; lower right panel). In addition, overexpression of SOD2 also blocked the decrease of aconitase enzyme activity after trauma and hemorrhage. As described previously, this higher aconitase activity correlates with lower levels of superoxide and/or peroxynitrite in the SOD2-overexpressing mouse after trauma and hemorrhage (Fig. 3D). These results indicate that increased hepatic ROS levels after trauma and hemorrhage are decreased in SOD2-overexpressing mice.
Overexpression of SOD2 had only a small effect on blood glucose levels after trauma alone (T90′) compared with WT animals (Fig. 3E, white bars). However, the increased expression of SOD2 significantly inhibited the development of hyperglycemia after trauma and hemorrhage, with blood glucose decreased from 349 mg/dl (WT-TH90′) to 248 mg/dl (SOD2-TH90′; Fig. 3E). Overexpression of SOD2 significantly decreased blood insulin levels after trauma alone (T90′) compared with WT animals (Fig. 3F). This suggests an increase in insulin sensitivity in the trauma-only animals with overexpression of SOD2. Furthermore, there was no significant rise in insulin levels after trauma and hemorrhage in SOD2-overexpressing mice, in contrast to the hyperinsulinemia that occurred after trauma and hemorrhage in WT animals (Fig. 3F).
Effects of SOD2 overexpression on whole-body glucose tolerance and insulin sensitivity after trauma and hemorrhage
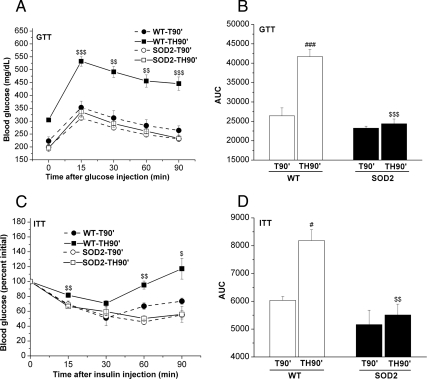
To further confirm that overexpression of SOD2 altered systemic glucose metabolism, we performed glucose and insulin tolerance tests on C57BL/6 (WT) and SOD2-overexpressing mice. After surgery in trauma-alone (T90′) or trauma and hemorrhage (TH90′) mice, glucose levels were measured. Before the injection of glucose, as expected, basal levels of glucose were significantly elevated in the WT mice after trauma and hemorrhage for 90 min, but this was not evident in the SOD2-overexpressing mice (see 0 min, Fig. 4A). After injection of glucose into the femoral vein, blood glucose levels were increased to 450–550 mg/dl 15, 30, 60, and 90 min after administration of glucose in the WT trauma and hemorrhage animals. However, these highly elevated blood glucose levels were not evident in mice overexpressing SOD2 after trauma and hemorrhage, resulting in significantly improved glucose tolerance (Fig. 4A). The area under the curve (AUC) was then calculated and the AUC0–90min of SOD2-overexpressing mice was significantly lower than in C57BL/6 (WT) mice after trauma and hemorrhage (Fig. 4B).
Fig. 4.
Overexpression of SOD2 increased glucose tolerance and insulin sensitivity after trauma and hemorrhage. WT mice or mice overexpressing SOD2 were subjected to trauma alone (T90′) or trauma and hemorrhage (TH90′) as previously described. However, in these mice, glucose tolerance tests (GTT) and insulin tolerance tests (ITT) were performed. A and B, In the glucose tolerance tests, after a 6-h fast, blood glucose was measured (the 0-min time point) after T90′ or TH90′, and then glucose (0.6 g/kg) was injected into the femoral vein, blood glucose levels were measured every 15 min (A), and the AUC were calculated as described (B). C and D, In the insulin tolerance tests, there was no fasting period, blood glucose was measured (the 0 min time point) after T90′ or TH90′, and then insulin (0.5 U/kg) was injected into the femoral vein, and blood glucose levels were measured (C) and analyzed (D). Data are presented as mean ± sem of three samples in each group. $, Statistics between WT-TH90′ and SOD2-TH90′; #, vs. WT-T90′. # or $, P < 0.05; ## or $$, P < 0.01; ### or $$$, P < 0.001.
In parallel with the effects of SOD2 overexpression in the glucose tolerance tests, insulin sensitivity was measured by insulin tolerance tests. Due to the lack of fasting necessary for the insulin tolerance test, there was greater variability of basal blood glucose levels between animals. However, after injection of insulin, blood glucose levels were decreased by 15 min in all groups. In WT, trauma and hemorrhage mice, there was somewhat less of a decrease in blood glucose levels soon after insulin injection, and the effects of the insulin injection were short-lived with significantly increased blood glucose levels at 60 and 90 min after insulin injection compared with the two trauma-only groups (Fig. 4C). However, blood glucose levels were greatly decreased in trauma and hemorrhage animals overexpressing SOD2 after insulin injection and were identical to animals that were not hemorrhaged. This indicates that overexpressing SOD2 improved whole-body insulin sensitivity to a level equal to animals that were not hemorrhaged (Fig. 4C). The AUC0–90min, which was highly elevated by trauma and hemorrhage in C57BL/6 (WT) mice, was normalized in the SOD2-overexpressing mice (Fig. 4D).
