Abstract
Although the antimalarial agent, artemisinin itself is not active against tuberculosis, conjugation to a mycobacterial specific siderophore (microbial iron chelator) analog induces significant and selective anti-tuberculosis activity, including activity against MDR and XDR strains of Mtb. The conjugate also retains potent antimalarial activity. Physicochemical and whole cell studies indicate that ferric to ferrous reduction of the iron complex of the conjugate initiates the expected bactericidal Fenton-type radical chemistry on the artemisinin component. Thus, this “Trojan Horse” approach demonstrates that new pathogen selective therapeutic agents can be generated in which the iron component of the delivery vehicle also participates in triggering the antibiotic activity. The result is that one appropriate conjugate has potent and selective activity against two of the most deadly diseases in the world.
Tuberculosis (TB) and malaria are the two most widespread and lethal infectious diseases in the world.1 Approximately one-third of the world’s population is presently infected with Mycobacterium tuberculosis (Mtb), the causative pathogen of TB, and 40% is affected by malaria. Each of these diseases causes about two million deaths worldwide every year.2 The development and spread of multidrug resistant (MDR) and extensively drug resistant (XDR) strains of Mtb have stimulated research efforts globally.3 As a consequence, the pipeline of potential new drugs has expanded,4,5,6 but no effective new antiTB drug has been marketed in decades.7 Plasmodium falciparum parasites that cause malaria also have become resistant to standard antimalarial drugs like quinine and chloroquine.8 Current chemotherapeutic treatment of both malaria and tuberculosis are inadequate. New leads for drug development are urgently needed. Herein, we report that an artemisinin conjugate (3; Fig. 1) of a mycobactin T analog not only retains antimalarial activity similar to that of artemisinin itself, but is exquisitely microbe selective and has remarkably potent activity against Mtb.
Figure 1.
A. Conjugate design. B. Synthesis, anti-Mtb and anti-malaria activity of mycobactin-artemisinin conjugate 3.
Artemisinin (1, also called qinghaosu) is a natural peroxide-containing sesquiterpene based on 1,2,4-trioxane, which is a highly active and relatively non-toxic antimalarial agent.9 Trioxane derivatives, dihydroartemisinin, artemether, and water-soluble sodium artesunate have been studied extensively, are widely used and efforts continue towards the development of efficient methods for their preparation.10 Mode of action studies indicate that ferrous iron [in the form of heme or iron(II) salts] triggers reductive cleavage of the peroxide bond in artemisinin to form oxygen-centered radicals. The oxy radicals then form carbon-centered radicals that ultimately lead to death of the malaria parasites. Related mechanistic work has been used to rationally design new antimalarial peroxides,11 including dimeric versions.12 Similarly, in cancer-related therapeutic applications, iron released from transferrin can react with artemisinin bound to a transferrin-receptor targeting peptide to generate free radicals that can kill cells.13 Since cancer cells overexpress transferrin receptors for iron uptake, the artemisinin-peptide conjugates and transferrin-artemisinin conjugates are selective and potent anticancer agents.14
Virulence of Mtb depends on its ability to assimilate iron and, like most microbes, it synthesizes and utilizes siderophores, low molecular weight iron chelators, to sequester iron.15 Based on natural and synthetic examples of siderophore-antibiotic conjugates that enable active transport of drugs into specifically targeted bacteria,16 we anticipated that, while artemisinin itself has no antituberculosis activity, attachment of an artemisinin derivative to an analog of mycobactin T (2),17 a Mtb siderophore critical for growth under iron restriction, might allow the peroxide drug to be actively assimilated by the pathogen. Subsequent reduction of the bound ferric ion to its lower affinity ferrous form might then induce Fenton-like chemistry from interaction of the ferrous iron with the nearby peroxide. The consequent generation of free radicals is then expected to cause intracellular damage selectively to the assimilating bacteria.18 To test this hypothesis, we synthesized an artemisinin conjugate (3; Fig. 1) of a mycobactin T analog and found that it not only retains antimalarial activity similar to that of artemisinin itself (Table 1), but is exquisitely microbe selective and has remarkably potent activity against Mtb (Table 2). The mycobactin-arteminisin conjugate is not only very active against H37Rv Mtb (MIC = 0.39 μg/mL), but also against both MDR (MIC’s = 0.16–1.25 μg/mL) and XDR Mtb (MIC’s = 0.078–0.625 μg/mL, Tables 2 and 3).19 Moreover, as described below, it is selectively active against Mtb relative to fast growing species of mycobacteria and a broad set of other Gram-positive and Gram-negative bacteria. Physicochemical and whole cell studies indicate that ferric to ferrous reduction of the iron complex of 3 does initiate the expected lethal radical producing Fenton chemistry on the artemisinin component.
