Extra-nuclear steroid receptors mediate steroid hormone functions in brain, bone, cancer, and the cardiovascular system through epigenetic modulation of genes and non-genomic effects.
Abstract
Steroid receptors existing outside the nucleus are increasingly being recognized in many organs and cell types, impacting the biology of bone, the heart and blood vessels, and the central nervous system. Some controversy exists as to the nature of the receptors at the plasma membrane. However, compelling evidence has been advanced that at least some classical steroid receptors mediate steroid ligand actions originating as signaling from the cell surface. Here I review the recent findings in this evolving field emphasizing the in vivo impact of these receptor pools with a focus on estrogen receptors.
Rapid responses to steroid hormones have been known for 60 yr (1, 2), but the nature of the receptors that mediate these responses has only recently been identified as plasma membrane (PM)-localized pools (reviewed in Ref. 3). In addition to classical steroid receptors (SRs) identified at the PM of target cells, new protein candidates have been proposed as SRs in a wide variety of models.
Extranuclear Classical SR
Classical nuclear receptors, estrogen receptor-α (ERα) and the progesterone receptors (PRA and PRB), have been demonstrated at the PM of breast cancer cells where they rapidly signal to cell proliferation and survival (4–7). Classical ERα at the membrane and not in the nucleus mediates 17β-estradiol (E2)-induced rapid signaling to kinase activation in the mouse (8) or in cells (7). Rapid signaling occurs because the membrane-localized ER is an atypical G protein-coupled receptor (GPCR), selectively activating discrete G protein α- and βγ-subunits to convey cell-specific and situational specific signals (9, 10).
Extranuclear PR associates through a proline-rich domain to interact with the SH3 domain of Src kinase, activating Src signaling to ERK MAPK that plays a role in breast cancer cell biology (11). Src activation by progestins may also require the physical association of membrane-localized PR and ER. Similarly, membrane-localized classical androgen receptors (ARs) have been demonstrated at the membrane of prostate cancer cells (12). Androgens rapidly signal through kinase cascades to proliferation in these cells (13), suggesting an important but not well-characterized role for membrane-localized AR. This receptor pool also signals in oocytes, indicating that extranuclear AR promotes meiosis through inhibiting cAMP generation (14, 15).
Regarding cytoplasmic pools of SR, classical ERα and -β have been found in mitochondria (16) and ERα has been found in the endoplasmic reticulum (17). The latter are capable of regulating calcium flux (17) and may play a role for endoplasmic reticulum stress responses impacting mitochondrial intrinsic apoptotic pathways (18).
Putative membrane SRs
Estrogen Receptor
Various additional proteins have been proposed to act as steroid receptors. Most prominently, GPR30 has been implicated in mediating estrogen action at the membrane (17, 19). Although many cell-based studies have implicated this orphan GPCR as an ER, few studies have supported this idea in vivo. A recent study from the laboratory of Terasawa and co-workers (20) showed that estrogen activated calcium oscillations in cultured hypothalamic neurons. Here, the ER antagonist, ICI182780, did not inhibit E2 action but caused oscillations itself. Further, the putative GPR30 agonist G1, acted like estrogen, and the estrogen effects were not seen upon small interfering RNA knockdown of GPR30 in the neurons. However, no studies in neurons from classical ERα or ERβ knockout mice were attempted, and G1 has now been shown to be an activator of a truncated ERα, ER36, at the PM of cancer cells (21).
A role for GPR30 may involve collaboration between membrane ER and this downstream orphan GPCR, showing dependence on both proteins (22). Support for the idea that GPR30 is not an ER comes from extensive testing of four different GPR30 knockout mice from three different laboratories. These investigators found no consistent phenotype in the GPR30 models (reviewed in Ref. 23) and little overlap with the extraordinary phenotypes of ERα and ERβ deletion mice (24–26).
In addition, various truncated ERs have been proposed to mediate the actions of E2 at the membrane (27, 28). The regulation of nitric oxide production by ERα46 has been described in immortalized human endothelial cells that do not express classical ERα66 (27), but this truncated receptor has not been found in wild-type mouse or human arteries in vivo. ERα46 has been described in breast cancer cell lines and may mediate suppression of transcription that occurs through the 66-kDa ERα (29). A membrane-localized ERα36 also has been described in ERα66-negative human breast cancer cell lines and has been reported to mediate various rapid signals and other estrogen actions in these cells (28). Because aromatase inhibitors are not effective in women with ERα66-negative breast cancers that purportedly express ERα36 and thus should mediate E2 actions, a role for the truncated receptor is controversial. Further, support for the importance of ERα36 in animal models is lacking.
