Chromatin structure and histone modifications control neuron-specific GnRH expression and its modulation in response to Protein Kinase C activation.
Abstract
GnRH, a neuropeptide produced by rare, specialized hypothalamic secretory neurons, is critical for reproduction. During development, GnRH gene expression increases as neurons migrate from the olfactory placode to the hypothalamus, with highest levels in the mature, postmitotic state. While neuronal differentiation is known to be controlled by chromatin modulations, the role of chromatin dynamics in GnRH gene regulation has not been studied. Here, we use mature and immature GnRH neuronal cell models to show that both neuron-specific and protein kinase C regulation of GnRH expression are mediated by chromatin structure and histone modifications. Only in GT1-7 mature GnRH neuronal cells did GnRH regulatory elements display high sensitivity to DNase and enrichment of active histone markers histone-H3 acetylation and H3 lysine 4 trimethylation (H3K4-Me3), as well as RNA polymerase II (RNAPII) binding and enhancer RNA transcription. In contrast, H3K9-Me2, a marker of inactive chromatin, was highest in nonneuronal cells, low in GT1-7 cells, and intermediate in immature GnRH neuronal cells. The chromatin of the GnRH gene was therefore active in mature GnRH neuronal cells, inactive in nonneuronal cells, but not fully inactive in immature GnRH neuronal cells. Activation of protein kinase C (PKC) potently represses GnRH expression. PKC activation caused closing of the chromatin and decreased RNAPII occupancy at the GnRH minimal promoter (−278/−97). At GnRH-Enhancer-1 (−2404/−2100), PKC activation decreased phosphorylated-RNAPII binding, enhancer RNA transcription, and H3 acetylation, and reciprocally increased H3K9-Me2. Chromatin modifications therefore participate in the dynamic regulation and specification of GnRH expression to differentiated hypothalamic neurons.
GnRH is a neuropeptide hormone produced by approximately 800–1000 highly specialized, terminally differentiated neurons in the mouse hypothalamus. These neurons have a scattered localization throughout the basal forebrain and medial preoptic area in the anterior hypothalamus, with axons projecting to the median eminence (1, 2). GnRH regulates reproductive function from the apex of the hypothalamic-pituitary-gonadal axis by controlling production of gonadotropins from the anterior pituitary, which in turn stimulate the production of gonadal steroid hormones. During development, mouse GnRH neurons originate in the olfactory placode and subsequently migrate through the nasal septum to the forebrain, to reach their scattered localizations in the hypothalamus. The differentiated and fully functional phenotype of the GnRH neuron is defined by the expression of the GnRH gene, and therefore GnRH expression is primarily restricted to this population of hypothalamic neurons (3–8). GnRH gene expression gradually increases as these neurons migrate and acquire their terminally differentiated phenotype (9). GnRH neurons then integrate signals from neurotransmitters, steroids, growth factors, and peptide hormones to regulate sexual development, puberty, and overall reproductive function. Proper development and function of the GnRH neuron are critical for normal reproductive function, as dysregulation leads to idiopathic hypogonadotropic hypogonadism (IHH), Kallmann Syndrome, precocious puberty, or infertility.
Using the GT1-7 immortalized neuronal cell line, a model of the mature, terminally differentiated GnRH neuron (10), the specification of GnRH transcription to these specialized hypothalamic neurons has been shown to be controlled by combinations of transcription factors acting on evolutionarily conserved regulatory regions of the rat GnRH gene. The 173-bp minimal promoter (GnRH-P) (11) is regulated by many homeodomain transcription factors including Oct-1 (12), Msx and Dlx (13), Pbx and Prep (14), Nkx2.1 (15), and Otx2 (16). In addition to GnRH-P, neuron-specific expression is regulated by three GnRH enhancers: GnRH-E1, located at −1863/−1571 bp upstream of the transcription start site (TSS) (3), and the newly characterized GnRH-E2, located at −3135/−2980 bp (17, 18), and GnRH-E3, located at −4199/−3895 bp (18). GnRH-E1 is a bona fide neuron-specific enhancer, as it increases GnRH expression only in mature GnRH neuronal cells (3). GnRH-E1 interacts with GnRH-E2, GnRH-E3, and GnRH-P to target and enhance expression in mature GnRH neuronal cells, while blunting expression in immature GnRH neuronal as well as in nonneuronal cells (18, 19). GnRH-E1 binds many transcription factors including Pbx and Prep (14), Nkx2.1 (15), and GATA-4 (20). All three enhancers are regulated by Oct-1 (17, 18, 21) and Msx1 and Dlx2 (13, 18).
During development, as well as in normal reproductive function, GnRH production is controlled by various stimuli, including steroid and peptide hormones, growth factors, metabolic factors, and neurotransmitters. Many of these ligands signal through the protein kinase C (PKC) pathway. Treatment of GT1–7 cells with a PKC activator, 12-O-tetradecanoyl-phorbol 13-acetate (TPA), results in a potent decrease in GnRH mRNA that occurs at the level of transcription (22–24). TPA-mediated repression maps to the −126/−73-bp region of GnRH-P and involves c-Fos, but no robust changes in transcription factor DNA-binding at this region were reported (11, 23, 25). TPA-mediated repression also occurs through multiple portions of GnRH-E1, and TPA causes detectable decreases in binding of Oct-1, Pbx/Prep, and Dlx2 to GnRH-E1 (26). TPA-mediated repression of GnRH is very dramatic, therefore these results suggest a broader molecular mechanism for this complex repression.
Neuronal cell differentiation is controlled not only by transcription factors acting on cis-regulatory elements, but also by epigenetic mechanisms that include the modulation of chromatin structure and histone modifications that have functional impacts on gene expression (27–30). Inactive genes typically display condensed chromatin that is resistant to DNaseI digestion and show hypoacetylation of histone H3 and hypermethylation at histone H3 lysine 9 (H3K9) (31–33). Active genes and regulatory elements are often in an open chromatin conformation to facilitate binding of regulatory proteins and display sensitivity to DNAseI digestion (34), acetylation at histone H3 (H3Ac), and trimethylation at histone H3-lysine 4 (H3K4) (31–33). Assembly of the preinitiation complex and basal transcriptional machinery, which includes RNA polymerase II (RNAPII), is highly associated with actively transcribed genes (35), and further, phosphorylation of the RNAPII carboxyl-terminal domain is associated with different stages of transcription. For example, RNAPII phosphorylated at serine 5 is mainly associated with transcriptional initiation and the 5′ ends of genes, and RNAPII phosphorylated at serine 2 is an elongating form of RNAPII (36).
Dynamic changes to chromatin structure and histone modifications are thought to promote the differentiation of neurons from neural progenitor and precursor cells (37, 38). GnRH gene expression has been shown to gradually increase during development, with the highest expression levels attained in the mature, postmitotic state (9). Prior studies of GnRH expression primarily focused on regulation through transcription factor binding to regulatory elements. The GnRH gene could also be regulated at the level of chromatin structure, as such mechanisms in the regulation of GnRH expression during neuronal differentiation have not been extensively studied. In addition, the changes in GnRH expression in response to hormones and neurotransmitters may also involve chromatin modulation.
In this study, we provide evidence for the involvement of epigenetic changes in chromatin structure and histone modifications in neuronal specification of GnRH expression by showing that GnRH regulatory elements are in an active chromatin state in mature GnRH neuronal cells, an inactive state in nonneuronal cells, and an intermediate state in immature GnRH neuronal cells. We additionally demonstrate changes to the GnRH gene chromatin in response to PKC activation that involve closing of the chromatin at GnRH-P and changes in active and inactive histone modifications at GnRH-E1. Our results indicate that chromatin structure and histone modifications on GnRH regulatory elements contribute to neuronal specification and dynamic regulation of GnRH expression.
Results
GnRH regulatory elements are sensitive to DNaseI in GT1-7 cell chromatin but not GN11 or NIH3T3 cell chromatin
To study neuronal specification of GnRH expression, model GnRH neuronal cell lines were used. The GT1-7 mouse cell line is a representative model of the mature, differentiated GnRH neuron that produces high levels of GnRH in a pulsatile manner (10). The GN11 mouse cell line is a model immature migratory GnRH neuron that produces very low levels of GnRH (39). The NIH3T3 mouse fibroblast cell line is a nonneuronal cell line and serves as a control cell that does not express GnRH. GnRH mRNA levels are highest in the GT1-7 cell line, and GN11 cells display low levels, similar to nonneuronal cells (18).