SOD2 overexpression reduced trauma- and hemorrhage-induced hepatic insulin resistance
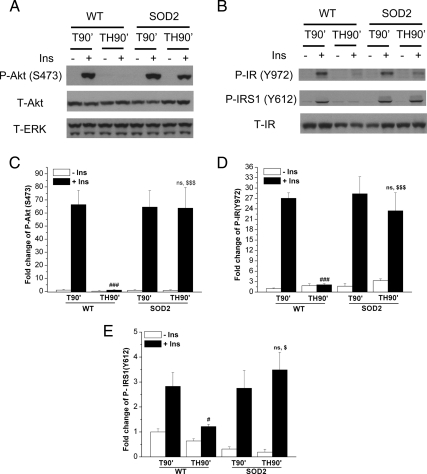
To determine whether the decreased ROS levels after trauma and hemorrhage in SOD2-overexpressing mice (Fig. 3) can prevent the acute development of hepatic insulin resistance, insulin-induced signaling was measured. In trauma-alone animals (T90′), there was no difference in insulin-induced P-Akt(S473) comparing WT and SOD2 mice. However, the large decrease of insulin-induced P-Akt(S473) after trauma and hemorrhage (TH90′), did not occur in SOD2-overexpressing mice (Fig. 5, A and C). We also examined other insulin signaling molecules and found that the insulin-induced tyrosine phosphorylation of the IR, P-IR(Y972), and IRS-1, P-IRS1(Y612), was almost completely normalized in SOD2-overexpressing mice after trauma and hemorrhage (Fig. 5, B, D and E). Also, there were no differences in the total levels of the Akt and IR proteins between WT and SOD2 mice (Fig. 5, A and B). Thus, the loss of insulin's ability to activate insulin signaling in the liver after trauma and hemorrhage was prevented by overexpression of SOD2.
Fig. 5.
Overexpression of SOD2 inhibited trauma- and hemorrhage-induced hepatic insulin resistance. After T90′ or TH90′, either saline (−Ins) or 1 U insulin (+Ins) was injected into the inferior vena cava, and after 4 min, the liver was removed and frozen in liquid nitrogen. A and B, Liver tissue lysates from WT or SOD2-overexpressing mice were subjected to Western blotting with P-Akt(S473), total (T)-Akt, T-ERK, P-IR (Y972), P-IRS1 (Y612), and T-IR antibodies. C–E, Autoradiographs were quantified by scanning densitometry. Data are presented as mean ± sem of samples from three to seven mice in each group. #, Statistics vs. WT-T90′+Ins; $, vs. WT-TH90′+Ins; ns, not significant vs. SOD2–T90′+Ins. # or $, P < 0.05; ### or $$$, P < 0.001.
MnTBAP and NAC prevented trauma- and hemorrhage-induced hepatic insulin resistance
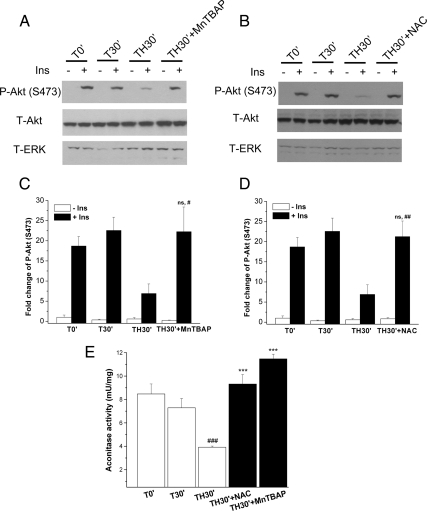
MnTBAP is a SOD-mimetic antioxidant, and NAC is a more general antioxidant. These two agents have been used to decrease ROS levels and improve insulin sensitivity in chronic insulin-resistant states (2, 3, 33). To further explore whether decreased ROS levels could prevent the development of hepatic insulin signaling after trauma and hemorrhage, the two antioxidants were separately administered to mice at doses similar to those previously used, specifically NAC (300 mg/kg) and MnTBAP (10 mg/kg) (34, 35) before hemorrhage. The liver was then harvested at 30 min (TH30′), the first time point after trauma and hemorrhage of a significant decrease of insulin-induced signaling (see Fig. 1G). Pretreatment with MnTBAP and NAC after trauma and hemorrhage could significantly increase aconitase activity compared with no treatment (TH30′), indicating damaging ROS levels were decreased by MnTBAP and NAC (Fig. 6E). There was a significant reversal of the decrease in insulin-induced P-Akt(S473) in liver after trauma and hemorrhage upon pretreatment with either MnTBAP (Fig. 6, A and C) or NAC (Fig. 6, B and D), resulting in almost complete recovery of insulin signaling compared with no treatment. These data further confirm that the acute development of hepatic insulin resistance after trauma and hemorrhage can be almost completely abolished by blocking the increase of damaging ROS levels.