Progesterone Receptor
There is evidence that progesterone may signal through additional membrane-localized receptors. A family of membrane-localized PR, products of separate genes distinct from classical PR, have been found in fish through mammals (30). These membrane PRs (mPRs) have been reported to signal in response to progesterone and may play roles in fish reproduction. Levine and colleagues (31) have recently reported that progesterone produces negative feedback regulation of GnRH in classical PR-knockout mice and in a cultured hypothalamic cell line. The latter was mediated by activation of the Gαi protein that resulted in decreased cAMP, reversed by small interfering RNA knockdown of mPRα. Thus, this new class of membrane PR may contribute to rapid progesterone signaling. However, mice created with mPR deletion(s) for the various members of this family have not yet been reported and are vital to demonstrate in vivo importance of these PR for progesterone action.
Another protein, PR membrane component-1, complexes with the plasminogen activator inhibitor 1 RNA-binding protein to function as a mPR (32). This receptor contributes to the antiapoptotic functions of progesterone in ovarian cancer cells and xenograft models undergoing cisplatin exposure (33). A similar survival effect of progesterone is mediated by this receptor in human granulose/luteal cells (34). Genetic deletion of this receptor in animal models has not been reported.
Signaling by Membrane SR
The point has been raised that membrane-localized, classical ERs have no intrinsic ability to signal, because the protein lacks structural domains comparable to tyrosine kinase receptors such as epidermal growth factor (EGF) or IGF-I receptors. However, this is true of all typical GPCRs such as the angiotensin, adrenergic, or dopaminergic receptors. Typical GPCRs often transactivate growth factor receptor tyrosine kinases at the membrane, such as the EGF receptor (35). For ERα, AR, and PR, there is convincing evidence that in some cell types, membrane SR transactivate the EGF or IGF-I receptors to stimulate kinase cascades impacting tumor biology (36–39).
In other situations, membrane-localized ERα collaborates in a still undefined fashion with typical GPCRs at the PM to transmit rapid signals impacting biology. A recent example of this by Spiegel and colleagues (40) showed that estrogen activated ERK MAPK in MCF-7 breast cancer cells. ERK activation was dependent upon sphinghosine 1-phosphate (S1P) generation via sphinghosine kinase. The ABCC1 and ABCG2 protein transporters mediated S1P export from the cell, with S1P presumably acting at S1P receptors that are known to contribute to breast cancer progression. These rapid actions of estrogen required ERα and not GPR30 (40). Previous work has suggested that membrane ERα collaborates with the S1P receptor, Edg-3, to transactivate EGF receptor for signaling through ERK (41). In specific situations, the collaboration could occur between membrane ERα and other GPCRs, including the aforementioned GPR30 (22).
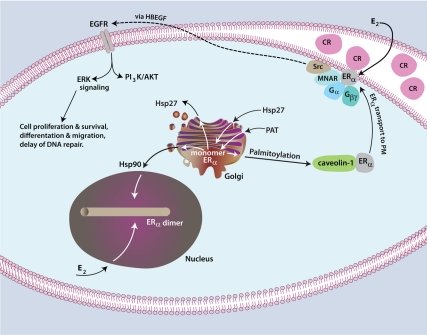
E2-induced phosphatidylinositol-3-kinase (PI3K) and ERK signaling in the hippocampus is important for object memory consolidation in female mice (42). Membrane-initiated steroid signaling by E2 through ERK to SMAD 1 phosphorylation prevents osteoblast generation yet preserves the viability of mature osteoblasts in vivo (43). Thus, the signalsome at the membrane of various target cells for steroid hormone action comprises varying and multiple proteins, sometimes including typical GPCRs, growth factor receptors, and classical steroid receptors (Fig. 1). Scaffolding of different proteins in the signalsome results in the generation of specific rapid signaling (reviewed in Ref. 44).
Fig. 1.
Model of ERα trafficking and signaling from the PM. In the Golgi, monomeric ERα is bound at cysteine 447 by Hsp27, promoting palmitoylation by an unknown palmitoylacyltransferase (PAT). I speculate Hsp27 opens up the ligand-binding domain structure to allow access by the PAT. Palmitoylation promotes monomeric ERα association with caveolin-1, the protein that transports ERα to caveolae rafts (CR) in the PM. ERα has been found within isolated CR but might adopt a tethering conformation to caveolin-1/CR in vivo. Caveolin-1 serves as a protein scaffold along with MNAR (modulator of nongenomic action of the estrogen receptor)/Pelp-1 to bring together components of the signalsome. ERα physically associates with and activates various Gα and Gβγ subunits depending upon estrogen binding that promotes receptor dimerization. G protein stimulation leads to multiple early signals including proximal kinase activation (e.g. Src) and cross talk to growth factor receptor tyrosine kinases (e.g. EGF receptor) in breast cancer cells. The growth factor receptor ultimately signals through multiple pathways to cell functions. Transport of ERα to the nucleus originates from the endoplasmic reticulum perhaps via the Golgi, chaperoned by Hsp90. Components of trafficking to the PM have been comparably established for classical PRs and ARs. HBEGF, Heparin-bound epidermal growth-factor.
