Abstract
Background
Common variation in the Fat Mass and Obesity related (FTO) gene is associated with increased body fat and susceptibility to type 2 diabetes (T2D). We hypothesized that this would also associate with metabolic phenotypes of insulin resistance, and increased risk of cardiovascular morbidity and mortality.
Methods and Results
FTO rs9939609 genotype was determined in 4897 patients with T2D in the prospective Genetics of Diabetes Audit and Research Study in Tayside Scotland (Go-DARTS) study. The A allele was associated with lower plasma HDL cholesterol (mean difference 0.03 mmol/L, p=0.008), higher triglycerides (0.1 mmol/L, p=0.007), higher atherogenic index of plasma (0.03, p=0.003) and, as expected, increased BMI (0.77 kg/m2, p=8.8×10−6). During a mean follow up of 3.6 years the A allele was also associated with increased risk (HR 2.36, CI 1.49-3.74, p=0.0002) of fatal and non fatal myocardial infarction (total 324 events) in a model including baseline age, gender, prevalent myocardial infarction, smoking status, statin and insulin use. This association diminished but remained significant when obesity related traits such as BMI, glycated haemoglobin and lipid parameters were also included (HR 2.01 CI 1.18-3.45, p=0.011). There was a strong interaction of FTO genotype and statin use and cardiovascular outcome (p=0.001) such that cardiovascular morbidity and mortality was completely abrogated in individuals prescribed statins.
Conclusion
The increased fat mass in carriers of the A allele of rs9939609 of FTO is associated not only with increased risk of T2D, but also with an increase in atherogenic lipid profile, and myocardial infarction in these patients. This variant may therefore in future contribute to more effective targeting of specific preventative therapy.
Keywords: Genetics, Myocardial Infarction, Diabetes Mellitus
Introduction
The increasing global burden of obesity is associated with an increasing prevalence of type 2 diabetes (T2D) and subsequently an increase in morbidity and mortality primarily due to ischemic heart disease1. It has been estimated that for every increase in a BMI measure of one unit there is an 8% increase in cardiovascular mortality2. Recently whole genome association studies have demonstrated that SNP rs9939609 in the Fat Mass and Obesity associated gene region (FTO) at 16q10 is strongly associated with both increased body weight and also susceptibility to T2D3 and this association has been confirmed in other studies4,5. The original association with T2D was found to be through an association with body mass index (BMI). Other SNPs in this region have also been identified with similar associations and it seems likely that their association is due to linkage disequilibrium within the region4. While the SNPs seem to be associated with raised BMI in multiple European populations3,4, the association appears to be absent in some non-European populations such as the African Americans4 the Chinese Han 6 and in oceanic populations7. It has been demonstrated that the region is also associated with other metabolic features related to being over weight. Healthy individuals possessing the A allele were found not only to have a raised body mass index but also an increased whole body resistance to insulin8. Given the firmly established relationship of increased BMI and T2D with cardiovascular morbidity and mortality we investigated the clinical impact of genetic variation in the FTO gene terms of risk of myocardial infarction in a large population of patients with T2D in Scotland known as Go-DARTS.
Methods
The Go-DARTS population has been described in previous studies9-11 and has been collected from the Tayside region of Scotland (population approximately 400,000) where all healthcare activity has, for the past 2 decades, been linked to a patient-unique identifier facilitating the creation of sophisticated regional health informatics systems. The Diabetes Audit and Research in Tayside Study (DARTS) is a comprehensive, well validated, region-wide clinical information system containing detailed data on effectively all patients with diabetes in the region12. The data is assimilated from multiple resources including the Community Health Index (CHI) which contains demographic data, Scottish Morbidity Records (SMR) detailing ICD (International Classification of Disease) coding for hospital admissions, with data from the General Registrars Office (GRO) detailing date and cause of death and general practitioner office records. Data has been verified through a continuous research nurse-led validation process. Other data sources include the regional biochemistry database containing all historical serum biochemistry assays and the regional dispensed prescribing database maintained by the University of Dundee’s Health Informatics Centre. This contains historical data on all medicines prescribed for all individuals in the region from 1993 to present.