Fig. 6.
NAC and MnTBAP prevented trauma- and hemorrhage-induced hepatic insulin resistance. WT mice were pretreated with NAC (300 mg/kg, ip) or MnTBAP (10 mg/kg, ip) before surgery. After 30 min of hemorrhage, either saline (−Ins) or 1 U insulin (+Ins) was injected into the inferior vena cava. A and B, Liver tissue lysates were subjected to Western blotting with P-Akt(S473), total (T)-Akt, and T-ERK antibodies. C and D, Autoradiographs were quantified by scanning densitometry. #, Statistics vs. TH30′+Ins; ns, not significant vs. T30′+Ins. E, Liver lysates were prepared, and aconitase activity was assayed; pretreatment with MnTBAP and NAC protected from the decrease in aconitase activity after trauma and hemorrhage. #, Statistics vs. T0′; *, statistics vs. TH30′. Data are presented as mean ± sem of samples from three to seven mice in each group. #, P < 0.05; ##, P < 0.01; ### or ***, P < 0.001.
The hemorrhage-induced increase of c-Jun N-terminal kinase (JNK) activation/phosphorylation was blocked by reducing hepatic ROS levels
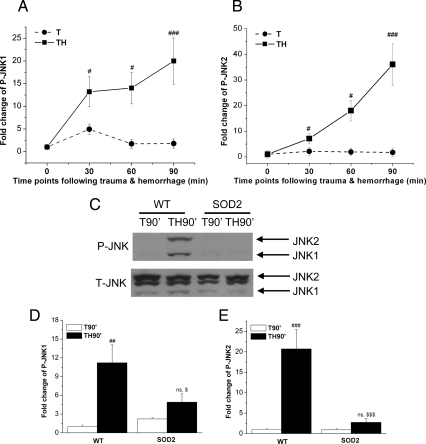
Activation of the JNK signaling pathway is one of many potential pathways involved in the development of insulin resistance in chronic diseases, such as type 2 diabetes, obesity, and the metabolic syndrome (36–38). It is also known that the JNK signaling pathway can be activated by ROS (3, 39). To investigate whether JNK was activated by trauma and hemorrhage, the time course of JNK1/2 activation/phosphorylation was determined. After trauma and hemorrhage, JNK1/2 phosphorylation was significantly increased by 30 min (TH30′), reaching a maximum by 90 min (TH90′) compared with trauma-only mice (Fig. 7, A and B, and Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). This indicates that the JNK signaling pathway is activated by trauma and hemorrhage, and the pattern of activation is similar to the development of the defect of hepatic insulin signaling and the accumulation of the ROS. To determine the involvement of increased damaging ROS levels, JNK phosphorylation was measured after trauma and hemorrhage in SOD2-overexpressing mice. By comparison with WT mice, phosphorylation of JNK1/2 was dramatically diminished in mice overexpressing SOD2 90 min after the combination of trauma and hemorrhage (TH90′; Fig. 7, C–E). Taken together, these data suggest that increased levels of damaging ROS play a role in activating JNK and in the development of acute insulin resistance in liver after trauma and hemorrhage in our mouse model of critical illness diabetes.
Fig. 7.
Trauma- and hemorrhage-induced activation of the JNK signaling pathway in liver was inhibited by a decrease of ROS. A and B, As described in the legend to Fig. 1, WT mice were subjected to trauma alone (T) or trauma and hemorrhage (TH), and at the 0-, 30-, 60-, and 90-min time points, the liver was removed and frozen in liquid nitrogen. Liver lysates were subjected to Western blotting with P-JNK1/2 antibodies, and autoradiographs were quantified by scanning densitometry. Data are presented as mean ± sem of samples from four to eight mice in each group. C, As described in the legend to Fig. 3, WT and SOD2-overexpressing mice underwent trauma alone (T90′) or trauma and hemorrhage (TH90′), and liver lysates were subjected to Western blotting with anti-P-JNK1/2 and T-JNK antibodies. D and E, Autoradiographs were quantified by scanning densitometry. Data are presented as mean ± sem of samples from four to six mice in each group. #, Statistics vs. time-matched trauma-only group T30′, T60′, or T90′; $, vs. WT-TH90′; ns, not significant vs. SOD2–T90′. # or $, P < 0.05; **, ##, or $$, P < 0.01; ### or $$$, P < 0.001.