Trafficking of Classical SR to the PM
Little is known about the mechanisms that underlie the localization of putative, nonclassical SRs such as mPR at the PM. In contrast, emerging evidence from the work of several laboratories has identified a conserved mechanism for classical sex SR trafficking to the PM.
Palmitoylation of SR
ERα46 was found to undergo palmitoylation on undetermined residues (27), a posttranslational modification that is well known to promote membrane localization of many proteins (45). Subsequent work from the Marino laboratory (46) showed that cysteine 447 in the human ERα ligand-binding (E) domain (amino acid 451 in mice) is the site of palmitoylation, required for membrane SR localization and rapid signaling. Additionally, this group showed that depalmitoylation of membrane-ERα occurs (47) but the functional impact is unclear at present.
Extending these studies, my laboratory described a palmitoylation motif of nine amino acids (445–453 in ERα) including the cysteine palmitoylation site. This motif was highly conserved in the E domains of ERα and -β isoforms, PR, and AR. By mutational analysis of the key residues in the palmitoylation motifs from the four sex SRs, we showed that palmitoylation via this motif was required to drive a small percentage of these receptors to the membrane, where upon steroid hormone ligation, the receptors rapidly signaled to cell biology (4). Interestingly, the glucocorticoid receptor also contains a comparable palmitoylation motif in the E domain, but the impact of this motif for membrane localization has not been reported.
Palmitoylation of ER occurs in cytoplasm and promotes a physical interaction with the caveolin-1 protein that serves as a key transporter for membrane localization of ERα (4, 7) (Fig. 1). This interaction is consistent with previous reports from several groups that membrane-localized ERs are found in caveolar rafts isolated from the membrane of endothelial and breast cancer cells. The classical vitamin D and mineralocorticoid receptors, members of the superfamily of SRs, also have been localized to signal from caveolae impacting target cell biology (reviewed in Ref. 44).
To identify additional proteins that facilitate membrane ER localization, we carried out a proteomic approach and showed that heat shock protein (Hsp)27 binds to the palmitoylation motif of ERα. Hsp27 was found to be necessary for ER palmitoylation, membrane trafficking, and rapid signaling to DNA synthesis (48). Palmitoylation preferentially occurred on the ERα monomer, promoting monomeric ER/caveolin-1 association and subsequent trafficking of the monomer to the PM. Endogenous ER at the PM exists primarily as a monomer in the absence of ligand, and E2 rapidly promotes dimerization of the membrane SR, a conformational change that is required for rapid signaling including G protein activation in seconds (49). Estrogen does not facilitate membrane localization but probably controls receptor acylation by inducing dimerization, thus limiting the amount of monomeric ER that is available to undergo palmitoylation. This may explain why only approximately 5% of receptors are found at the PM but 100% have the structural motif that theoretically allows palmitoylation and membrane trafficking. Hsp27 also facilitates palmitoylation, membrane localization, and rapid signaling by PRs and ARs in breast and prostate cancer cells, respectively (48). Thus, eliminating this small heat shock protein selectively interferes with only membrane (and not nuclear) functions of the SRs and allows a clearer understanding of what cell effects originate from membrane sex SR signaling.
Mitochondrial Functions
There is continuing development of a role for mitochondrial SR in functions affecting cell fate. Recent work from the laboratory of Srinivas (50) indicates that mitochondrial ERβ sequesters the proapoptotic protein BAD and prevents apoptosis of lung cancer in response to chemotherapy. In ligand-independent fashion, ERβ prevents Bcl-2 and Bcl-xl binding to BAD, thereby inducing survival. These investigators previously reported that ERβ is mainly expressed in cytoplasm of lung cancer cells and mediates the proliferative effects of estrogen through rapid activation of cAMP, AKT, and ERK (51). Interestingly, these investigators showed that expression of exogenous ERβ in cells mislocalizes the receptor to the nucleus (compared with the endogenous protein that is found to be extranuclear) and therefore could create experimental artifacts.
Cardiovascular System
Both ERα and ERβ have been localized to the PM of endothelial cells (10, 52) and cardiomyocytes (53). In vivo, membrane-localized ERα mediates arterial vasodilation 2 min after E2 administration, resulting from ERK and PI3K-dependent generation of nitric oxide due to endothelial nitric oxide synthase (eNOS) stimulation (54). E2 stimulation of eNOS has been well defined in many in vitro models (55) but whether ERβ coregulates this effect in vivo is unclear (reviewed in Ref. 56).