Table 1.
Activity of conjugate 3 against Plasmodium falciparum strains [IC50 in μg/mL].
| Strain | HB3 | 7G8 | 3D7 | Dd2 |
|---|---|---|---|---|
| Conjugate 3 | 0.0047 | 0.0051 | 0.0040 | 0.0041 |
| Artemisinin | 0.0013 | 0.0004 | 0.0036 | 0.0022 |
Abbreviations: HB3=Honduras (chloroquine sensitive, low-level pyrimethamine-resistant), Dd2=Indochina (multi-drug resistant), 7G8=Brazil (low level chloroquine resistant), 3D7=Airport (sensitive to sulfadoxine).
Table 2.
Activity of conjugate 3 against eight different Mtb multi-drug resistant (MDR) clinical strains19 (MIC in μg/mL).
| Strain 1 HRESP |
Strain 2 HREZSP |
Strain 3 HCPTTh |
Strain 4 HREKP* |
Strain 5 HRERb |
Strain 6 HREZ SKPTh |
Strain 7 HREZRb Th |
|---|---|---|---|---|---|---|
| 1.25 | 0.625 | 0.625 | 0.625 | 0.3125 0.156* 1.25* |
0.625 | 0.3125 |
The Mtb strains are subsets of those previously reported.19
Table 3.
Activity of conjugate 3 against Mtb extensively drug resistant (XDR) strains19 and M. smegmatis (MIC in μg/mL).
| Strain 8 HRESPOCTh |
Strain 9 HREPKOTh |
Strain 10 HRESPO |
Strain 11 HRESPOCTh |
M. smegmatis |
|---|---|---|---|---|
| 0.31 | 0.078 | 0.625 | 0.31 | >5 |
Abbreviations for Tables 2&3: Resistance to: H=isoniazid, R=rifampicin, E=ethambutol, Z=pyrazinamide, S=streptomycin, C=cycloserine, T=ethionamide, K=kanamycin, P=p-aminosalicylic acid, Rb=rifabutin, Th=thioacetazone, O=ofloxacin.
The synthesis of mycobactin-artemisinin conjugate 3 is summarized in Figure 1. Since mycobactin T has no functional group for use in linking to the artemisinin component, we resynthesized our previously reported mycobactin analog 5,20 which incorporates a diaminopropionic acid as a replacement for the natural β-hydroxybutyrate. Artemisinin is remarkably amenable to chemical modification despite containing inherently sensitive functionality. Thus, a sequence analogous to previous reports21 gave N-hydroxysuccinimide (NHS) active ester 4. Treatment of mycobactin analog 5 with trifluoroacetic acid to remove the Boc group followed by neutralization and direct treatment with 4 gave the desired conjugate 3. (Supporting Information).
As indicated, conjugate 3 not only retained efficacy against Plasmodium falciparum (Table 1) but also was remarkably potent against Mtb H37Rv [MIC 0.338 μM = 0.39 μg/mL], while artemisinin (1) itself and all synthetic intermediates to active ester 4 (Supporting Information) had no activity against Mtb. Further studies revealed that conjugate 3 was similarly potent against MDR and XDR strains of Mtb (Tables 2 and 3). Thus, by using a mycobacterial siderophore to deliver an artemisinin derivative and provide a source of reactive iron to Mtb we have retained its antimalarial activity and enabled it to be a potent antitubercularculosis agent.