Discussion
Critical illness diabetes is common after injury, infection, and critical illness (40–42) and is characterized by hyperglycemia, hyperinsulinemia, and acute insulin resistance. Because the liver is the major site of insulin-regulated glucose production, defects of hepatic insulin signaling can contribute to the development of hyperglycemia. Controlling this hyperglycemia has recently become a therapeutic target in surgical ICU, with data supporting the use of intensive insulin therapy (8, 42). However, a negative outcome of intensive insulin therapy is the increased frequency of hypoglycemic incidents (11, 14). These patient-based data have been performed in the absence of a basic understanding of the mechanisms of development of critical illness diabetes. Thus, a greater understanding of the mechanisms leading to critical illness diabetes and the associated insulin resistance are necessary to develop additional/alternative therapeutic approaches to control injury/critical illness-induced hyperglycemia.
A mouse model of critical illness diabetes, using surgical trauma and hemorrhage, was used for the first time to determine changes of blood glucose and insulin levels after injury and to examine whether this resulted in defects of insulin signaling in the liver. Trauma alone caused a rise in blood glucose levels, but the combination of trauma and hemorrhage induced even higher blood glucose levels. Serum insulin levels were not significantly increased by trauma alone or at early times after the combination of trauma and hemorrhage, but became significant at later times, coincident with worsening hyperglycemia and an increasingly severe defect in hepatic insulin signaling. The defects of hepatic insulin signaling, the hyperglycemia and the hyperinsulinemia, all indicate an acute development of insulin resistance in mice. Supporting this was the decreased capacity of the mice to handle glucose and respond to insulin, as measured in the glucose and insulin tolerance tests, respectively.
One potential contributing factor for the development of acute insulin resistance after the combination of trauma and hemorrhage is increased ROS. Numerous studies have established a role of increased damaging ROS in the chronic insulin-resistant state observed in type 2 diabetes, obesity, and the metabolic syndrome (1, 2, 4, 43). However, the development of a chronic insulin-resistant state can take months, years, or decades to develop, whereas the acute insulin resistance of critical illness diabetes can occur much more rapidly, within hours of injury or critical illness as measured in ICU patients or within minutes as measured in our animal injury studies (25–29). The data in the present study demonstrated that damaging ROS levels increased significantly, as early as 15–30 min after trauma and hemorrhage, and there was a strong correlation between ROS levels, as measured by decreased aconitase activity and DHE staining, and hepatic insulin resistance.
We then investigated whether a blockade of ROS accumulation after injury could prevent the rapid development of a defect in hepatic insulin signaling. Overexpressing SOD2 greatly improves the ROS-scavenging capacity of the mitochondria, decreasing ROS levels. Another approach is treatment with MnTBAP, which can mimic SOD2 overexpression, whereas NAC is a less specific ROS scavenging agent. Overexpression of SOD2 and treatment with the antioxidants all resulted in the same effects, little development of hepatic insulin resistance and, when measured, a sensitization to insulin and a reduction in the development of hyperglycemia. Thus, our data suggest that damaging ROS plays an important role in the acute development of hepatic insulin resistance after injury. Our previous findings indicate that elevated TNF-α results in hepatic insulin resistance after trauma and hemorrhage and resuscitation at a time point at least 2 h later than the data presented here (27). Combining the data from our past and present studies indicates that defects of hepatic insulin signaling can be blocked by decreasing ROS at early time points during the hemorrhage period and reversed by reduction of TNF-α at later time points after resuscitation.
Hyperglycemia may be a risk factor for numerous complicating conditions after injury or critical illness (42, 44, 45). Consistent with our findings of decreased defects of insulin signaling, there was a normalization of blood glucose levels and a reduction of insulin levels in SOD2-overexpressing mice. In addition, overexpression of SOD2 improved whole-body insulin sensitivity and glucose tolerance after trauma and hemorrhage. Thus, there are demonstrable effects on liver (this study), but it is not yet known whether treatments to reduce damaging ROS can alter insulin action in other insulin target tissues.
The JNK pathway plays a role in multiple diseases/syndromes of chronic insulin resistance (36, 37, 46–48), and increased levels of ROS may, in part, act by activating the JNK pathway (3, 39). The presented data demonstrate that trauma and hemorrhage resulted in a rapid activation of the JNK signaling pathway in liver, which coincided with the decrease of insulin-induced signaling. In addition, decreasing ROS, either by overexpressing SOD2 or treatment with MnTBAP or NAC, prevented the injury-induced increase in JNK1/2 phosphorylation/activation. In chronic insulin-resistant states, such as obesity-associated insulin resistance, JNK1 may play a more significant role than JNK2 (36, 46). However, in the present study, both JNK1 and JNK2 were activated after injury, and both were normalized when ROS levels were decreased. Taken together, these finding suggest that ROS may activate the JNK signaling pathway, resulting in the development of acute insulin resistance in liver and altered glucose homeostasis after injury.