Additional evidence further supports the importance of rapid signaling by membrane ER. The endogenous selective ER modulator, 27-hydroxycholesterol, acts both at the cell membrane and nuclear ER to antagonize E2 actions (57). The selective ER modulator impairs eNOS activity induced by ERα or ERβ, NOS gene expression in vivo, and vasodilation and replenishment of the endothelium after acute vascular injury (the latter mediated in part by eNOS activity).
A recent paper indicated that the activation function 1 of ERα is dispensable for the ability of E2 to prevent aortic atherosclerosis in a transgenic mouse (58). The authors showed that E2 increased the production of endothelial nitric oxide and accelerated endothelial healing after injury, comparably in activation function 1-deleted and wild-type mice. These data collectively suggest that rapid signaling by E2 from membrane ERα is highly likely to contribute to some of these effects, whereas the longer-term prevention of atherogenesis could represent E2 action at both membrane and nuclear ER pools.
Addressing this important issue, new data have recently been published establishing that signaling exclusively by membrane ERα rescues acute vascular injury in mice Shaul and colleagues (59) showed that administration of an estrogen-dendrimer conjugate (EDC) (60) that does not transit to the nucleus activates a Gαi-dependent increase of nitric oxide upon binding ERα. EDC administration to mice prevented neointimal formation in the carotid artery after mechanical injury and endothelial layer denudation upon electrical arterial injury. In contrast, neither uterine growth nor breast cancer xenograft tumor growth was stimulated by the EDC, in contrast to E2. The latter results establish that the EDC did not engage nuclear ERα, required for the growth-stimulating effects by E2 in these targets.
Increasingly, a clear role for ERβ in preventing cardiac hypertrophy is supported from in vivo models. Administration of 8β-VE2 (Bayer Pharmaceutical, Berlin, Germany), a specific ERβ agonist, lowered the blood pressure and prevented cardiac hypertrophy in spontaneously hypertensive rats that inexorably progress to these aspects of cardiovascular disease (61).
In mouse models, E2 inhibition of angiotensin-induced cardiac hypertrophy and fibrosis occurred in wild-type and ERα knockout mice but not in ERβ knockout mice (62). The ability of E2/ERβ to block protein phosphatase 2B (calcineurin) activity restrained the NFATc3 transcription factor from translocating to the nucleus, thus preventing hypertrophic gene induction. E2/ERβ effects occurred through rapid PI3K activation to the MCIP1 gene up-regulation, the protein product inhibiting the catalytic activity of calcineurin in cytoplasm (53, 62). E2/ERβ up-regulation of natriuretic peptide production in the heart also opposed the hypertrophic signaling by angiotensin II receptors through ERK MAPK, and at least some of these actions were likely to be initiated by the ERβ pool demonstrated at the PM of cardiomyocytes (53). In addition, E2 binding to membrane ERβ produced in cardiac fibroblasts mediates the prevention of fibrosis in mice, preventing further progression of heart disease (63). This occurs when E2/ERβ inhibit TGF-β signaling through c-jun N-terminal kinase to SMAD transcription factor phosphorylation. As a result, the SR prevents collagen gene transcription (63).
Epigenetic Signaling by Membrane SRs
Regulation of transcription by SR signaling from the membrane affects multiple processes that often impact nuclear SR function (64). A novel role for rapid SR signaling to the epigenetic regulation of gene transcription has been suggested from several recent papers.
Kim et al. (65) showed that PTH signaling through protein kinase C phosphorylated a DNA glycosylase, MBD4. This promoted the ability of the MBD4 protein to incise methylated DNA, leading to demethylation of a nuclear vitamin D receptor-repressed gene (CYP27B1). Thus, it is possible that rapid signaling by steroid receptors, GPCRs, or growth factor receptors can act in an epigenetic fashion to promote increased or decreased gene transcription regulated by members of the nuclear SR superfamily.
Supporting this idea, Bredfeldt et al. (66) recently reported that xenoestrogens such as diethylstilbestrol induces a rapid, PI3K-dependent phosphorylation of the histone methyltransferase, EZH2, both in cultured uterine cells and in the developing, postnatal rat uterus in vivo. Rapid signaling through PI3K was mediated by membrane-localized ERα. As a result of EZH2 phosphorylation, methyltransferase activity directed against PR and IGF-binding protein 5 genes at the repressive histone 3 mark on lysine 27 was inhibited, resulting in gene derepression. These results indicate that rapid signaling from a membrane sex SR to an epigenetic alteration facilitates gene transcription regulated by nuclear sex SRs. Perhaps chromatin remodeling through epigenetic reprogramming by membrane-initiated diethylstilbestrol signaling contributes to the vaginal cancer seen in the offspring of xenoestrogen-treated pregnant women. This steroid hormone action might also occur independently of nuclear SR engagement to impact the modulation of some genes.