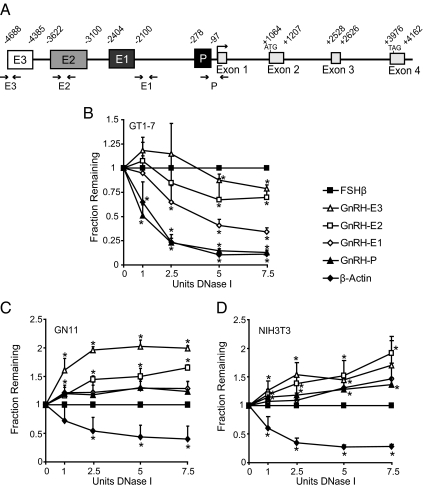
Specification of GnRH expression to mature GnRH neurons could be mediated through the regulation of chromatin structure at GnRH regulatory elements. Chromatin that is in an open, active conformation displays increased sensitivity to DNaseI treatment compared with chromatin that is in a predominantly closed, inactive conformation (34). To study the chromatin structure of the GnRH gene, quantitative DNaseI sensitivity assays (40) were performed using the model GnRH neuronal cell lines. Nuclei from GT1-7, GN11, and NIH3T3 cells were treated with increasing concentrations of DNaseI, and DNA was analyzed by quantitative real-time PCR (qPCR) using primers specific to GnRH regulatory elements (GnRH-P, GnRH-E1, GnRH-E2, GnRH-E3) in the mouse gene (Fig. 1A). Sensitivity to DNaseI would result in a decreased abundance of a particular PCR product as DNaseI concentration is increased. Quantities of PCR products corresponding to GnRH regulatory elements were measured relative to a gene that is inactive in all three cell types, FSHβ.
Fig. 1.
GnRH regulatory elements are sensitive to DNase in GT1-7 cells. A, Schematic diagram of the mouse GnRH gene showing relative locations of GnRH-E3 (E3), GnRH-E2 (E2), GnRH-E1 (E1), GnRH-P (P), exons, and introns. The coding sequence is indicated by start and stop codons (ATG, TAG). Small arrowheads indicate location of PCR primers used in DNaseI sensitivity and ChIP assays. B–D, DNaseI sensitivity assays in (B) GT1-7, (C) GN11, and (D) NIH3T3 cells. DNA from nuclei treated with increasing concentrations of DNaseI was analyzed by qPCR with primers specific to GnRH regulatory elements. Amplicon quantities were normalized to the inactive FSHβ gene, and qPCR data are presented as the mean fraction of DNA remaining relative to FSHβ ± sd. *, Significantly different from FSHβ by Student's t test (P < 0.05).
In GT1-7 cells, quantities of amplicons corresponding to GnRH-P, as well as β-actin, a gene active in all cell types, were significantly lower than the inactive FSHβ gene upon treatment with only 1 U of DNaseI (Fig. 1B). Quantities of GnRH-E1 were significantly lower than FSHβ with 2.5 U of DNaseI. The levels of GnRH-E2 and GnRH-E3 became significantly lower than FSHβ upon treatment with 5 U DNaseI. These results indicate that GnRH regulatory elements are sensitive to DNaseI digestion in GT1-7 cells. GnRH-P and β-Actin were the most sensitive to DNaseI, followed by GnRH-E1, then GnRH-E2 and GnRH-E3, therefore indicating an open chromatin conformation.
In GN11 and NIH3T3 cells, amplicon quantities corresponding to GnRH regulatory elements were not significantly lower than FSHβ at any concentration of DNaseI treatment, and in some cases were, in fact, significantly higher, and thus more resistant to DNAseI treatment compared with the negative control FSHβ (Fig. 1, C and D). Only the positive control, β-actin, showed significantly less signal than FSHβ with as low as 2.5 or 1 U of DNaseI treatment in GN11 or NIH3T3 cells, respectively. These results indicate that GnRH regulatory elements in GN11 and NIH3T3 cells do not display sensitivity to DNaseI compared with an inactive gene, indicating a closed chromatin conformation.
GnRH regulatory elements display histone markers of active chromatin only in GT1-7 cells
Histone modifications may influence chromatin structure to mediate neuron-specific expression through GnRH regulatory elements. Chromatin immunoprecipitation (ChIP) was used to analyze histone modifications on the GnRH gene in GT1-7, GN11, and NIH3T3 cells. Chromatin was sonicated to 300–500 bp to distinguish between regulatory elements of the mouse GnRH gene that are roughly 1000 bp or more apart, and primers corresponding to GnRH-P and the three enhancer regions (Fig. 1A) were used for qPCR.
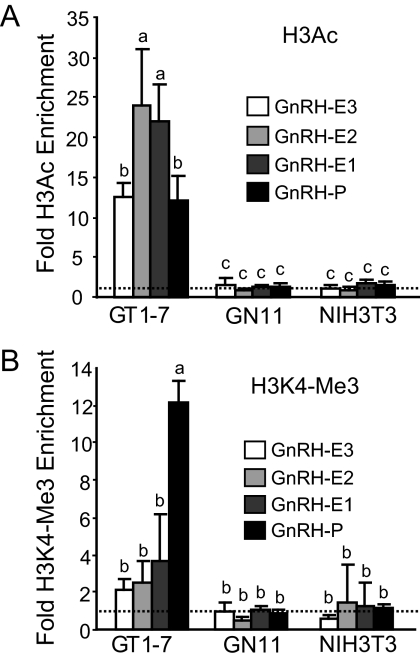
Histone H3 acetylation at lysine 9 and 14 is associated with actively transcribed genes (31, 32, 41). ChIP using an antibody recognizing H3 acetylation at H3K9 and H3K14 (H3Ac) followed by qPCR showed that in GT1-7 cells, all of the GnRH regulatory elements showed significantly higher levels of H3Ac enrichment compared with the very low enrichment levels in GN11 and NIH3T3 cells (Fig. 2A).
Fig. 2.
GnRH regulatory elements display histone markers of active chromatin in GT1-7 cells. ChIP analysis using antibodies specific to (A) H3Ac and (B) H3K4-Me3. Immunoprecipitated chromatin from GT1-7, GN11, and NIH3T3 cells was analyzed by qPCR using primers specific to GnRH regulatory elements. Data are presented as fold enrichment relative to IgG, with values normalized to the FSHβ negative control gene (dotted line), mean ± sd. Levels not assigned the same letter are significantly different by two-way ANOVA and post hoc least squares mean differences Tukey Kramer HSD (P < 0.05).
The trimethylated form of histone H3 lysine 4 (H3K4-Me3) is another marker of active genes that is primarily associated with promoters and transcription start sites (42). We previously showed this modification to be highly enriched only at GnRH-P in GT1-7 cells as a distinguishing chromatin signature for an active promoter (18). We then compared enrichment of this modification in GN11 and NIH3T3 cells. ChIP using an antibody to H3K4-Me3 showed that this modification is not significantly enriched at any of the GnRH regulatory elements in GN11 and NIH3T3 cells (Fig. 2B). Thus, in GT1-7 cells, but not in GN11 or NIH3T3 cells, GnRH regulatory elements display a primarily active chromatin state.
RNA polymerase II and serine 5-phosphorylated RNA polymerase II bind GnRH regulatory elements and transcribe enhancer RNA in GT1-7 cells, but not GN11 or NIH3T3 cells
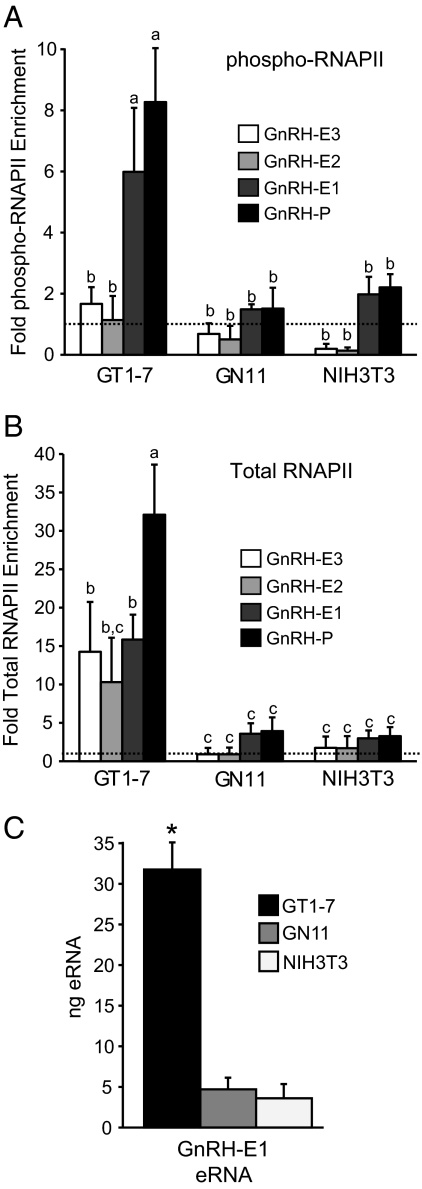
RNAPII mediates transcription of mRNA in eukaryotes. Once the preinitiation complex consisting of RNAPII and the basal transcriptional machinery is assembled, phosphorylation of serine 5 of the RNAPII carboxyl-terminal domain typically marks the initiation of transcription (36). The serine 5-phophorylated form of RNAPII (phospho-RNAPII) is associated with promoters of actively transcribed genes (36). Phospho-RNAPII occupancy at GnRH regulatory elements was compared in GT1-7, GN11, and NIH3T3 cells using an antibody specific to phospho-RNAPII. In GT1-7 cells, GnRH-P and GnRH-E1 showed equivalently high levels of phospho-RNAPII enrichment. The distal enhancers, GnRH-E2 and GnRH-E3, showed significantly lower levels of phospho-RNAPII enrichment, as did all GnRH regulatory elements in GN11 and NIH3T3 cells (Fig. 3A), consistent with low or absent transcription in these two cell lines (18).