In summary, our data indicate that ROS rapidly increase after trauma and hemorrhage, and damaging ROS plays a dominant role in the ensuing development of acute hepatic insulin resistance and dysregulation of glucose metabolism. Blocking the increase of ROS can prevent the acute development of hepatic insulin resistance and reduce the hyperglycemia and hyperinsulinemia after injury. Due to the potential of an increased incidence of hypoglycemic events with intensive insulin therapy in the ICU, the present study suggests that antioxidant therapy may be a useful therapeutic approach to control hyperglycemia after injury, infection, or critical illness.
Materials and Methods
Animal model of trauma hemorrhage
WT C57BL/6 and SOD2-overexpressing transgenic mice (originally developed by Dr. Ye-Shih Ho) (32), 12–13 wk old, were fasted 6 h before experiments but were allowed water ad libitum. Mice were anesthetized with isoflurane (Mallinckrodt Veterinary, Mundelein, IL) inhalation. After mice were clipped and shaved, they were restrained in a supine position and were kept anesthetized by continuous inhalation of 1.5% isoflurane and 98.5% air throughout the surgical procedure. A 2-cm ventral midline laparotomy was performed representing soft tissue trauma. The abdomen was then closed in layers using 6-0 Ethilon sutures (Ethicon, Somerville, NJ), and the wounds were bathed with 1% lidocaine (Elkins-Sinn, Cherry Hill, NJ) throughout the surgical procedure to reduce postoperative pain. Polyethylene-10 catheters (Clay-Adams, Parsippany, NJ) were placed in the right and left femoral arteries for bleeding and monitoring of mean arterial pressure (MAP; Micro-Med, Louisville, KY), respectively. Mice were bled to a MAP of 30–35 mm Hg within 10 min, and once MAP reached 35 mm Hg, the timing of the hemorrhage period began. All procedures were carried out in accordance with the guidelines set forth in the Animal Welfare Act and the Guild for the Care and Use of Laboratory Animals by the National Institutes of Health.
Study design
Due to the considerable trauma incurred during anesthesia, catheterizations, and opening of the abdominal cavity to perform the insulin injections (see below), it was impossible to have a completely trauma-free control group. Thus, the baseline animals chosen in these experiments were the trauma-alone mice (T0′) that were subjected to anesthesia, laparotomy, and catheterization and then killed immediately after saline or insulin injection. Other trauma-only groups were subjected to the same procedures but killed at 15 min (T15′), 30 min (T30′), 60 min (T60′), or 90 min (T90′) after catheterization. The trauma and hemorrhage groups were subjected to the same procedures as the trauma groups, followed by hemorrhage and killed at 15 min (TH15′), 30 min (TH30′), 60 min (TH60′), or 90 min (TH90′).
Drug administration
NAC was purchased from Sigma Chemical Co. (St. Louis, MO) and was dissolved in saline. For NAC pretreatment, mice received one injection of NAC (300 mg/kg, ip) 30 min before surgery. In separate groups of mice, pretreatment was with MnTBAP (Calbiochem, San Diego, CA; dissolved in saline) via a single injection (10 mg/kg, ip) 60 min before surgery.
Tissue harvesting procedures
At the 0-, 15-, 30-, 60-, or 90-min time points, the abdominal cavity of the animal was reopened, the inferior vena cava was exposed, and insulin (1 U; 100 μl) or saline was injected into the inferior vena cava. Four minutes after the injection, the liver was removed, snap frozen, and stored in liquid nitrogen.
Genotyping
The genotype of transgenic mice was determined by PCR analysis of genomic DNA isolated from mouse ear by the QiaAmp DNA 250 MiniKit (QIAGEN, Valencia, CA). The SOD2 transgene PCR primers were 5′-CTCCGGCTTTGGGGTATCT-3′ (forward) and 5′-GTGTCCCCGTTCCTTATTG-3′ (reverse). Genomic DNA was amplified for 35 cycles at 95 C for 15 sec and at 60 C for 60 sec. PCR products were visualized on a 2% agarose gel stained with ethidium bromide.
Measurement of fasting blood insulin and glucose levels
Blood glucose levels were measured by glucose meter (Freestyle Freedom, Alameda, CA) from femoral artery sampling, and serum insulin levels were determined using a rat insulin RIA kit (Millipore Corp., St. Charles, MO). For this, 0.1 ml blood was collected from the femoral artery, centrifuged at 5000 × g for 10 min and stored at −80 C until analysis.