Long-term memory may be modulated by glucocorticoids acting at a putative membrane-associated receptor pool. Roozendaal and colleagues (67) showed that emotion-arousing memory for object recognition occurred in mice through functions of the hippocampus and insular cortex in the brain and was greatly facilitated by systemic glucocorticoid administration. Memory consolidation required glucocorticoid-induced cAMP and cAMP response-element binding protein interactions with cAMP response-element binding protein-binding protein. Glucocorticoids caused cAMP response-element binding protein-binding protein -related histone acetylation in the brain regions mentioned and subsequent remodeling of chromatin that correlated to memory changes in vivo. Memory enhancement was also caused by glucocorticoid-BSA injections into discrete brain regions. Injection of a histone deacetylase inhibitor into the brain selectively enhanced object memory retention dependent on glucocorticoid receptors in the specific brain areas implicated (67). Thus, epigenetic modulation by steroid-generated cyclic nucleotides influences long-term memory consolidation in vivo.
Perspective
Emerging data now support clear roles for extranuclear SR in modulating many aspects of biology and the response to injury. This has especially become evident in the bone, central nervous and cardiovascular systems, and some cancers. However, extranuclear SR actions often require collaboration with nuclear SR, particularly when modulation of gene transcription is critical to the overall biological functions of steroids. The transcriptional interactions are complex, involving rapid signaling to coactivator/repressor recruitment to the transcriptosome, phosphorylation of nuclear SR that leads to tethering induction at both proximal promoters and distal enhancer sites for genes (68, 69), modification of histones and DNA, the latter at sites of methylation, acetylation, and sumoylation (70), and the regulation of micro-RNAs. These latter functions implicate membrane SR signaling to epigenetic regulation through multiple mechanisms. A comprehensive review of the impact from ERK activation by PR for histone/chromatin modifications at the mouse mammary tumor virus promoter was recently published (71). Interestingly, rapid signaling by mPR through ERK and the MSK1 kinase displaces the repressive complex containing HP1γ protein from the mouse mammary tumor virus promoter, allowing chromatin remodeling that leads to coactivator and RNA polymerase II recruitment.
Recent studies of the role of membrane SR signaling in transcription have unveiled additional mechanisms. Madak-Erdogan et al. (72) used the EDC compound to exclusively bind to membrane ER and activate rapid signaling such as ERK. DNA arrays from MCF-7 cells exposed to the EDC compound indicated that some genes appear to be regulated without recruiting nuclear ERα to target gene promoters. The mechanisms involved are not clear but could include recruitment of transcription factors or interactions between nuclear ER and DNA-binding proteins that would not be established from chromatin immunoprecipitation assays. Using several different functional mutants of PR, Quiles et al. (73) support this idea, showing that some genes regulated by progesterone and PRB do not require nuclear PR recruitment to the promoter. Furthermore, these investigators find that the vast majority of genes modulated by progesterone in breast cancer cells stably transfected to express WT PRB require both membrane and nuclear PR. Additionally, some genes are stimulated by progestin in cells expressing a PR mutant that prevents interaction between PR with ER, thus eliminating Src and ERK MAPK signaling. These genes are likely regulated by additional rapid signals that do not require physical association with membrane ER but recruit immediate-early genes/transcription factors to target promoters.
Unrelated to transcription, there are well-described, rapid signaling effects of extranuclear SR that impact enzyme and other protein activities, regulating cell fate, migration/invasion, and DNA repair. An example of the latter is the ability of membrane ERα to signal through AKT to block the activation of ataxia telangiectasia-mutated and Rad3-related kinase and the resulting signal transduction cascade (74). Activation of this cascade induces G1/S and G2/M cell cycle checkpoints and DNA repair protein recruitment to the site of radiation-induced DNA damage. As a result of E2 inhibiting ataxia telangiectasia-mutated and Rad3-related and downstream kinase signaling, DNA repair is delayed and cell cycle progression inappropriately progresses. This leads to additional chromosome damage in breast cancer and normal mammary epithelial cells (74).
In vivo proof that selective engagement of membrane-localized ER by steroid hormone protects against vascular injury indicates a new approach to identifying the specific roles of the extranuclear SR (59). In addition, various ER mutant mice that show altered transcriptional ability but retain rapid membrane signaling can be used to establish unanticipated functions of extranuclear ER (8, 75). The challenges for the future include developing unique reagents that allow dissection of the functions of SR at cytoplasmic organelles (mitochondria, endoplasmic reticulum, endosomes) in cells and in vivo. Only then will we understand the true integrative functions of SRs, because organs/cells almost invariably express multicompartment endogenous SR.