Over the past 10 years an increasing number of patients with T2D in the region have been approached to provide a sample of blood for genetic studies with consent for the genetic information to be linked anonymously to their clinical data in DARTS and associated data sets. Many of these individuals were recruited under the auspices of the Wellcome Trust UK type 2 Diabetes Case Control consortium and contributed significantly to the replication phase for the WTCCC GWA studies for T2D3. The linkage of this genotypic data from this large group of patients with T2D together with the rich longitudinal clinical datasets constitutes the Genetics of DARTS (Go-DARTS) study. We studied 4786 individuals with a diagnosis of T2D who were recruited to Go-DARTS between October 1997 and March 2006 and with an age of recruitment less than 85 years of age.
Genotyping
Genotyping of rs9939609 was performed as previously described using a combination of TAQMAN allelic discrimination and KASPAR genotyping (Kbiosciences)13.
Statistics
Each individual in Go-DARTS often has multiple measures of clinical parameters recorded over a period of time during the course of their clinical management. We therefore obtained a single integrated estimate as a mean value of multiple measures for each individual (intra-individual mean), obtained within a three year period around the date the individual was recruited into the Go-DARTS study. These clinical parameters were Body Mass Index, total Cholesterol, HDL cholesterol, triglycerides, glycated hemoglobin, systolic and diastolic blood pressure. All such intra-individual mean values were adjusted for age and gender. In the case of triglycerides and triglyceride-related values which can vary widely depending on post-prandial state, intra-individual means were also adjusted for intra-individual variation as the standard deviation of the intra-individual range. Atherogenic index of plasma14 was determined by log10([Triglycerides]/[HDLc]). Analysis of variance was used to determine the impact of genotype on clinical parameters.
In order to perform a prospective study of the impact of the FTO variant on cardiovascular disease in T2D we studied only individuals who had at least 1 year of follow up data from recruitment to the genetic study with Cox’s regression being used to estimate the relative rates of cardiovascular endpoints. The model included exposure to both statin and insulin therapy. These were simply dichotomised as ever having at least two prescriptions dispensed during the study period or never having had a prescription. Other variables included in the model were smoking (recorded as ever smokers and never smokers) as well as a history of a previous myocardial infarction. In this study a combined endpoint of fatal and non-fatal myocardial infarction was used as determined by a hospital discharge event with myocardial infarction as the diagnosis or cause of death being recorded as myocardial infarction. All data manipulation and statistical analysis were executed using STATA v9.0
Results
The baseline characteristics of the 4897 Go-DARTS participants genotyped for FTO rs9939609 who fulfilled the entry criteria for the study were are shown in table 1. The rare (A) allele had a frequency of 0.424 SE 0.005. Allele frequencies at this locus in this population did not deviate from Hardy-Weinberg equilibrium (p=0.96). Adjusted means by FTO genotype for BMI, pulse pressure, mean arterial pressure, glycated hemoglobin, HDL cholesterol, triglycerides, total cholesterol and atherogenic index of plasma are provided in table 2. Inspection of the data indicated a dominant/co-dominant impact of the variant on all clinical parameters investigated and therefore the significance levels for both models are given. As expected there was a strong association of the FTO variant with BMI, with the adjusted mean for individuals carrying the A allele (dominant model) being 0.77 kgm−2 greater than TT homozygotes (p=8.8×10−6). Similarly, the A allele was associated with an adjusted mean HDL cholesterol that was 0.03 mmolL−1 lower (p=0.008) and an adjusted mean triglyceride 0.1 mmolL−1 higher (p=0.002) compared to TT homozygotes. There was no association of FTO with total cholesterol. Although individuals with the A allele were more likely to be have been exposed to statin medication (p=0.002) (table 3), accounting for this did not alter this lack of association. To further emphasise the atherogenicity of the lipid profile we determined the atherogenic index15 and found that the A allele was associated with a significantly higher index (0.03, p=0.003). We found no significant association of genotype with glycated haemoglobin parameters or treatment with insulin (data not shown).
Table 1.