Glucose and insulin tolerance test
For the glucose tolerance tests, mice were fasted for 6 h before the surgery (trauma alone; T90′) or trauma and hemorrhage (TH90′). Basal levels (0′) were measured from femoral arterial blood followed by injection of glucose (0.6 g/kg) into the femoral vein, and blood glucose levels were measured at 15, 30, 60, and 90 min. The insulin tolerance tests were performed without fasting. After basal blood glucose levels were measured (0′), insulin (0.5 U/kg) was injected into the femoral vein, and blood glucose levels were measured at 15-min intervals.
Aconitase activity
ROS levels were assessed by indirect measurement of aconitase activity as previously described (49). Aconitase is specifically inactivated by superoxide and peroxynitrite, and lower aconitase activity correlates with increased ROS levels. Aconitase activity was measured in a NADP-isocitrate dehydrogenase-coupled enzyme system with citrate and NADP as substrates. The rate of reduced NADP production, followed spectrophotometrically at 340 nm, correlates with the rate of citrate utilization by aconitase, and aconitase activity was expressed as milliunits activity per milligram protein.
In situ measurement of superoxide
DHE (Sigma), a fluorescent dye, was used to evaluate production of superoxide in situ (4). Unfixed frozen livers were sectioned (5 μm), and DHE (20 μmol) was applied to the surface of each tissue section. The slides were incubated at 37 C for 20 min and washed with PBS, and images were captured with a fluorescent microscope (IX70; Olympus, Tokyo, Japan) with a 585-nm long pass filter.
Western immunoblot analysis
Liver tissue from each animal (0.2 g) was homogenized in extraction buffer, as described previously, and stored at −80 C until use (25–27, 50). The protein concentration of tissue lysates was determined by the bicinchoninic acid method (Pierce Biotechnology, Rockford, IL), and 30 μg of protein per lane was resolved by 10% SDS-PAGE and transferred to nitrocellulose paper. The Western transfers were immunoblotted with the following primary antibodies: anti-P-Y972-IR, anti-P-Y612-IRS-1, and anti-P-JNK1/2 (Invitrogen Biosource International, Carlsbad, CA), anti-total IRβ and anti-total JNK (Santa Cruz Biotechnology, Santa Cruz, CA), anti-total human SOD2 and anti-total mouse SOD2 (Fitzgerald, Concord, MA), and anti-P-S473-Akt, anti-total Akt, and anti-total ERK (Cell Signaling Technology, Danvers, MA). Horseradish peroxidase-conjugated secondary antibody was then added for detection of bound antibody by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) or Supersignal Femto Maximum Sensitivity Substrate reagent (Pierce). The blots were stripped using Reblot reagent (Chemicon International, Inc., Temecula, CA) for 15 min and then reprobed with a different antibody.
Densitometric and statistical analysis
Enhanced chemiluminescence images of immunoblots were scanned and quantified using Zero D-Scan (Scanalytics Corp., Fairfax, VA). Data are presented as mean ± sem. Data were analyzed by ANOVA or Student's t test using the InStat statistical program by GraphPad Software, Inc. (San Diego, CA).
Acknowledgments
We thank the University of Alabama Clinical Nutrition Research Center's (P30 DK56336) and the Diabetes Research and Training Center's (P60 DK079626) Bioanalytical Redox Biology Core. We also thank Dr. Gin Chuang, Melissa Pompilius, and David Westbrook for their assistance and insightful discussions.
This research is supported by grants from the National Institutes of Health (DK 62071), the Department of Defense (W81XWH-0510387), and the Veterans Administration Merit Review to J.L.M. and HL 77419 to S.W.B.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- Area under the curve
- DHE
- dihydroethidium
- ICU
- intensive care unit
- IR
- insulin receptor
- IRS
- IR substrate
- JNK
- c-Jun N-terminal kinase
- MAP
- mean arterial pressure
- NADPH
- nicotinamide adenine dinucleotide phosphate
- P-
- phospho-
- ROS
- reactive oxygen species
- SOD
- superoxide dismutase
- WT
- wild type.