Acknowledgments
This work was supported by grants from the Research Service of the Department of Veteran's Affairs and National Institutes of Health Grant CA-10036.
Disclosure Summary: No conflicts of interest were reported by the author and he has nothing to disclose.
Footnotes
- AR
- Androgen receptor
- E2
- 17β-estradiol
- EDC
- estrogen-dendrimer conjugate
- EGF
- epidermal growth factor
- eNOS
- endothelial nitric oxide synthase
- ER
- estrogen receptor
- GPCR
- G protein-coupled receptor
- Hsp
- heat shock protein
- mPR
- membrane PR
- PI3K
- phosphatidylinositol-3-kinase
- PM
- plasma membrane
- PR
- progesterone receptor
- S1P
- sphinghosine 1-phosphate
- SR
- steroid receptor.
References
- 1. Selye H. 1950. Stress and the general adaptation syndrome. Br J Med 1:1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Szego CM, Davis JS. 1967. Adenosine 3,5 -monophosphate in rat uterus: acute elevation by estrogen. Proc Natl Acad Sci USA 58:1711–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levin ER. 2009. Plasma membrane estrogen receptors. Trends Endocrinol Metab 20:477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pedram A, Razandi M, Sainson RCA, Kim JK, Hughes CC, Levin ER. 2007. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem 282:22278–22288 [DOI] [PubMed] [Google Scholar]
- 5. Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. 2007. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol 21:359–375 [DOI] [PubMed] [Google Scholar]
- 6. Skildum A, Faivre E, Lange CA. 2005. Progesterone receptors induce cell cycle progression via activation of mitogen-activated protein kinases. Mol Endocrinol 19:327–339 [DOI] [PubMed] [Google Scholar]
- 7. Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. 2003. Identification of a structural determinant for the membrane localization of ERα. Mol Cell Biol 23:1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pedram A, Razandi M, Kim JK, O'Mahony F, Lee EY, Luderer U, Levin ER. 2009. Developmental phenotype of a membrane only estrogen receptor α (MOER) mouse. J Biol Chem 284:3488–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Razandi M, Pedram A, Greene GL, Levin ER. 1999. Cell membrane and nuclear estrogen receptors derive from a single transcript: studies of ERα and ERβ expressed in CHO cells. Mol Endocrinol 13:307–319 [DOI] [PubMed] [Google Scholar]
- 10. Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW. 2007. Direct interactions with Gαi and Gβγ mediate nongenomic signaling by estrogen receptor β. Mol Endocrinol 21:1370–1380 [DOI] [PubMed] [Google Scholar]
- 11. Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. 2001. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell 8:269–280 [DOI] [PubMed] [Google Scholar]
- 12. Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. 2000. Steroid-induced androgen receptor-oestradiol receptor β-Src complex triggers prostate cancer cell proliferation. EMBO J 19:5406–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bagchi G, Wu J, French J, Kim J, Moniri NH, Daaka Y. 2008. Androgens transduce the Gαs-mediated activation of protein kinase A in prostate cells. Cancer Res 68:3225–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lutz LB, Cole LM, Gupta MK, Kwist KW, Auchus RJ, Hammes SR. 2001. Evidence that androgens are the primary steroids produced by Xenopus laevis ovaries and may signal through the classical androgen receptor to promote oocyte maturation. Proc Natl Acad Sci USA 98:13728–31333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gill A, Jamnongjit M, Hammes SR. 2004. Androgens promote maturation and signaling in mouse oocytes independent of transcription: a release of inhibition model for mammalian oocyte meiosis. Mol Endocrinol 18:97–104 [DOI] [PubMed] [Google Scholar]
- 16. Chen JQ, Delannoy M, Cooke C, Yager JD. 2004. Mitochondrial localization of ERα and ERβ in human MCF-7 cells. Am J Physiol Endocrinol Metab 28:E1011–E1022 [DOI] [PubMed] [Google Scholar]
- 17. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. 2005. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630 [DOI] [PubMed] [Google Scholar]
- 18. Chami M, Oulès B, Szabadkai G, Tacine R, Rizzuto R, Paterlini-Bréchot P. 2008. Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol Cell 32:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr 2000. Estrogen induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, gpr30, and occurs via transactivation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 [DOI] [PubMed] [Google Scholar]
- 20. Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. 2009. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol 23:349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang ZY. 2010. Involvement of estrogen receptor variant ERα36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol 24:709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levin ER. 2009. GPR30: ER or collaborator? Endocrinology 150:1563–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Langer G, Bader B, Meøli L, Insensee J, Delbeck M, Noppinger PR, Otto C. 2010. A critical review of fundamental controversies in the field of GPR30 research. Steroids 75:603–610 [DOI] [PubMed] [Google Scholar]