Fig. 3.
Serine 5-phosphorylated-RNAPII and total RNAPII occupancy at GnRH regulatory elements. ChIP analysis using antibodies specific to (A) Ser5-phosphorylated-RNAPII (phospho-RNAPII) and (B) total RNAPII. Immunoprecipitated chromatin from GT1-7, GN11, and NIH3T3 cells was analyzed by qPCR using primers specific to GnRH regulatory elements. Data are presented as fold enrichment relative to IgG, with values normalized to the FSHβ negative control gene (dotted line), mean ± sd. Levels not assigned the same letter are significantly different by two-way ANOVA and post hoc least squares mean differences Tukey Kramer HSD (P < 0.05). C, eRNA is produced from GnRH-E1 in GT1-7 cells. RT-qPCR analysis of RNA from GT1-7, GN11, and NIH3T3 cells using primers specific to GnRH-E1. Nanogram quantities of GnRH-E1 eRNA were normalized to GAPDH mRNA and are presented mean ± sd. *, Significantly different from GN11 and NIH3T3 by one-way ANOVA and post hoc Tukey Kramer HSD (P < 0.05).
Genome-wide studies have revealed RNAPII binding to inactive genes, suggesting a promoter pausing or gene poising mechanism of gene regulation (36, 43). To determine whether this mechanism could be involved in GnRH gene regulation, ChIP using an antibody that recognizes total RNAPII was performed on chromatin from GT1-7, GN11, and NIH3T3 cells. In GT1-7 cells, RNAPII was highly enriched at GnRH-P, and also bound at slightly lower levels at all three GnRH enhancer regions. RNAPII levels at GnRH-P, GnRH-E1, and GnRH-E3 were significantly higher in GT1-7 than GN11 and NIH3T3 cells, which showed no RNAPII enrichment at any of the elements (Fig. 3B). This suggests that a promoter pausing or poising mechanism is not occurring in GN11 or NIH3T3 cells on the GnRH gene. These results are consistent with the GnRH mRNA expression pattern among the three cell lines (18) and also suggest an additional regulatory role for RNAPII at the GnRH enhancer regions in GT1-7 cells.
There is accumulating evidence for RNAPII binding at enhancer regions (36, 44). It also has been recently reported that RNAPII at enhancers of neuronal genes transcribes a novel class of enhancer RNAs (eRNA) that are typically less than 2 kb, are nonpolyadenylated, and positively correlate with levels of mRNA expression from specifically regulated promoters (45). Because the activated form (serine 5-phosphorylated) of RNAPII bound to GnRH-E1, we hypothesized that eRNA may be transcribed from GnRH-E1. RNA from GT1-7, GN11, and NIH3T3 cells was reverse transcribed using random priming and subjected to qPCR using primers specific to GnRH-E1. We detected an eRNA transcript from GnRH-E1 at significantly higher levels in GT1-7 cells than in GN11 and NIH3T3 cells (Fig. 3C). No transcripts were observed in a no-reverse transcriptase control (data not shown). This correlated with the phospho-RNAPII binding pattern at the GnRH gene among these three cell lines and also indicates a possible function for RNAPII enhancer binding in GnRH gene regulation.
GnRH regulatory elements display a histone marker of inactive chromatin in GN11 and NIH3T3 cells
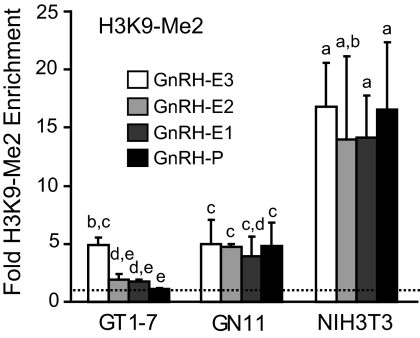
The dimethylated form of H3K9 (H3K9-Me2) is associated with inactive or repressed genes (31, 33, 46, 47). Enrichment of this modification was analyzed in GT1-7, GN11, and NIH3T3 cells by ChIP with an antibody to H3K9-Me2 (Fig. 4). Nonneuronal NIH3T3 cells displayed significantly higher enrichment of H3K9-Me2 at GnRH regulatory elements compared with each region in GT1-7, as well as in GN11 cells. In GN11 cells, H3K9-Me2 enrichment was similar to (GnRH-E3, GnRH-E1) or higher (GnRH-E2, GnRH-P) than in GT1-7 cells. Overall, H3K9-Me2 enrichment at GnRH regulatory elements in GN11 cells fell between the high levels in NIH3T3 cells and the low levels in GT1-7 cells. These results confirmed a mostly active chromatin state in GT1-7 cells, an inactive chromatin state in NIH3T3 cells, as shown additionally by low H3 acetylation and DNaseI sensitivity, but reveal an intermediate state in GN11 cells, not active enough for DNaseI sensitivity, H3 acetylation, H3K4 trimethylation or RNAPII binding, but not inactive enough to be fully occupied by dimethylated H3K9.
Fig. 4.
GnRH regulatory elements display a histone marker of inactive chromatin in GN11 or NIH3T3 cells. ChIP analysis using an antibody specific to H3K9-Me2. Immunoprecipitated chromatin from GT1-7, GN11, and NIH3T3 cells was analyzed by qPCR using primers specific to GnRH regulatory elements. Data are presented as fold enrichment relative to IgG, with values normalized to the β-actin negative control gene (dotted line), mean ± sd. Levels not assigned the same letter are significantly different by two-way ANOVA and post hoc least squares mean differences Tukey Kramer HSD (P < 0.05).
TPA represses GnRH expression
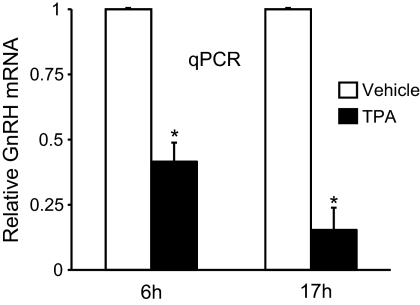
GnRH expression in mature, differentiated neurons is subject to regulation by many hormones, neurotransmitters, and other signaling molecules. TPA, an activator of the PKC pathways, has been shown to repress GnRH expression. Time course studies by Northern blotting and reporter assays show TPA-mediated repression of GnRH mRNA as early as 6 h, with the effects more pronounced at later time points (i.e., 17 h or 24 h) (22, 24, 26). Because TPA-mediated repression of endogenous GnRH mRNA had not been analyzed by qPCR in GT1-7 cells, RNA from GT1-7 cells treated for 6 h and 17 h with 100 nm TPA was subject to reverse-transcription and qPCR to quantify the repression. After 6 h of TPA treatment, GnRH mRNA levels were significantly reduced by 60% and were further reduced by 90% after 17 h of treatment (Fig. 5), supporting previous observations.
Fig. 5.
TPA represses GnRH gene expression. RT-qPCR analysis of GnRH mRNA from GT1-7 cells treated with 100 nm TPA for 6 h or 17 h. Picogram quantities of GnRH mRNA were normalized to GAPDH mRNA and are presented relative to vehicle, mean ± sd. *, Significantly different from vehicle by Student's t test (P < 0.05).