Population characteristics
| Mean | SD | |
|---|---|---|
| Age (yrs) | 63.7 | 11.7 |
| Female (%) | 55.3 | - |
| Prevalent MI (%) | 10.9 | - |
| Statin prescription (%) | 69.8 | - |
| Insulin Prescription (%) | 32.4 | |
| BMI (kgm−2) | 30.7 | 5.9 |
| Systolic BP (mmHg) | 140.9 | 7.8 |
| Diastolic BP (mmHg) | 77.7 | 7.7 |
| Glycated Haemoglobin (%) | 7.7 | 1.3 |
| Triglycerides (mmolL−1) | 2.4 | 1.6 |
| Total cholesterol (mmolL−1) | 4.8 | 0.9 |
| HDL cholesterol (mmolL−1) | 1.3 | 0.4 |
Table 2.
Influence of FTO genotype on clinical parameters. All values adjusted for age and gender. (Measures including triglycerides also adjusted for intrinsic intraindividual variation; aip – atherogenic index of plasma)
| TT | TA | AA | dominant | co-dominant | ||||
|---|---|---|---|---|---|---|---|---|
| Genotype count (%) | 1625 (33.2) | 2391(48.8) | 881(18) | |||||
| Mean | SE | Mean | SE | Mean | SE | |||
| BMI (kgm−2) | 30.16 | 0.14 | 30.80 | 0.12 | 31.29 | 0.19 | 8.8×10−6 | 8.5×10−7 |
| Pulse pressure (mmHg) | 62.77 | 0.25 | 63.31 | 0.17 | 63.84 | 0.31 | 0.021 | 0.026 |
| Mean arterial Pressure (mmHg) | 98.5 | 0.2 | 98.8 | 0.1 | 99.2 | 0.2 | 0.017 | 0.049 |
| Glycated Hemoglobin (%) | 7.7 | 0.03 | 7.7 | 0.02 | 7.8 | 0.04 | 0.127 | 0.185 |
| HDL(mmolL−1) | 1.32 | 0.008 | 1.30 | 0.005 | 1.29 | 0.01 | 0.008 | 0.035 |
| Triglycerides (mmolL−1) | 2.36 | 0.03 | 2.44 | 0.02 | 2.52 | 0.03 | 0.002 | 0.002 |
| Total Cholesterol (mmolL−1) | 4.83 | 0.02 | 4.84 | 0.01 | 4.85 | 0.03 | 0.718 | 0.554 |
| aip | 0.20 | 0.006 | 0.22 | 0.004 | 0.24 | 0.08 | 0.003 | 0.002 |
Table 3.
Individuals receiving a prescription for a statin during the course of the study in entire cohort
| Statin Prescription | TT | TA/AA |
|---|---|---|
| No | 478 (30%) | 823 (25%) |
| Yes | 1,140 (71%) | 2,433 (75%) |
| Total | 1,618 | 3,256 |
OR 1.24 CI 1.09-1.42 P=0.002
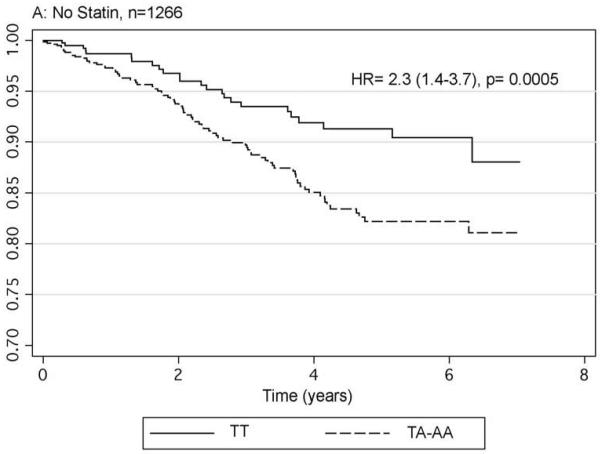
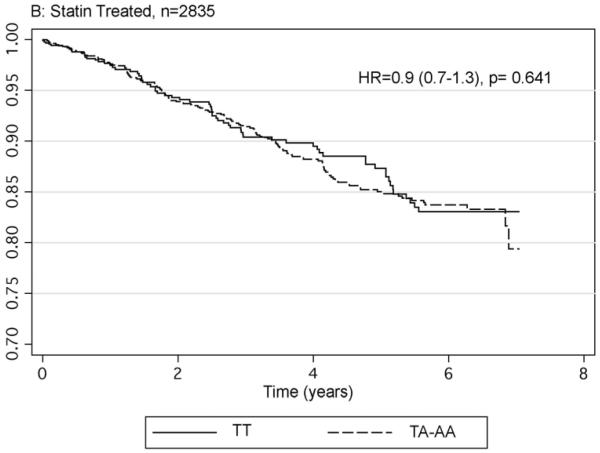
The impact of FTO genotype on cardiovascular morbidity and mortality was modelled by determining the relative hazard of possession of the A allele of rs9939609 for developing a fatal or non-fatal myocardial infarction following recruitment in the 4101 individuals with complete data. Age, gender, smoking status, prevalent myocardial infarction and having a history of statin and insulin use were included in the initial model. The model included an interaction term of genotype with statin use. During a mean follow-up of 3.6 years, there were a total of 324 events with 94 (6.82%) occurring in the 1377 TT homozygotes and 230 (8.44%) in the 2724 individuals with the A allele. The hazard ratio for possession of the A allele compared to TT homozygotes in this model was 2.36 CI 1.49-3.74, p=0.0002. With the subsequent inclusion of obesity related parameters ie. BMI, glycated haemoglobin, mean arterial pressure, HDL cholesterol, triglycerides and total cholesterol in the model the impact of FTO genotype was reduced but remained significant (HR 2.01 CI 1.18-3.45, p=0.011). As a result of the finding of a significant interaction term (p=0.001) for statin use by FTO genotype we stratified the data according to statin use. Figure 1 gives the Kaplan-Meier hazard functions for possession of the A allele compared to the TT homozygote in these two groups showing that the increased risk is seen in the 1266 non-statin users (HR 2.3 CI 1.4-3.7, p=0.0005), but is abolished by statin use (HR 0.9 CI 0.7-1.3 p=0.64).