- 24. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. 1993. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. 2000. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- 26. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. 1998. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li L, Haynes MP, Bender JR. 2003. Plasma membrane localization and function of the estrogen receptor α variant (ER46) in human endothelial cells. Proc Natl Acad Sci USA 100:4807–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. 2006. A variant of estrogen receptor-α, hER-36: transduction of estrogen and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci USA 103:9063–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F. 2000. Identification of a new isoform of the human estrogen receptor-α (hER-α) that is encoded by distinct transcripts and that is able to repress hER-α activation function 1. EMBO J 19:4688–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomas P. 2008. Characteristics of membrane progestin receptor α (mPRα) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol 29:292–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. 2009. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology 150:3833–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peluso JJ, Romak J, Liu X. 2008. Progesterone receptor membrane component-1 (PGRMC1) is the mediator of progesterone's antiapoptotic action in spontaneously immortalized granulose cells as revealed by PGRMC1 small interfering ribonucleic acid treatment and functional analysis of PGRMC1 mutations. Endocrinology 149:534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peluso JJ, Gawkowska A, Liu X, Shioda T, Pru JK. 2009. Progesterone receptor membrane component-1 regulates the development and cisplatin sensitivity of human ovarian tumors in athymic nude mice. Endocrinology 150:4846–4854 [DOI] [PubMed] [Google Scholar]
- 34. Peluso JJ, Liu X, Gawkowska A, Johnston-MacAnanny E. 2009. Progesterone activates a progesterone receptor membrane component 1-dependent mechanism that promotes human granulose/luteal cell survival but not progesterone secretion. J Clin Endocrinol Metab 94:2644–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Biesen T, Luttrell LM, Hawes BE, Lefkowitz RJ. 1996. Mitogenic signaling via G protein-coupled receptors. Endocr Rev 17:698–714 [DOI] [PubMed] [Google Scholar]
- 36. Razandi M, Pedram A, Park ST, Levin ER. 2003. Proximal events in ER signaling from the plasma membrane. J Biol Chem 278:2701–2712 [DOI] [PubMed] [Google Scholar]
- 37. Santen RJ, Fan P, Zhang Z, Bao Y, Song RX, Yue W. 2009. Estrogen signals via an extra-nuclear pathway involving IGF-1R and EGFR in tamoxifen-sensitive and -resistant breast cancer cells. Steroids 74:586–594 [DOI] [PubMed] [Google Scholar]
- 38. Sen A, O'Malley K, Wang Z, Raf GV, DeFranco DB, Hammes SR. 2010. Paxillin regulates androgen and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells. J Biol Chem 285:28787–28795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Faivre EJ, Lange CA. 2007. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol 27:466–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takabe K, Kim RH, Allegood JC, Mitra P, Ramachandran S, Nagahashi M, Harikumar KB, Hait NC, Milstien S, Spiegel S. 2010. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J Biol Chem 285:10477–10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, Bernal A, Derian CK, Ullrich A, Vadas MA, Xia P. 2006. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol 173:301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. 2010. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci 30:4390–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Almeida M, Martin-Millan M, Ambrogini E, Bradsher R, III, Han L, Chen XD, Roberson PK, Weinstein RS, O'Brien CA, Jilka RL, Manolagas SC. 2010. Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the ERα. J Bone Miner Res 25:769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hammes SR, Levin ER. 2007. Extra-nuclear steroid receptors: nature and function. Endocr Rev 28:726–741 [DOI] [PubMed] [Google Scholar]
- 45. Baekkeskov S, Kanaani J. 2009. Palmitoylation cycles and regulation of protein function (Review). Mol Membr Biol 26:42–54 [DOI] [PubMed] [Google Scholar]
- 46. Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. 2005. Palmitoylation-dependent estrogen receptor α membrane localization regulation by 17-β-estradiol. Mol Biol Cell 16:231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Galluzzo P, Ascenzi P, Bulzomi P, Marino M. 2008. The nutritional flavanone naringenin triggers antiestrogenic effects by regulating estrogen receptor α-palmitoylation. Endocrinology 149:2567–2575 [DOI] [PubMed] [Google Scholar]
- 48. Razandi M, Pedram A, Levin ER. 2010. Heat shock protein 27 is required for sex steroid receptor trafficking to and functioning at the plasma membrane. Mol Cell Biol 30:3249–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. 2004. Plasma membrane estrogen receptors exist and function as dimers. Mol Endocrinol 18:2854–2865 [DOI] [PubMed] [Google Scholar]