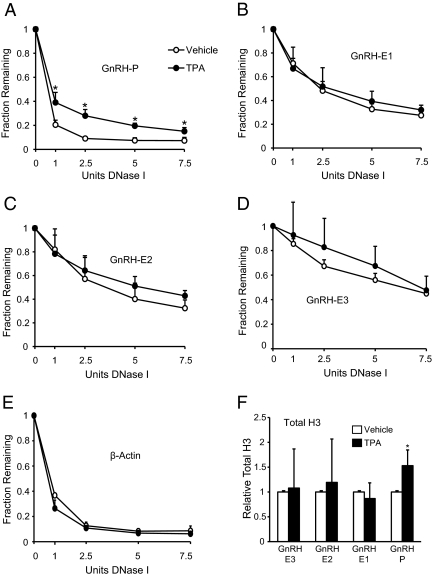
TPA causes closing of chromatin at GnRH-P
The molecular mechanisms of TPA-mediated GnRH repression are complex, involving multiple transcription factors acting through GnRH-P and GnRH-E1 (11, 26). The small changes in transcription factor binding do not fully explain the potent repression of GnRH by TPA. This repression could be mediated at a more global level through alteration of the overall chromatin structure of the GnRH gene. To study the effects of TPA on the overall chromatin structure of the GnRH gene, DNaseI sensitivity assays were performed on GT1-7 cells treated with vehicle or 100 nm TPA. The 6-h treatment time point was chosen because it was the earliest a decrease in GnRH mRNA was previously observed, suggesting that an effect on the chromatin structure likely had preceded that time point. Nuclei from vehicle and TPA-treated GT1-7 cells were subject to DNaseI digestion with increasing amounts of DNaseI, and DNA was analyzed by qPCR using primers specific to GnRH regulatory elements. TPA caused a significant decrease in DNaseI sensitivity and therefore a closing of the chromatin, only at GnRH-P (Fig. 6A). None of the GnRH enhancer regions (Fig. 6, B–D), β-Actin (Fig. 6E), or FSHβ (data not shown), exhibited significant changes in DNaseI sensitivity with TPA treatment. These trends were also maintained at the 17-h time point (data not shown). Closing of the chromatin at GnRH-P in response to TPA suggests a change in nucleosome density. This was confirmed by performing ChIP using an antibody to total histone H3 on chromatin from GT1-7 cells treated for 6 h with either vehicle or 100 nm TPA. qPCR analysis of immunoprecipitated chromatin showed a significant 50% increase in total H3 occupancy only at GnRH-P, and not at the GnRH enhancer regions (Fig. 6F), consistent with the results of the DNaseI sensitivity assay. Taken together, these results show that TPA causes closing of the chromatin at GnRH-P.
Fig. 6.
TPA causes closing of the chromatin at GnRH-P. A–E, DNaseI sensitivity assays in GT1-7 cells treated with 100 nm TPA for 6 h. Nuclei from TPA-treated (black circles) or vehicle-treated (white circles) cells were incubated with increasing concentrations of DNaseI, and DNA was analyzed by qPCR with primers specific to (A) GnRH-P, (B) GnRH-E1, (C) GnRH-E2, (D) GnRH-E3, and (E) β-actin. Amplicon quantities were normalized to the inactive FSHβ gene, and qPCR data are presented as the mean fraction of DNA remaining relative to FSHβ ± sd. *, Significantly different from vehicle by Student's t test (P < 0.05). F, ChIP analysis using an antibody specific to total histone H3. Immunoprecipitated chromatin from GT1-7 cells treated for 6 h with vehicle (white) or 100 nm TPA (black) was analyzed by qPCR using primers specific to GnRH regulatory elements. Data are presented as fold enrichment relative to vehicle, mean ± sd. *, Significantly different from vehicle by Student's t test (P < 0.05).
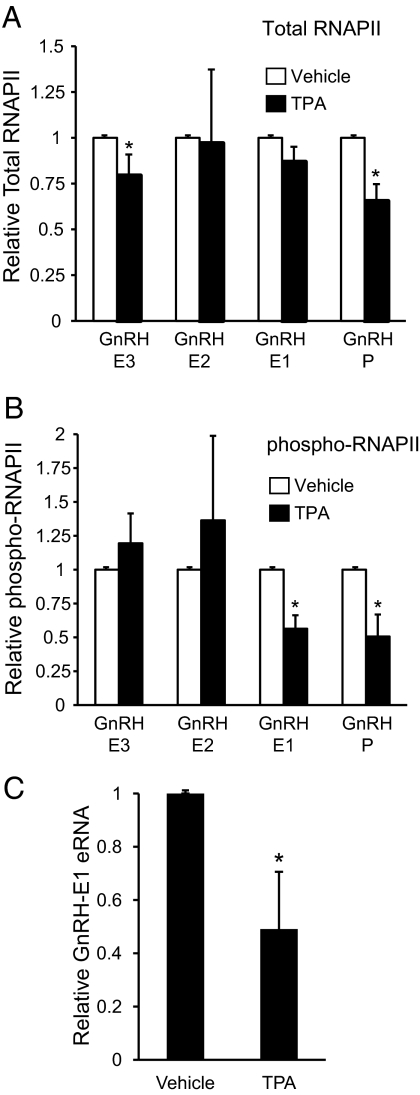
TPA decreases total RNAPII and phospho-RNAPII levels at the GnRH gene
Closing of the chromatin at GnRH-P with TPA treatment suggested a decreased accessibility for binding of the basal transcriptional machinery, including RNAPII. To determine changes in RNAPII occupancy in response to TPA treatment, ChIP using antibodies to both total RNAPII and phospho-RNAPII was performed on GT1-7 chromatin from cells treated for 6 h with vehicle or 100 nm TPA. TPA treatment caused a significant 40% reduction of total RNAPII occupancy at GnRH-P, no significant changes at GnRH-E1 or GnRH-E2, and a small reduction at GnRH-E3 (Fig. 7A). In contrast, phospho-RNAPII levels were significantly reduced by 50% not only at GnRH-P but also at GnRH-E1 (Fig. 7B). The decrease in phospho-RNAPII at GnRH-P is likely attributable to decreased overall occupancy of RNAPII, and the decrease at GnRH-E1 is likely attributable to a specific change in phosphorylation state.
Fig. 7.
TPA decreases total RNAPII and phospho-RNAPII occupancy at the GnRH gene. ChIP analysis using antibodies specific to (A) total RNAPII and (B) phospho-RNAPII. Immunoprecipitated chromatin from GT1-7 cells treated for 6 h with vehicle (white) or 100 nm TPA (black) was analyzed by qPCR using primers specific to GnRH regulatory elements. Data are presented as fold enrichment relative to vehicle, mean ± sd. *, Significantly different from vehicle by Student's t test (P < 0.05). C, TPA decreases eRNA from GnRH-E1. RT-PCR analysis of RNA from GT1-7 cells treated for 6 h with either vehicle or 100 nm TPA using primers specific to GnRH-E1. Nanogram quantities of eRNA were normalized to GAPDH mRNA and are presented relative to vehicle, mean ± sd. *, Significantly different from vehicle by Student's t test (P < 0.05).
The observed decrease in phospho-RNAPII at GnRH-E1 suggested that TPA treatment could influence the transcription of eRNA from GnRH-E1. RT-qPCR using RNA from GT1-7 cells treated for 6 h with either vehicle or 100 nm TPA was performed using primers specific to GnRH-E1. TPA significantly decreased the levels of eRNA from GnRH-E1 (Fig. 7C), likely caused by decreased phosphorylation of RNAPII at GnRH-E1 in response to TPA.
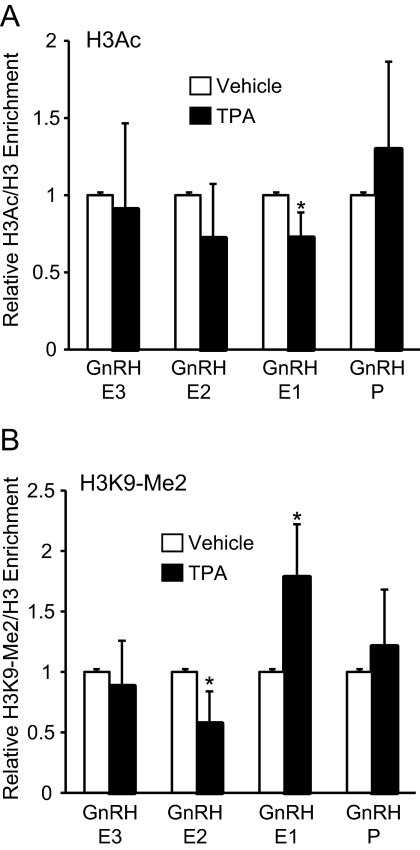
TPA alters enrichment of active and inactive histone H3 modifications at GnRH-E1
TPA may repress GnRH expression by changing histone H3 modifications at GnRH regulatory elements. ChIP was performed on GT1-7 cells treated for 6 h with either vehicle or 100 nm TPA using antibodies to an active histone marker, H3Ac, and an inactive marker, H3K9-Me2. To correct for changes in nucleosome density to specifically analyze changes in the histone modification levels in response to TPA treatment, enrichment of the modified H3 was measured relative to total H3 for each treatment condition. Changes to both active and inactive histone H3 modifications were observed only at GnRH-E1. TPA treatment caused a significant 30% decrease in H3Ac enrichment (Fig. 8A) and a significant 80% increase of H3K9-Me2 (Fig. 8B) at GnRH-E1. H3Ac enrichment at GnRH-P, GnRH-E2, and GnRH-E3 did not change with TPA treatment. TPA caused a slight decrease of H3K9-Me2 enrichment at GnRH-E2, but no significant changes were observed at GnRH-E3 or GnRH-P.
Fig. 8.
TPA alters enrichment of active and inactive histone H3 modifications at GnRH-E1. ChIP analysis using antibodies specific to (A) H3Ac and (B) H3K9-Me2. Immunoprecipitated chromatin from GT1-7 cells treated for 6 h with vehicle (white) or 100 nm TPA (black) was analyzed by qPCR using primers specific to GnRH regulatory elements. Data are presented as the ratio of modified H3/total H3 relative to vehicle, mean ± sd. *, Significantly different from vehicle by Student's t test (P < 0.05).