Fig 1.
Hazard functions for fatal and non-fatal myocardial infarction by FTO genotype in those without satins (panel A) and those prescribed statins (panel B). Hazard ratios provided are adjusted for age, gender, BMI, smoking status and history of myocardial infarction
Discussion
We have demonstrated in this large prospective longitudinal study of patients with T2D that variation in the FTO gene is not only associated with increased BMI, but also with a specific dyslipidemic phenotype that is characteristic of insulin resistance. This in turn is associated with an increase in risk of myocardial infarction and cardiovascular death. These observations come from a population with established T2D, and the genotypic effect of the FTO variant on lipids is therefore over and above the dyslipidemic profile and cardiovascular risk we would expect in this population. This suggests that within the spectrum of phenotypic subtypes of T2D the FTO variant is associated with one that is marked by greater insulin resistance and at greater cardiovascular risk.
In determining metabolic phenotype we have been able to exploit the availability of multiple measures over time in Go-DARTS to determine a mean value for an individual and in this way accommodate the temporal fluctuation in single values that may obscure genuine associations. The risk allele for increased weight and T2D is associated with a lower plasma HDL cholesterol level, raised plasma triglycerides and therefore a raised atherogenic index, and as expected this is largely driven by the effects of FTO on body weight. Interestingly the FTO variant appears to be associated with overall fat mass in terms of both central abdominal obesity and also subcutaneous fat3 and therefore not associated with a body fat distribution typically associated with the insulin resistant state. On the other hand the clear association with the risk of development of T2D implicates insulin resistance. Furthermore, in a study of 1286 healthy individuals in whom diabetes had been excluded the FTO A allele was found to be associated with peripheral insulin resistance8 as measured by hyperinsulinemic euglycemic clamp. The insulin resistance however was again entirely dependent on body weight.
Carriers of the FTO A allele were over twice as likely to have a myocardial infarction or cardiovascular death than the TT homozygotes. The strength of this association appears to be reduced although remains significant by including obesity-related parameters in the model. This observation appears initially to be divergent from the association of FTO genotype with T2D, which is completely explained by the effect of the FTO genotype on BMI. In this case it is likely to be due to the correction of the obesity driven dyslipidemia in the individuals who receive statins, thus disconnecting obesity risk from cardiovascular risk. This is consistent with previous observations that, for a similar cholesterol reduction statins are more beneficial in patients who have a low HDL cholesterol and high triglyceride 16.