References
- 1. Abdul-Ghani MA, Jani R, Chavez A, Molina-Carrion M, Tripathy D, Defronzo RA. 2009. Mitochondrial reactive oxygen species generation in obese non-diabetic and type 2 diabetic participants. Diabetologia 52:574–582 [DOI] [PubMed] [Google Scholar]
- 2. Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J. 2008. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118:789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Houstis N, Rosen ED, Lander ES. 2006. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440:944–948 [DOI] [PubMed] [Google Scholar]
- 4. Kumashiro N, Tamura Y, Uchida T, Ogihara T, Fujitani Y, Hirose T, Mochizuki H, Kawamori R, Watada H. 2008. Impact of oxidative stress and peroxisome proliferator-activated receptor γ coactivator-1α in hepatic insulin resistance. Diabetes 57:2083–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abilés J, de la Cruz AP, Castaño J, Rodríguez-Elvira M, Aguayo E, Moreno-Torres R, Llopis J, Aranda P, Argüelles S, Ayala A, de la Quintana AM, Planells EM. 2006. Oxidative stress is increased in critically ill patients according to antioxidant vitamins intake, independent of severity: a cohort study. Crit Care 10:R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, Stepto N, Wu B, Mitchell CA, Tonks NK, Watt MJ, Febbraio MA, Crack PJ, Andrikopoulos S, Tiganis T. 2009. Reactive oxygen species enhance insulin sensitivity. Cell Metab 10:260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Veal EA, Day AM, Morgan BA. 2007. Hydrogen peroxide sensing and signaling. Mol Cell 26:1–14 [DOI] [PubMed] [Google Scholar]
- 8. Hansen TK, Thiel S, Wouters PJ, Christiansen JS, Van den Berghe G. 2003. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab 88:1082–1088 [DOI] [PubMed] [Google Scholar]
- 9. Vogelzang M, Nijboer JM, van der Horst IC, Zijlstra F, ten Duis HJ, Nijsten MW. 2006. Hyperglycemia has a stronger relation with outcome in trauma patients than in other critically ill patients. J Trauma 60:873–877; discussion 878–879 [DOI] [PubMed] [Google Scholar]
- 10. Latorre JG, Chou SH, Nogueira RG, Singhal AB, Carter BS, Ogilvy CS, Rordorf GA. 2009. Effective glycemic control with aggressive hyperglycemia management is associated with improved outcome in aneurysmal subarachnoid hemorrhage. Stroke 40:1644–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia BR, Tasker RC, Ramos Garcia PC, Piva JP, Dias XL. 2007. Glycemic control and insulin therapy in sepsis and critical illness. J Pediatr (Rio J) 83:S128–S136 [DOI] [PubMed] [Google Scholar]
- 12. Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. 2004. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation 109:1497–1502 [DOI] [PubMed] [Google Scholar]
- 13. Fahy BG, Sheehy AM, Coursin DB. 2009. Glucose control in the intensive care unit. Crit Care Med 37:1769–1776 [DOI] [PubMed] [Google Scholar]
- 14. Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. 2009. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 15. Nakae J, Accili D. 1999. The mechanism of insulin action. J Pediatr Endocrinol Metab 12(Suppl 3):721–731 [PubMed] [Google Scholar]
- 16. White MF. 1997. The insulin signalling system and the IRS proteins. Diabetologia 40:S2–S17 [DOI] [PubMed] [Google Scholar]
- 17. Biddinger SB, Kahn CR. 2006. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol 68:123–158 [DOI] [PubMed] [Google Scholar]
- 18. Pessin JE, Saltiel AR. 2000. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest 106:165–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guzy RD, Mack MM, Schumacker PT. 2007. Mitochondrial complex III is required for hypoxia-induced ROS production and gene transcription in yeast. Antioxid Redox Signal 9:1317–1328 [DOI] [PubMed] [Google Scholar]
- 20. Nohl H, Gille L, Staniek K. 2005. Intracellular generation of reactive oxygen species by mitochondria. Biochem Pharmacol 69:719–723 [DOI] [PubMed] [Google Scholar]
- 21. Ikai I, Ozaki N, Shimahara Y, Wakashiro S, Tokunaga Y, Tanaka A, Ozawa K. 1989. Significance of hepatic mitochondrial redox potential on the concentrations of plasma amino acids following hemorrhagic shock in rats. Circ Shock 27:63–72 [PubMed] [Google Scholar]
- 22. Lehnert M, Arteel GE, Smutney OM, Conzelmann LO, Zhong Z, Thurman RG, Lemasters JJ. 2003. Dependence of liver injury after hemorrhage/resuscitation in mice on NADPH oxidase-derived superoxide. Shock 19:345–351 [DOI] [PubMed] [Google Scholar]
- 23. Akgür FM, Brown MF, Zibari GB, McDonald JC, Epstein CJ, Ross CR, Granger DN. 2000. Role of superoxide in hemorrhagic shock-induced P-selectin expression. Am J Physiol Heart Circ Physiol 279:H791–H797 [DOI] [PubMed] [Google Scholar]
- 24. Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. 2003. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol 140:445–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma Y, Wang P, Kuebler JF, Chaudry IH, Messina JL. 2003. Hemorrhage induces the rapid development of hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol 284:G107–G115 [DOI] [PubMed] [Google Scholar]