TPA therefore caused changes to the chromatin at GnRH-P and GnRH-E1 to mediate repression. TPA treatment resulted in closing of the chromatin and decreased RNAPII occupancy at GnRH-P, and at GnRH-E1, TPA decreased RNAPII phosphorylation, eRNA transcription, and H3Ac enrichment, and increased H3K9-Me2 enrichment.
Discussion
Specification of neuropeptide hormone gene expression to highly specialized hypothalamic neurons, as well as regulation by physiological stimuli, requires a complex orchestrated process involving cis-regulatory elements, DNA-binding transcription factors, coactivators and corepressors, and more global epigenetic regulation through chromatin structure and histone modifications. This study provides evidence for the involvement of chromatin structure and histone modifications in the specification of GnRH transcription to mature differentiated GnRH neurons and in the dynamic regulation of GnRH transcription by PKC activation.
Chromatin structure of the GnRH gene mediates specification of expression to differentiated GnRH neurons
Previous studies of neuron-specific GnRH expression have focused on combinations of transcription factor binding to GnRH regulatory elements. However, it is well known that chromatin structure is an important determinant of cell- and tissue-specific gene expression and, in particular, neuronal specification (37, 38). We used model GnRH neuronal cell lines to demonstrate that the chromatin architecture of the GnRH cis-regulatory elements provides an additional higher order level of regulation of cell-specific expression (Fig. 9A). GnRH is highly expressed in GT1-7 mature GnRH neuronal cells but is expressed at very low levels in GN11 immature GnRH neurons and nonneuronal cells (18). In GT1-7 cells, and, therefore, most likely in mature differentiated GnRH neurons, the GnRH gene is in an active chromatin formation as demonstrated by sensitivity of GnRH regulatory elements to DNAseI, high enrichment of H3Ac and H3K4-Me3, high levels of RNAPII and phospho-RNAPII occupancy, and low enrichment of a marker of inactive chromatin, H3K9-Me2. The association of RNAPII binding with H3K4-Me3 and H3Ac enrichment at GnRH-P only in GT1-7 cells is consistent with the reported enrichment of these three modifications at active tissue-specific promoters in a genome-wide study of mouse embryonic stem cells and adult organs (48). GnRH-E2 and GnRH-E3 were not as sensitive to DNaseI as GnRH-E1 and GnRH-P, suggesting that GnRH-P and GnRH-E1 need to be more open than the two upstream enhancer regions to promote critical transcription factor binding for neuron-specific expression, consistent with their critical roles in targeting expression exclusively to GnRH neurons in vivo (8). GnRH-E3, though in an active chromatin conformation, also displayed some enrichment of the marker of inactive chromatin, H3K9-Me2. Because GnRH-E2 and GnRH-E3 function through similar mechanisms (18), it is possible that GnRH-E3 is not required to be fully active to mediate neuron-specific GnRH expression. However, we cannot rule out the possibility that GnRH-E3 may become more active in other specific physiological conditions or play a greater role in vivo.
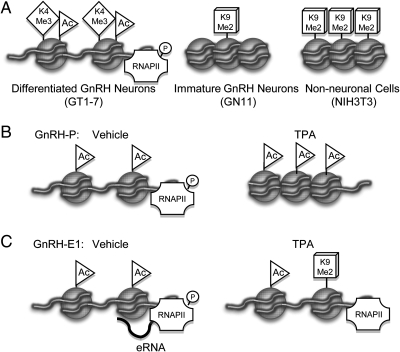
Fig. 9.
Model of chromatin modulation of GnRH gene expression. A, Schematic diagram of chromatin structure and histone modifications at GnRH-P in differentiated GnRH neurons [active, open chromatin, enrichment of Acetylated H3 (Ac) and H3K4-Me3 (K4Me3), and Ser5-phosphorylated RNAPII], immature GnRH neurons [inactive, closed chromatin, with some H3K9-Me2 (K9Me2) enrichment], and nonneuronal cells (inactive, closed chromatin, high K9-Me2 enrichment). B and C, Schematic diagram of chromatin modulation of (B) GnRH-P and (C) GnRH-E1, upon activation of PKC signaling with TPA. At GnRH-P, TPA causes closing of the chromatin with associated increased density of H3 and decreased RNAPII binding. At GnRH-E1, TPA causes a decrease in H3Ac, an increase in K9Me2, and a combined decrease of Ser5-phosphorylation of RNAPII and eRNA transcription.
In contrast, in the nonneuronal cell line NIH3T3, GnRH regulatory elements are insensitive to DNAseI and display low levels of H3Ac, H3K4-Me3, and RNAPII but have high levels of H3K9-Me2. Therefore, this region of the genome is in an inactive chromatin formation that would not facilitate the binding of transcription factors or the transcriptional machinery to the GnRH gene, thereby keeping GnRH expression repressed in this nonneuronal cell line. These observations provide evidence that chromatin structure of the GnRH gene is involved in neuron-specific expression.
GN11 cells very likely represent an immature premigratory GnRH neuron very early in development. GN11 cells were derived from a tumor in the olfactory bulb (39), and exhibit migratory properties in vitro (49). They express neuronal markers but do not fire action potentials (50), and GnRH mRNA levels are as low in GN11 cells as in nonneuronal cells (18). In vivo, during development, GnRH expression increases sharply as the GnRH neurons cross the cribriform plate into the forebrain (9). The chromatin state of the GnRH gene is in an intermediate state in GN11 cells. GnRH regulatory elements in GN11 cells display a lack of sensitivity to DNaseI and no significant enrichment of H3Ac, H3K4-Me3, and RNAPII, suggesting a closed conformation similar to nonneuronal cells. However, GnRH regulatory elements in GN11 cells display enrichment of H3K9-Me2 at intermediate levels, lower than in NIH3T3 cells but higher than in GT1-7 cells, suggesting that the chromatin in GN11 cells is not fully inactive. Early in development, the GnRH gene likely displays this intermediate chromatin state, possibly indicating early specification of its neuronal cell fate, and that the GnRH gene may be poised and ready to transition from an inactive to active state during neuronal migration and maturation.
Changes in histone methylation status are thought to be involved in neural cell and astrocyte differentiation. In progenitor cells, the glial fibrillary acidic protein gene is hypermethylated at H3K9 and inactive, but upon differentiation into an astrocytic lineage H3K9 methylation decreases, and H3K4 methylation increases as this gene is up-regulated (51). Similarly, the conversion of oligodentrocyte precursor cells to a neural cell fate involves the replacement of H3K9 methylation with H3K4 methylation and H3K9 acetylation at the Sox2 promoter (52). Because H3K9 methylation in GN11 cells was lower than in NIH3T3 cells, our data suggest demethylation of H3K9 as an early step in developmental regulation of GnRH gene expression, and the gradual up-regulation of GnRH expression likely involves the dynamic activity of histone modifying and chromatin remodeling enzymes at various stages of GnRH neuronal migration and differentiation. Treatment of GN11 cells with trichostatin A, an HDAC inhibitor, did not result in a significant increase of GnRH mRNA (data not shown). This suggests that other changes to the chromatin, such as DNA methylation, which has been recently reported to be involved in in vitro primate GnRH neuronal maturation (53), may be necessary before histone acetylation, and further that GnRH regulation likely involves a complex combination of many factors during development and migration.
TPA modulates chromatin modifications to repress GnRH gene expression
Hormones, neurotransmitters, and other various ligands induce changes in gene expression to mediate physiologic effects. These effects, for example, in the action of estrogen, thyroid, and growth hormones, are mediated in part by epigenetic alterations of the chromatin landscape of target genes that involve changes in histone acetylation, methylation, and RNAPII binding (54–58). TPA, a PKC activator, causes a dramatic decrease in GnRH mRNA (22, 24, 25) that is regulated at the transcriptional level. Previous studies have shown that TPA-mediated repression maps to GnRH-E1 and GnRH-P (11, 25, 26). In vitro reporter assays and EMSA analyses have shown that repression at GnRH-E1 involves small changes in transcription factor binding at various sites, but repression at GnRH-P does not cause major changes in transcription factor binding.