In this observational study the strong modulating influence of statin therapy with FTO genotype-associated outcome raises several important questions. We chose the simple metric of having had two prescriptions for a stain dispensed for pragmatic reasons given the known significant impact statins have on cardiovascular event rates, however there are many potential biases that could influence this metric. For example, we have demonstrated that the risk allele of FTO was associated with a significantly higher level of prescribing of statins in spite of no differences in cholesterol levels by genotype. However, no association was found between cholesterol levels and FTO genotype in either individuals prescribed statins or those not prescribed statins (data not shown) and thus the increased tendency to receive a statin seems unlikely to be due to this indication in those carrying the risk allele. This suggests that the increased statin prescribing may reflect other clinical aspects or risk associated with the obese phenotype which is supported by the finding that this allele was associated with significantly increased tendency to be prescribed other medications such as the fibrates and thiazolidinedines (data not shown). This observation may therefore further support the major finding of this study that FTO genotype is associated with a metabolic phenotype which in turn is associated with greater cardiovascular risk. Thus the observed impact of statins may be partly due to the fact that the more at risk individuals have a greater number of protective medications being prescribed. In such an observational study such potential biases are challenging to tease out and as such this finding would require to be replicated in a randomised prospective study of statin use. It would also be appropriate to consider replication of this finding in a non-diabetic population, however, given the assumed biological association of this FTO variant with outcome is through an engendered metabolic phenotype the event rate in the non-diabetic population is likely to be very low requiring very large numbers of individuals. This is a likely reason that FTO has not been a striking feature from GWAS of early-onset cardiovascular disease. At present the mechanism of action of the FTO variant on increasing weight gain and increased insulin resistance is uncertain. There are no features suggesting that rs9939609 is the causal variant and indeed there are many other variants that are in complete linkage disequilibrium with this variant and are therefore linked to obesity and diabetes. However, based on the rapid fall off of linkage disequilibrium with other SNPs beyond 47-kb it has been concluded that the functional variant is likely to lie within this area of the FTO gene3. Indeed rs8050136 has recently been implicated in modulating the binding of a transcription factor CUTL1, and CUTL1 knockdown by siRNA was shown to repress both FTO expression and expression of the neighbouring gene FTM17. This SNP lies 4kb from rs9939609 and these variants are in complete linkage disequilibrium in the Caucasian population (D’ of 1.00 and R2 of 0,996)3. Through the use of bioinformatics and recombinant functional studies it has recently been demonstrated that the FTO gene encodes a nucleic acid demethylase18,19. Although the physiologically relevant substrate for this enzyme has yet to be determined, expression appears to be highest in the brain and particularly in hypothalamic nuclei involved in energy balance18. Furthermore expression was found to be modulated by feeding and fasting, and correlated with the expression of various peptide hormones known to modulate eating behaviour including Neuropeptide Y20. The neighbouring gene FTM which encodes a ciliary protein, appears to be co-regulated with FTO in the arcuate nucleus and may also play a role in the observed phenotypes17. This molecular data supports direct observations from our own group that the eating behaviour of young children is modulated by FTO genotype21. Indeed the FTO genotype has now been associated with weight gain from the age of two weeks post birth22. This together with the present study clearly indicates that this genotype is having a significant role in human health from the cradle to the grave, and highlights recent concerns regarding the increased levels of childhood obesity and subsequent cardiovascular morbidity and mortality23.
Conclusion
We have demonstrated in a large group of patients with T2D that the A allele of rs9939609 in the FTO gene is associated not only with a raised BMI but also with a tendency to a dyslipidemic phenotype seen typically in the insulin resistance syndrome. This translates into an increased risk of myocardial infarction.
Prescription with statins appeared to ameliorate this association indicating that it is possible for intervention to prevent the serious life threatening consequences of this genotype.
Common variation in the FTO gene has recently emerged as a major determinant of human obesity. Obesity is in turn associated with an increased risk of developing Type 2 Diabetes with all its associated detriments to health. Recent evidence indicates that the FTO gene regulates obesity by modulating food preference, with a recent study showing that children, aged 7 to 9, eat 100 extra calories at a single meal when they carry a particular FTO gene variant. These extra calories come from preferentially eating more energy dense foods and may be mediated by differential regulation of hypothalamic gene expression by the protein encoded by FTO. In the current report, a group of patients being managed for Type 2 Diabetes who have inherited this same variant of the FTO gene that is associated with overeating are on average even more obese and have an unhealthier metabolic phenotype compared to patients not having this variant. This leads in turn to a greater risk of myocardial infarction and death. Therefore, this gene has an impact on individuals from early childhood through old age predisposing them to a greater risk of ill health probably largely through the eating habits it engenders. Interestingly we find that in patients who have been prescribed lipid lowering therapy in the form of statins this association is not evident. This suggests that variation in the FTO gene may contribute in the future to targeting specific therapies and interventions those individuals at greatest risk.