- 50. Zhang GF, Yanamala N, Lathrop KL, Zhang L, Klein-Seetharaman J, Srinivas H. 2010. Ligand-independent anti-apoptotic function of estrogen receptor β in lung cancer cells. Mol Endocrinol 24:1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang G, Liu X, Farkas AM, Parwani AV, Lathrop KL, Lenzner D, Land SR, Srinivas H. 2009. Estrogen receptor β functions through nongenomic mechanisms in lung cancer cells. Mol Endocrinol 23:146–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pedram A, Razandi M, Levin ER. 2006. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol 20:1996–2009 [DOI] [PubMed] [Google Scholar]
- 53. Pedram A, Razandi M, Aitkenhead M, Levin ER. 2005. Estrogen inhibits cardiomyocyte hypertrophy in vitro: antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem 280:26339–26348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guo X, Razandi M, Pedram A, Kassab G, Levin ER. 2005. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors α and β. J Biol Chem 280:19704–19710 [DOI] [PubMed] [Google Scholar]
- 55. Kim KH, Bender JR. 2009. Membrane-initiated actions of estrogen on the endothelium. Mol Cell Endocrinol 308:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chow RW, Handelsman DJ, Ng MK. 2010. Rapid actions of sex steroids in the endothelium. Endocrinology 151:2411–2422 [DOI] [PubMed] [Google Scholar]
- 57. Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 2007. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med 13:1185–1192 [DOI] [PubMed] [Google Scholar]
- 58. Billon-Galés A, Fontaine C, Filipe C, Douin-Echinard V, Fouque MJ, Flouriot G, Gourdy P, Lenfant F, Laurell H, Krust A, Chambon P, Arnal JF. 2009. The transactivating function 1 of estrogen receptor α is dispensable for the vasculoprotective actions of 17β-estradiol. Proc Natl Acad Sci USA 106:2053–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. 2010. Non-nuclear estrogen receptor α signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest 120:2319–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. 2006. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol 20:491–502 [DOI] [PubMed] [Google Scholar]
- 61. Jazbutyte V, Arias-Loza PA, Hu K, Widder J, Govindaraj V, von Poser-Klein C, Bauersachs J, Fritzemeier KH, Hegele-Hartung C, Neyses L, Ertl G, Pelzer T. 2008. Ligand-dependent activation of ER lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized SHR. Cardiovasc Res 77:774–781 [DOI] [PubMed] [Google Scholar]
- 62. Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. 2008. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor β to inhibit calcineurin. Endocrinology 149:3361–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pedram A, Razandi M, O'Mahony F, Lubahn D, Levin ER. Estrogen receptor β inhibits cardiac fibrosis. Mol Endocrinol 10.1210/me.2010-0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Björnström L, Sjöberg M. 2005. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 19:833–842 [DOI] [PubMed] [Google Scholar]
- 65. Kim MS, Kondo T, Takada I, Youn MY, Yamamoto Y, Takahashi S, Matsumoto T, Fujiyama S, Shirode Y, Yamaoka I, Kitagawa H, Takeyama K, Shibuya H, Ohtake F, Kato S. 2009. DNA demethylation in hormone-induced transcriptional derepression. Nature 461:1007–1012 [DOI] [PubMed] [Google Scholar]
- 66. Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. 2010. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol 24:993–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA. 2010. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. J Neurosci 30:5037–5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Faivre EJ, Daniel AR, Hillard CJ, Lange CA. 2008. Progesterone receptor rapid signaling mediates ser 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol 22:823–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Subtil-Rodriguez A, Millan-Ariño L, Quiles I, Ballaré C, Beato M, Jordan A. 2008. Progesterone induction of the 11β-hydroxysteroid dehydrogenase type 2 promoter in breast cancer cells involves coordinated recruitment of STAT5A and progesterone receptor to a distal enhancer and polymerase tracking. Mol Cell Biol 28:3830–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Daniel AR, Lange CA. 2009. Protein kinases mediate ligand-independent derepression of sumoylated progesterone receptors in breast cancer cells. Proc Natl Acad Sci USA 106:14287–14292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Guillermo P, Vicent A, Silvina N, Roser Z, Ballare C, Clausell J, Beato M. Minireview: role of kinases and chromatin remodeling in progesterone signaling to chromatin. Mol Endocrinol 1210/me.2010-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS. 2008. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol 22:2116–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Quiles I, Millán-Ariño L, Subtil-Rodriguez A, Miñana B, Spinedi N, Ballaré C, Beato M, Jordan A. 2009. Mutational analysis of progesterone receptor functional domains in stable cell lines delineates sets of genes regulated by different mechanisms. Mol Endocrinol 23:809–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Pedram A, Razandi M, Evinger AJ, Kim JK, Lee E, Levin ER. 2009. Estrogen inhibits ATR signaling to cell cycle checkpoints and DNA repair. Mol Biol Cell 20:3374–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. 2002. An estrogen receptor (ER)α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol 16:2188–2201 [DOI] [PubMed] [Google Scholar]