We demonstrated that TPA-mediated repression of GnRH involves higher-order reorganization of the chromatin structure (Fig. 9, B and C). The observed changes occurred only at GnRH-P and GnRH-E1, consistent with previous mapping studies. These changes in chromatin structure also occurred relatively rapidly before the earliest observed mRNA decrease at 6 h. At GnRH-P, TPA caused an overall closing of the chromatin and increased nucleosome density that also likely prevented RNAPII from binding. GnRH-P is therefore altered at a more global level. The previous observations that transcription factor binding was not dramatically altered (11) is likely attributable to the fact that in vitro EMSA assays are typically not performed on chromatinized templates, and such techniques would not detect more global alterations as would ChIP. TPA decreased H3Ac and increased H3K9-Me2 enrichment at GnRH-E1, causing a change in the histone modification status that could influence the recruitment of transcription factors, as previously described, as well as coactivators and corepressors to modulate repression. Induction of c-fos upon PKC activation (24, 25) could induce the expression or recruitment of coregulators and histone-modifying factors to mediate these changes. This pattern of chromatin dynamics could be a mechanism for repression of GnRH mRNA by melatonin (59), which signals through PKC pathway, and other hormones, neurotransmitters, metabolic factors, and growth factors could cause similar changes in chromatin structure and histone modifications to regulate GnRH expression. For example, estrogen directly represses GnRH mRNA expression (60) and controls negative-feedback suppression of GnRH during the estrous cycle (61), and regulation of GnRH expression during the estrous cycle could also involve dynamic chromatin modulation.
RNAPII binding to GnRH enhancers suggests additional complexity in GnRH regulation
ChIP analysis of total RNAPII and phospho-RNAPII binding in GT1-7 cells revealed additional complexity in GnRH gene regulation by the three enhancer regions. RNAPII binding was observed at all three GnRH enhancer regions. High enrichment of phospho-RNAPII was observed at GnRH-P, consistent with transcriptional initiation, but we also demonstrated an equal level of enrichment at GnRH-E1. Numerous genome-wide studies report that RNAPII binding to enhancer regions facilitates transcription by many functional mechanisms (36, 44). The observation of RNAPII at enhancers could be the result of looping of the chromatin between a promoter and enhancer to facilitate preinitiation complex recruitment. Alternatively, the preinitiation complex and RNAPII might assemble to enhancers early in the process of transcriptional activation and then track along the DNA to the promoter. A recent study showed that RNAPII at a large number of active neuronal enhancers transcribes eRNA (45). We showed that eRNA is transcribed from GnRH-E1 in GT1-7 cells, likely as a consequence of serine 5 phosphorylation of RNAPII on GnRH-E1. While the exact function of these eRNAs is unclear, eRNAs are thought to be necessary for activating promoters by facilitating an open chromatin structure for transcription factor binding or may be part of a mechanism of RNAPII tracking from an enhancer to a promoter (36, 44).
Our results suggest a RNAPII tracking mechanism at the GnRH gene. At GnRH-P, upon TPA treatment, the decreased levels of both total RNAPII and phospho-RNAPII levels suggest that the closed chromatin does not facilitate preinitiation complex assembly. However, at GnRH-E1, only phospho-RNAPII levels decreased with TPA treatment, accompanied by a corresponding decrease in eRNA from GnRH-E1. Before phosphorylation, RNAPII may bind to the two upstream enhancers and track along the DNA until it arrives at GnRH-E1. RNAPII could be subsequently phosphorylated and could transcribe eRNA as it tracks to GnRH-P. Activation of PKC likely causes decreased tracking of RNAPII from GnRH-E1, as suggested by the decrease in eRNA, and this could explain the decrease of total RNAPII at GnRH-P. An analogous tracking mechanism requiring phosphorylation of RNAPII at an enhancer has been shown in androgen receptor-mediated activation of the prostate specific antigen gene (62).
In summary, our results suggest the presence of multiple regulatory mechanisms acting on the GnRH gene to specify expression to differentiated neurons in the hypothalamus and also to regulate its expression in response to hormones, neurotransmitters, and growth factors. The role of coordinate regulation through combinations of homeodomain factors acting on GnRH regulatory elements has been extensively studied (3, 11–16, 18–20). Chromatin modulation of the GnRH gene provides an additional level of regulation that could be used at varying degrees throughout the development and adult functioning of the GnRH neuron. During development and migration of the GnRH neurons, the chromatin at the GnRH gene likely begins in a relatively inactive state and gradually opens and adopts an active conformation once in the postmitotic and postmigratory states. The chromatin dynamics would then be regulated by physiological stimuli to control levels of GnRH expression for proper reproductive development and function.
Materials and Methods
Cell culture
GT1-7, GN11 (kindly provided by Sally Radovick), and NIH3T3 (ATCC) cell lines were cultured in DMEM with 4.5% glucose, 10% fetal bovine serum, and 1× penicillin-streptomycin in 5% CO2 at 37 C. For experiments involving TPA (524400; Calbiochem/EMD4Biosciences, San Diego, CA), cells were serum starved for 24 h in serum-free media (DMEM with 4.5% glucose, 0.1% BSA, and 1× penicillin-streptomycin), before treatment with either 100 nm TPA or vehicle (ethanol) in serum-free media for 6 h or 17 h where indicated.
DNase sensitivity assay
GT1-7, GN11, and NIH3T3 cells were lysed in hypotonic buffer [20 mm Tris-HCl (pH 7.4), 10 mm NaCl, 1 mm MgCl2, 10 mm NaF, 0.5 mm EDTA, 0.1 mm EGTA with protease inhibitors]. Nuclei were isolated by centrifugation at 5000 rpm for 5 min at 4 C. Intact nuclei were resuspended in 1× DNaseI Reaction Buffer (Promega, Madison, WI) containing 2% glycerol. Equal amounts of nuclei were added to increasing quantities of DNaseI (Promega) in 1× DNaseI Reaction Buffer, ranging from 0 units (U) to 7.5 U, and incubated at 37 C for 5 min. DNaseI was inactivated using DNaseI Stop Solution (Promega) and incubation at 65 C for 10 min. Treated nuclei were lysed in Nuclei Lysis Buffer [100 mm TrisHCl (pH 8.0), 5 mm EDTA, 200 mm NaCl, 0.2% SDS] followed by RNase A and proteinase K digestion. Genomic DNA was then isolated by extraction twice with phenol/chloroform/isoamyl alcohol and once with chloroform. Samples were handled with wide-mouth pipette tips to minimize DNA shearing. DNA was then ethanol precipitated and resuspended in TE buffer followed by 55 C incubation for one hour. Samples were analyzed by qPCR using primers specific to the mouse GnRH regulatory elements (18) that were designed to detect only the endogenous mouse GnRH sequence and not the rat and human transgenes used to generate the GT1-7 (10) and GN11 (39) cell lines, respectively. The primer locations are as follows: GnRH-E3, −4706/−4533; GnRH-E2, −3579/−3427; GnRH-E1, −1814/−1653; GnRH-P, −173/+53. Mouse FSHβ (63) and β-actin promoters (64) were used as negative and positive controls for DNase sensitivity, respectively. Forty nanograms of DNA from each treatment condition was quantitated relative to a standard curve of dilutions of undigested DNA. Data from each primer set were normalized to the inactive gene, FSHβ. Data represent mean ± sd of at least three independent experiments. Statistical analyses were performed using Student's t test with P < 0.05 to indicate significance.
ChIP
ChIP assays were performed as previously described (18, 63). Chromatin was sonicated to an average length of 300–500 bp using a Branson Sonifier 250 (Branson Ultrasonics Corp., Danbury, CT). Antibodies recognizing specific histone modifications were as follows: anti–acetyl-Histone-H3 (06-599; Millipore, Temecula, CA), anti–trimethyl-Histone H3-Lys4 (07-473; Millipore), anti-RNA Polymerase II-phosphorylated-serine 5 (ab5131; Abcam, Cambridge, MA), anti-RNA Polymerase II 8WG16 (MMS-126R; Covance, Emeryville, CA), anti–dimethyl-Histone H3-Lys9 (ab1220; Abcam), anti-Histone H3 (ab1791; Abcam). Immunoprecipitated DNA and DNA from input chromatin were analyzed for sequences of interest by qPCR using primers specific to GnRH regulatory elements that have been described (18). Primers specific to the mouse FSHβ promoter (63) and the mouse β-actin promoter (64) were used as negative and positive controls where indicated. DNA from immunoprecipitated samples was quantified relative to a standard curve representing percent of input chromatin. For ChIP assays comparing GT1-7, GN11, and NIH3T3 chromatin, the fold enrichment of antibody signal over IgG was calculated for each primer set, and data from each independent experiment were normalized to the indicated negative control gene. For ChIP assays on TPA-treated GT1-7 cells, data for total H3, phospho-RNAPII, and RNAPII are presented as fold enrichment of antibody signal relative to vehicle, and for histone H3 modifications, data are presented as the ratio of modified H3/total H3 relative to vehicle. Data are presented as mean ± sd of at least three independent experiments. Statistical analyses were performed as indicated in figure legends using either two-way ANOVA followed by post hoc analysis by Tukey-Kramer Honestly Significant Difference (HSD), for studies comparing the three cell lines, or by Student's t test, for TPA studies, with P < 0.05 to indicate significance.