Acknowledgements
We would like to thank Alison Bell at the Health Informatics Centre, University of Dundee and Ritchie McAlpine of the Clinical Technology Centre, Ninewells hospital for their help in provision of the clinical data
Funding Sources
This work was funded by the Wellcome Trust, UK together with a local charitable grant from the TENOVUS foundation, UK
Footnotes
Disclosures
None
Doney et al The FTO gene and myocardial infarction
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control. 2002;13:325–32. doi: 10.1023/a:1015288615472. [DOI] [PubMed] [Google Scholar]
- 2.Li TY, Rana JS, Manson JE, Willett WC, Stampfer MJ, Colditz GA, Rexrode KM, Hu FB. Obesity as compared with physical activity in predicting risk of coronary heart disease in women. Circulation. 2006;113:499–506. doi: 10.1161/CIRCULATIONAHA.105.574087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–94. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orru M, Usala G, Dei M, Lai S, Maschio A, Busonero F, Mulas A, Ehret GB, Fink AA, Weder AB, Cooper RS, Galan P, Chakravarti A, Schlessinger D, Cao A, Lakatta E, Abecasis GR. Genome-Wide Association Scan Shows Genetic Variants in the FTO Gene Are Associated with Obesity-Related Traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dina C, Meyre D, Gallina S, Durand E, Korner A, Jacobson P, Carlsson LM, Kiess W, Vatin V, Lecoeur C, Delplanque J, Vaillant E, Pattou F, Ruiz J, Weill J, Levy-Marchal C, Horber F, Potoczna N, Hercberg S, Le Stunff C, Bougneres P, Kovacs P, Marre M, Balkau B, Cauchi S, Chevre JC, Froguel P. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet. 2007;39:724–6. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Wu Y, Loos RJ, Hu FB, Liu Y, Wang J, Yu Z, Lin X. Variants in FTO gene are not associated with obesity in a Chinese Han population. Diabetes. 2007 doi: 10.2337/db07-1130. [DOI] [PubMed] [Google Scholar]
- 7.Ohashi J, Naka I, Kimura R, Natsuhara K, Yamauchi T, Furusawa T, Nakazawa M, Ataka Y, Patarapotikul J, Nuchnoi P, Tokunaga K, Ishida T, Inaoka T, Matsumura Y, Ohtsuka R. FTO polymorphisms in oceanic populations. J Hum Genet. 2007 doi: 10.1007/s10038-007-0198-2. [DOI] [PubMed] [Google Scholar]
- 8.Pascoe L, Tura A, Patel SK, Ibrahim IM, Ferrannini E, Zeggini E, Weedon MN, Mari A, Hattersley AT, McCarthy MI, Frayling TM, Walker M. Common variants of the novel type 2 diabetes genes, CDKAL1 and HHEX/IDE, are associated with decreased pancreatic {beta}-cell function. Diabetes. 2007 doi: 10.2337/db07-0634. [DOI] [PubMed] [Google Scholar]
- 9.Doney AS, Fischer B, Leese G, Morris AD, Palmer CN. Cardiovascular risk in type 2 diabetes is associated with variation at the PPARG locus: a Go-DARTS study. Arterioscler Thromb Vasc Biol. 2004;24:2403–7. doi: 10.1161/01.ATV.0000147897.57527.e4. [DOI] [PubMed] [Google Scholar]
- 10.Doney AS, Lee S, Leese GP, Morris AD, Palmer CN. Increased cardiovascular morbidity and mortality in type 2 diabetes is associated with the glutathione S transferase theta-null genotype: a Go-DARTS study. Circulation. 2005;111:2927–34. doi: 10.1161/CIRCULATIONAHA.104.509224. [DOI] [PubMed] [Google Scholar]
- 11.Kimber CH, Doney AS, Pearson ER, McCarthy MI, Hattersley AT, Leese GP, Morris AD, Palmer CN. TCF7L2 in the Go-DARTS study: evidence for a gene dose effect on both diabetes susceptibility and control of glucose levels. Diabetologia. 2007;50:1186–91. doi: 10.1007/s00125-007-0661-9. [DOI] [PubMed] [Google Scholar]