- 26. Ma Y, Toth B, Keeton AB, Holland LT, Chaudry IH, Messina JL. 2004. Mechanisms of hemorrhage-induced hepatic insulin resistance: role of tumor necrosis factor-α. Endocrinology 145:5168–5176 [DOI] [PubMed] [Google Scholar]
- 27. Xu J, Kim HT, Ma Y, Zhao L, Zhai L, Kokorina N, Wang P, Messina JL. 2008. Trauma and hemorrhage-induced acute hepatic insulin resistance: dominant role of tumor necrosis factor-α. Endocrinology 149:2369–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li L, Thompson LH, Zhao L, Messina JL. 2009. Tissue-specific difference in the molecular mechanisms for the development of acute insulin resistance after injury. Endocrinology 150:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thompson LH, Kim HT, Ma Y, Kokorina N, Messina JL. 2008. Acute muscle-type specific insulin resistance following injury. Mol Med 11–12:715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hausladen A, Fridovich I. 1996. Measuring nitric oxide and superoxide: rate constants for aconitase reactivity. Methods Enzymol 269:37–41 [DOI] [PubMed] [Google Scholar]
- 31. Hausladen A, Fridovich I. 1994. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J Biol Chem 269:29405–29408 [PubMed] [Google Scholar]
- 32. Ho YS, Vincent R, Dey MS, Slot JW, Crapo JD. 1998. Transgenic models for the study of lung antioxidant defense: enhanced manganese-containing superoxide dismutase activity gives partial protection to B6C3 hybrid mice exposed to hyperoxia. Am J Respir Cell Mol Biol 18:538–547 [DOI] [PubMed] [Google Scholar]
- 33. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. 2004. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114:1752–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mollen KP, McCloskey CA, Tanaka H, Prince JM, Levy RM, Zuckerbraun BS, Billiar TR. 2007. Hypoxia activates c-Jun N-terminal kinase via Rac1-dependent reactive oxygen species production in hepatocytes. Shock 28:270–277 [DOI] [PubMed] [Google Scholar]
- 35. Gauuan PJ, Trova MP, Gregor-Boros L, Bocckino SB, Crapo JD, Day BJ. 2002. Superoxide dismutase mimetics: synthesis and structure-activity relationship study of MnTBAP analogues. Bioorg Med Chem 10:3013–3021 [DOI] [PubMed] [Google Scholar]
- 36. Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. 2002. A central role for JNK in obesity and insulin resistance. Nature 420:333–336 [DOI] [PubMed] [Google Scholar]
- 37. Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. 2008. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322:1539–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Delibegovic M, Zimmer D, Kauffman C, Rak K, Hong EG, Cho YR, Kim JK, Kahn BB, Neel BG, Bence KK. 2009. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes 58:590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. 2005. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120:649–661 [DOI] [PubMed] [Google Scholar]
- 40. Carter EA. 1998. Insulin resistance in burns and trauma. Nutr Rev 56:S170–S176 [DOI] [PubMed] [Google Scholar]
- 41. Lange MP, Dahn MS, Jacobs LA. 1985. The significance of hyperglycemia after injury. Heart Lung 14:470–472 [PubMed] [Google Scholar]
- 42. van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. 2001. Intensive insulin therapy in the critically ill patients. N Engl J Med 345:1359–1367 [DOI] [PubMed] [Google Scholar]
- 43. Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. 2000. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787–790 [DOI] [PubMed] [Google Scholar]
- 44. Aberegg SK. 2006. Intensive insulin therapy in the medical ICU. N Engl J Med 354:2069–2071 [PubMed] [Google Scholar]
- 45. Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. 2006. Intensive insulin therapy in the medical ICU. N Engl J Med 354:449–461 [DOI] [PubMed] [Google Scholar]
- 46. Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. 2006. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci USA 103:10741–10746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nakatani Y, Kaneto H, Kawamori D, Hatazaki M, Miyatsuka T, Matsuoka TA, Kajimoto Y, Matsuhisa M, Yamasaki Y, Hori M. 2004. Modulation of the JNK pathway in liver affects insulin resistance status. J Biol Chem 279:45803–45809 [DOI] [PubMed] [Google Scholar]
- 48. Napoli R, Gibson L, Hirshman MF, Boppart MD, Dufresne SD, Horton ES, Goodyear LJ. 1998. Epinephrine and insulin stimulate different mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Diabetes 47:1549–1554 [DOI] [PubMed] [Google Scholar]
- 49. Gardner PR, Raineri I, Epstein LB, White CW. 1995. Superoxide radical and iron modulate aconitase activity in mammalian cells. J Biol Chem 270:13399–13405 [DOI] [PubMed] [Google Scholar]
- 50. Zhai L, Messina JL. 2009. Age and tissue specific differences in the development of acute insulin resistance following injury. J Endocrinol 203:365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]