Reverse transcription-qPCR
Total RNA from GT1-7, GN11, and NIH3T3 cells, as well as from vehicle and TPA-treated GT1-7 cells, was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to manufacturer's recommendations. cDNA was obtained by reverse transcription of 2 μg RNA from each cell line using random hexameric primers and Superscript III First Strand Synthesis System (Invitrogen, San Diego, CA). For analysis of TPA-mediated repression, cDNA was subject to qPCR using primers specific to the coding sequences for mouse GnRH (65) in addition to the coding sequence of housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (65). Standard curves were generated using serial dilutions of plasmid DNA containing the qPCR amplicons cloned into pCR2.1 (Invitrogen). Quantities of GnRH were normalized to GAPDH and are presented relative to vehicle. Data are represented as mean ± sd from three independent RNA samples. Statistical analyses were performed using Student's t test, with P < 0.05 to indicate significance. For analysis of eRNA, cDNA was subject to qPCR using primers to GnRH-E1 (18). Standard curves were generated using dilutions of genomic DNA, and quantities of eRNA were normalized to GAPDH. For studies comparing three cell lines, data are represented as mean ± sd from three independent RNA samples and statistical analyses were performed using one-way ANOVA followed by post hoc Tukey Kramer HSD, with P < 0.05 to indicate significance. For TPA studies, data are represented relative to vehicle as mean ± sd from three independent RNA samples. Statistical analyses were performed using Student's t test, with P < 0.05 to indicate significance.
qPCR
For reverse-transcription-qPCR and analysis of DNA from DNase sensitivity and ChIP assays, qPCR was performed using the iQ5 Real-Time PCR Detection System and Software and iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) according to the manufacturer's recommendations.
Statistical analyses
JMP 8 (SAS Institute, Inc., Cary, NC) software was used for statistical analyses.
Acknowledgments
We thank Mark Lawson for assistance with statistical analyses, Bing Ren for valuable discussions, Sally Radovick for kindly providing GN11 cells, Aida Sun and Susan L. Mayo for technical assistance, Christine Glidewell-Kenney for critical reading of the manuscript, and members of the Mellon laboratory for insightful discussions.
This work was supported by National Institutes of Health (NIH) Grants R01 DK044838 and R01 HD020377 (to P.L.M.) and by National Institute of Child Health and Human Development/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to P.L.M.). A.K.I. was partially supported by NIH Grants F32 HD058427 and T32 DK007494. M.J.B. was partially supported by NIH Grants F32 HD058460 and T32 HD007203.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- ChIP
- Chromatin immunoprecipitation
- eRNA
- enhancer RNA
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- H3Ac
- acetylated histone H3
- H3K4
- histone H3-lysine 4
- H3K4-Me3
- trimethylated form of histone H3 lysine 4
- H3K9-Me2
- dimethylated form of histone H3 lysine 9
- HSD
- Honesly Significant Difference
- phospho-RNAPII
- serine 5-phophorylated form of RNAPII
- PKC
- protein kinase C
- qPCR
- quantitative real-time PCR
- RNAPII
- RNA polymerase II
- TPA
- 12-O-tetradecanoyl-phorbol 13-acetate.
References
- 1. Schwanzel-Fukuda M, Pfaff DW. 1989. Origin of luteinizing hormone-releasing hormone neurons. Nature 338:161–164 [DOI] [PubMed] [Google Scholar]
- 2. Wray S, Grant P, Gainer H. 1989. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA 86:8132–8136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whyte DB, Lawson MA, Belsham DD, Eraly SA, Bond CT, Adelman JP, Mellon PL. 1995. A neuron-specific enhancer targets expression of the gonadotropin-releasing hormone gene to hypothalamic neurosecretory neurons. Mol Endocrinol 9:467–477 [DOI] [PubMed] [Google Scholar]
- 4. Kepa KJ, Wang C, Neeley CI, Raynolds MV, Gordon DF, Wood WM, Wierman ME. 1992. Structure of the rat gonadotropin releasing hormone (rGnGH) gene promoter and functional analysis in hypothalamic cells. Nucl Acids Res 20:1393–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pape JR, Skynner MJ, Allen ND, Herbison AE. 1999. Transgenics identify distal 5′- and 3′-sequences specifying gonadotropin-releasing hormone expression in adult mice. Mol Endocrinol 13:2203–2211 [DOI] [PubMed] [Google Scholar]
- 6. Skynner MJ, Slater R, Sim JA, Allen ND, Herbison AE. 1999. Promoter transgenics reveal multiple gonadotropin-releasing hormone-I-expressing cell populations of different embryological origin in mouse brain. J Neurosci 19:5955–5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. 2000. Genetic targeting of green fluorescent protein to gonadotropin- releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- 8. Lawson MA, MacConell LA, Kim J, Powl BT, Nelson SB, Mellon PL. 2002. Neuron-specific expression In vivo by defined transcription regulatory elements of the gonadotropin-releasing hormone gene. Endocrinology 143:1404–1412 [DOI] [PubMed] [Google Scholar]
- 9. Simonian SX, Herbison AE. 2001. Regulation of gonadotropin-releasing hormone (GnRH) gene expression during GnRH neuron migration in the mouse. Neuroendocrinology 73:149–156 [DOI] [PubMed] [Google Scholar]
- 10. Mellon PL, Windle JJ, Goldsmith P, Padula C, Roberts J, Weiner RI. 1990. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 5:1–10 [DOI] [PubMed] [Google Scholar]
- 11. Eraly SA, Mellon PL. 1995. Regulation of GnRH transcription by protein kinase C is mediated by evolutionarily conserved, promoter-proximal elements. Mol Endocrinol 9:848–859 [DOI] [PubMed] [Google Scholar]
- 12. Eraly SA, Nelson SB, Huang KM, Mellon PL. 1998. Oct-1 binds promoter elements required for transcription of the gonadotropin-releasing hormone gene. Mol Endocrinol 12:469–481 [DOI] [PubMed] [Google Scholar]
- 13. Givens ML, Rave-Harel N, Goonewardena VD, Kurotani R, Berdy SE, Swan CH, Rubenstein JL, Robert B, Mellon PL. 2005. Developmental regulation of gonadotropin-releasing hormone gene expression by the MSX and DLX homeodomain protein families. J Biol Chem 280:19156–19165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rave-Harel N, Givens ML, Nelson SB, Duong HA, Coss D, Clark ME, Hall SB, Kamps MP, Mellon PL. 2004. TALE homeodomain proteins regulate gonadotropin-releasing hormone gene expression independently and via interactions with Oct-1. J Biol Chem 279:30287–30297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee BJ, Cho GJ, Norgren RB, Jr, Junier MP, Hill DF, Tapia V, Costa ME, Ojeda SR. 2001. TTF-1, a homeodomain gene required for diencephalic morphogenesis, is postnatally expressed in the neuroendocrine brain in a developmentally regulated and cell-specific fashion. Mol Cell Neurosci 17:107–126 [DOI] [PubMed] [Google Scholar]
- 16. Kelley CG, Lavorgna G, Clark ME, Boncinelli E, Mellon PL. 2000. The Otx2 homeoprotein regulates expression from the gonadotropin-releasing hormone proximal promoter. Mol Endocrinol 14:1246–1256 [DOI] [PubMed] [Google Scholar]
- 17. Givens ML, Kurotani R, N R-H, Miller NLG, Mellon PL. 2004. Phylogenetic footprinting reveals functional upstream regions of the gonadotropin-releasing hormone gene that enhance cell-specific expression. Mol Endocrinol 18:2950–2966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Iyer AK, Miller NL, Yip K, Tran BH, Mellon PL. 2010. Enhancers of GnRH Transcription embedded in an upstream gene use homeodomain proteins to specify hypothalamic expression. Mol Endocrinol 24:1949–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson SB, Lawson MA, Kelley CG, Mellon PL. 2000. Neuron-specific expression of the rat gonadotropin-releasing hormone gene is conferred by interactions of a defined promoter element with the enhancer in GT1-7 cells. Mol Endocrinol 14:1509–1522 [DOI] [PubMed] [Google Scholar]
- 20. Lawson MA, Buhain AR, Jovenal JC, Mellon PL. 1998. Multiple factors interacting at the GATA sites of the gonadotropin-releasing hormone neuron-specific enhancer regulate gene expression. Mol Endocrinol 12:364–377 [DOI] [PubMed] [Google Scholar]
- 21. Clark ME, Mellon PL. 1995. The POU homeodomain transcription factor Oct-1 is essential for activity of the gonadotropin-releasing hormone neuron-specific enhancer. Mol Cell Biol 15:6169–6177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bruder JM, Drebs WD, Nett TM, Wierman ME. 1992. Phorbol ester activation of the protein kinase C pathway inhibits gonadotropin-releasing hormone gene expression. Endocrinology 131:2552–2558 [DOI] [PubMed] [Google Scholar]
- 23. Bruder JM, Wierman ME. 1994. Evidence for transcriptional inhibition of GnRH gene expression by phorbol ester at a proximal promoter region. Mol Cell Endocrinol 99:177–182 [DOI] [PubMed] [Google Scholar]