- 12.Morris AD, Boyle DI, MacAlpine R, Emslie-Smith A, Jung RT, Newton RW, MacDonald TM, DARTS/MEMO Collaboration The diabetes audit and research in Tayside Scotland (DARTS) study: electronic record linkage to create a diabetes register. Bmj. 1997;315:524–8. doi: 10.1136/bmj.315.7107.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freathy RM, Timpson NJ, Lawlor DA, Pouta A, Ben-Shlomo Y, Ruokonen A, Ebrahim S, Shields B, Zeggini E, Weedon MN, Lindgren CM, Lango H, Melzer D, Ferrucci L, Paolisso G, Neville MJ, Karpe F, Palmer CN, Morris AD, Elliott P, Jarvelin MR, Smith GD, McCarthy MI, Hattersley AT, Frayling TM. Common variation in the FTO gene alters diabetes-related metabolic traits to the extent expected given its effect on BMI. Diabetes. 2008;57:1419–26. doi: 10.2337/db07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobiasova M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)) Clin Biochem. 2001;34:583–8. doi: 10.1016/s0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- 15.Dobiasova M. Atherogenic index of plasma [log(triglycerides/HDL-cholesterol)]: theoretical and practical implications. Clin Chem. 2004;50:1113–5. doi: 10.1373/clinchem.2004.033175. [DOI] [PubMed] [Google Scholar]
- 16.Ballantyne CM, Olsson AG, Cook TJ, Mercuri MF, Pedersen TR, Kjekshus J. Influence of low high-density lipoprotein cholesterol and elevated triglyceride on coronary heart disease events and response to simvastatin therapy in 4S. Circulation. 2001;104:3046–51. doi: 10.1161/hc5001.100624. [DOI] [PubMed] [Google Scholar]
- 17.Stratigopoulos G, Padilla S, Leduc CA, Watson E, Hattersley AT, McCarthy MI, Zeltser LM, Chung WK, Leibel RL. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol. 2008 doi: 10.1152/ajpregu.00839.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerken T, Girard CA, Tung YC, Webby CJ, Saudek V, Hewitson KS, Yeo GS, McDonough MA, Cunliffe S, McNeill LA, Galvanovskis J, Rorsman P, Robins P, Prieur X, Coll AP, Ma M, Jovanovic Z, Farooqi IS, Sedgwick B, Barroso I, Lindahl T, Ponting CP, Ashcroft FM, O’Rahilly S, Schofield CJ. The Obesity-Associated FTO Gene Encodes a 2-Oxoglutarate Dependent Nucleic Acid Demethylase. Science. 2007 doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez-Pulido L, Andrade-Navarro MA. The FTO (fat mass and obesity associated) gene codes for a novel member of the non-heme dioxygenase superfamily. BMC Biochem. 2007;8:23. doi: 10.1186/1471-2091-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredriksson R, Hagglund M, Olszewski PK, Stephansson O, Jacobsson JA, Olszewska AM, Levine AS, Lindblom J, Schioth HB. The obesity gene, FTO, is of ancient origin, upregulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology. 2008 doi: 10.1210/en.2007-1457. [DOI] [PubMed] [Google Scholar]
- 21.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2558–66. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Bermejo A, Petry CJ, Diaz M, Sebastiani G, de Zegher F, Dunger DB, Ibanez L. The Association Between the FTO Gene and Fat Mass in Humans Develops by the Postnatal Age of Two Weeks. J Clin Endocrinol Metab. 2008 doi: 10.1210/jc.2007-2343. [DOI] [PubMed] [Google Scholar]
- 23.Ludwig DS. Childhood obesity--the shape of things to come. N Engl J Med. 2007;357:2325–7. doi: 10.1056/NEJMp0706538. [DOI] [PubMed] [Google Scholar]