- 24. Wetsel WC, Eraly SA, Whyte DB, Mellon PL. 1993. Regulation of gonadotropin-releasing hormone by protein kinases A and C in immortalized hypothalamic neurons. Endocrinology 132:2360–2370 [DOI] [PubMed] [Google Scholar]
- 25. Bruder JM, Spaulding AJ, Wierman ME. 1996. Phorbol ester inhibition of rat gonadotropin-releasing hormone promoter activity: role of fos and jun in the repression of transcription. Mol Endocrinol 10:35–44 [DOI] [PubMed] [Google Scholar]
- 26. Tang Q, Mazur M, Mellon PL. 2005. The protein kinase C pathway acts through multiple transcription factors to repress gonadotropin-releasing hormone gene expression in hypothalamic GT1-7 neuronal cells. Mol Endocrinol 19:2769–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feng J, Fouse S, Fan G. 2007. Epigenetic regulation of neural gene expression and neuronal function. Pediatr Res 61:58R–63R [DOI] [PubMed] [Google Scholar]
- 28. Hsieh J, Gage FH. 2004. Epigenetic control of neural stem cell fate. Curr Opin Genet Dev 14:461–469 [DOI] [PubMed] [Google Scholar]
- 29. Hsieh J, Gage FH. 2005. Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol 17:664–671 [DOI] [PubMed] [Google Scholar]
- 30. Barrera LO, Ren B. 2006. The transcriptional regulatory code of eukaryotic cells–insights from genome-wide analysis of chromatin organization and transcription factor binding. Curr Opin Cell Biol 18:291–298 [DOI] [PubMed] [Google Scholar]
- 31. Kouzarides T. 2007. Chromatin modifications and their function. Cell 128:693–705 [DOI] [PubMed] [Google Scholar]
- 32. Li B, Carey M, Workman JL. 2007. The role of chromatin during transcription. Cell 128:707–719 [DOI] [PubMed] [Google Scholar]
- 33. Sims RJ, 3rd, Nishioka K, Reinberg D. 2003. Histone lysine methylation: a signature for chromatin function. Trends Genet 19:629–639 [DOI] [PubMed] [Google Scholar]
- 34. Gross DS, Garrard WT. 1988. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem 57:159–197 [DOI] [PubMed] [Google Scholar]
- 35. Kim TH, Barrera LO, Qu C, Van Calcar S, Trinklein ND, Cooper SJ, Luna RM, Glass CK, Rosenfeld MG, Myers RM, Ren B. 2005. Direct isolation and identification of promoters in the human genome. Genome Res 15:830–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koch F, Jourquin F, Ferrier P, Andrau JC. 2008. Genome-wide RNA polymerase II: not genes only! Trends Biochem Sci 33:265–273 [DOI] [PubMed] [Google Scholar]
- 37. Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. 2004. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci USA 101:16659–16664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siebzehnrubl FA, Buslei R, Eyupoglu IY, Seufert S, Hahnen E, Blumcke I. 2007. Histone deacetylase inhibitors increase neuronal differentiation in adult forebrain precursor cells. Exp Brain Res 176:672–678 [DOI] [PubMed] [Google Scholar]
- 39. Radovick S, Wray S, Lee E, Nicols DK, Nakayama Y, Weintraub BD, Westphal H, Cutler J, G B, Wondisford FE. 1991. Migratory arrest of gonadotropin-releasing hormone neurons in transgenic mice. Proc Natl Acad Sci USA 88:3402–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McArthur M, Gerum S, Stamatoyannopoulos G. 2001. Quantification of DNaseI-sensitivity by real-time PCR: quantitative analysis of DNaseI-hypersensitivity of the mouse beta-globin LCR. J Mol Biol 313:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jenuwein T, Allis CD. 2001. Translating the histone code. Science 293:1074–1080 [DOI] [PubMed] [Google Scholar]
- 42. Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD, Barrera LO, Van Calcar S, Qu C, Ching KA, Wang W, Weng Z, Green RD, Crawford GE, Ren B. 2007. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 39:311–318 [DOI] [PubMed] [Google Scholar]
- 43. Kim TH, Barrera LO, Zheng M, Qu C, Singer MA, Richmond TA, Wu Y, Green RD, Ren B. 2005. A high-resolution map of active promoters in the human genome. Nature 436:876–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Szutorisz H, Dillon N, Tora L. 2005. The role of enhancers as centres for general transcription factor recruitment. Trends Biochem Sci 30:593–599 [DOI] [PubMed] [Google Scholar]
- 45. Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, Harmin DA, Laptewicz M, Barbara-Haley K, Kuersten S, Markenscoff-Papadimitriou E, Kuhl D, Bito H, Worley PF, Kreiman G, Greenberg ME. 2010. Widespread transcription at neuronal activity-regulated enhancers. Nature 465:182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bernstein BE, Meissner A, Lander ES. 2007. The mammalian epigenome. Cell 128:669–681 [DOI] [PubMed] [Google Scholar]
- 47. Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M. 2008. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 132:958–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barrera LO, Li Z, Smith AD, Arden KC, Cavenee WK, Zhang MQ, Green RD, Ren B. 2008. Genome-wide mapping and analysis of active promoters in mouse embryonic stem cells and adult organs. Genome Res 18:46–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maggi R, Pimpinelli F, Molteni L, Milani M, Martini L, Piva F. 2000. Immortalized luteinizing hormone-releasing hormone neurons show a different migratory activity in vitro. Endocrinology 141:2105–2112 [DOI] [PubMed] [Google Scholar]
- 50. Pimpinelli F, Redaelli E, Restano-Cassulini R, Curia G, Giacobini P, Cariboni A, Wanke E, Bondiolotti GP, Piva F, Maggi R. 2003. Depolarization differentially affects the secretory and migratory properties of two cell lines of immortalized luteinizing hormone-releasing hormone (LHRH) neurons. Eur J Neurosci 18:1410–1418 [DOI] [PubMed] [Google Scholar]
- 51. Song MR, Ghosh A. 2004. FGF2-induced chromatin remodeling regulates CNTF-mediated gene expression and astrocyte differentiation. Nat Neurosci 7:229–235 [DOI] [PubMed] [Google Scholar]
- 52. Kondo T, Raff M. 2004. Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes Dev 18:2963–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kurian JR, Keen KL, Terasawa E. 2010. Epigenetic changes coincide with in vitro primate GnRH neuronal maturation. Endocrinology 151:5359–5368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kininis M, Chen BS, Diehl AG, Isaacs GD, Zhang T, Siepel AC, Clark AG, Kraus WL. 2007. Genomic analyses of transcription factor binding, histone acetylation, and gene expression reveal mechanistically distinct classes of estrogen-regulated promoters. Mol Cell Biol 27:5090–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chia DJ, Rotwein P. 2010. Defining the epigenetic actions of growth hormone: acute chromatin changes accompany GH-activated gene transcription. Mol Endocrinol 24:2038–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chia DJ, Young JJ, Mertens AR, Rotwein P. 2010. Distinct alterations in chromatin organization of the two IGF-I promoters precede growth hormone-induced activation of IGF-I gene transcription. Mol Endocrinol 24:779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang D, Xia X, Weiss RE, Refetoff S, Yen PM. 2010. Distinct and histone-specific modifications mediate positive versus negative transcriptional regulation of TSHalpha promoter. PLoS One 5:e9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Welboren WJ, van Driel MA, Janssen-Megens EM, van Heeringen SJ, Sweep FC, Span PN, Stunnenberg HG. 2009. ChIP-Seq of ERα and RNA polymerase II defines genes differentially responding to ligands. EMBO J 28:1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roy D, Belsham DD. 2002. Melatonin receptor activation regulates GnRH gene expression and secretion in GT1-7 GnRH neurons. Signal transduction mechanisms. J Biol Chem 277:251–258 [DOI] [PubMed] [Google Scholar]
- 60. Roy D, Angelini NL, Belsham DD. 1999. Estrogen directly represses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-α (ERα)- and ERβ-expressing GT1-7 GnRH neurons. Endocrinology 140:5045–5053 [DOI] [PubMed] [Google Scholar]
- 61. Dorling AA, Todman MG, Korach KS, Herbison AE. 2003. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology 78:204–209 [DOI] [PubMed] [Google Scholar]
- 62. Wang Q, Carroll JS, Brown M. 2005. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell 19:631–642 [DOI] [PubMed] [Google Scholar]
- 63. Coss D, Jacobs SB, Bender CE, Mellon PL. 2004. A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. J Biol Chem 279:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huh YH, Ryu JH, Chun JS. 2007. Regulation of type II collagen expression by histone deacetylase in articular chondrocytes. J Biol Chem 282:17123–17131 [DOI] [PubMed] [Google Scholar]
- 65. Sasson R, Dearth RK, White RS, Chappell PE, Mellon PL. 2006. Orexin A induces GnRH gene expression and secretion from GT1-7 hypothalamic GnRH neurons. Neuroendocrinology 84:353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]