Conjugate 3 was not active against a broad set of bacteria at the highest levels tested (2 mM), including Gram positive bacteria (Bacillus subtilis ATCC 6633 and Staphylococcus aureus SG 511) and Gram negative bacteria (Serratia marcescens SG 621, Klebsiella pneumoniae ATCC 10031, Escherichia coli ATCC 25922, E. coli DC0, E. coli DC2, a permeability mutant, P. aeruginosa KW 799/WT, P. aeruginosa KW 799/61, a permeability mutant, P. aeruginosa SG 137, P. aeruginosa ATCC 27853 and Stenotrophomonas maltophilia GN 12873, Supporting Information). As a further indication of the unique antimalarial and antiTB selectivity of 3, it was tested and found to have negligible activity against a number of fast growing strains of mycobacteria [M. vaccae IMET 10670 MIC = 50 μg/mL (43 μM), M. smegmatis SG987 MIC = 200 μg/mL (173 μM), M. aurum SB66 MIC = 12.5 μg/mL (43.3 μM), M. fortuitum B MIC = 100 μg/mL, (87 μM) Supporting Information]. Thus, the antibiotic activity of conjugate 3 is microbe selective, as anticipated.
In order to determine if the remarkable activity of 3 depends on its ability to bind iron, we synthesized and tested the activity of a derivative (6) in which the iron binding hydroxamates were protected. This modification reduced antimalarial activity by >20 to 100 fold. The activity against all strains of Mtb was completely lost (>12.5 μM). To determine the effect of conjugation to a different siderophore, we prepared an artemisinin conjugate (7) of desferrioxiamine (DFO), a natural siderophore derived from Streptomyces pilosus that is therapeutically used for treatment of iron overload and also has efficacy for revival of patients in malaria-induced comas.22 We also synthesized a DFO derivative (8) in which all three of the hydroxamates were acetylated to negate iron binding. While with IC50 values ranging from 0.16 to >2.2 μM, both of the DFO-derived conjugates were more active against P. falciparum than DFO itself, but much less active than conjugate 3 or artemisinin and they were completely inactive against Mtb, since Mtb probably has no uptake route for the siderophore (DFO) based on studies with M. smegmatis.23
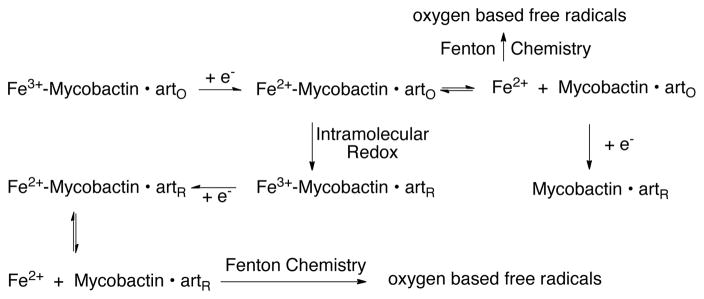
Since our intent was to use the mycobactin to deliver iron and the redox sensitive agent, artemisinin, to Mtb and induce damaging Fenton chemistry during the attempt of the pathogen to reductively assimilate the iron, we studied the related physicochemical properties of the conjugate (3). A cyclic voltammogram for commercially available iron loaded mycobactin J, used as a control, is shown in Figure 3. The strongly negative cathodic peak current is consistent with that observed for other high iron affinity siderophore complexes.24 The absence of a return oxidation wave is indicative of an irreversible Fe3+/Fe2+ couple, with dissociation of the labile ferrous ion subsequent to reduction. A cyclic voltammogram for the iron-loaded mycobactin-artemisinin conjugate (3) is also shown in Figure 3, along with that of artemisinin alone. Both voltammograms show irreversible behavior, consistent with the literature report for artemisinin.25 The iron loaded conjugate shows a shoulder and a main peak, which we assign to the Fe-mycobactin and artemisinin components, respectively. The Fe-mycobactin component in the conjugate is shifted negative ~100 mV relative to the control Fe-mycobactin J and the peak current is increased from 7 μA/mM to 18 μA/mM. The main peak attributable to the artemisinin component of the conjugate is shifted slightly more negative and the peak current increased from 9 μA/mM to 19 μA/mM. These results suggest that the combination of Fe-loaded mycobactin and artemisinin within the conjugate results in a chemical interaction between the two redox active components, apparently subsequent to the reduction of the Fe3+ to Fe2+ in the mycobactin binding site. This is consistent with the increased currents, suggesting a chemical event subsequent to the electrochemical reduction event (i.e. a modified EC process). We hypothesize that reduction of the bound ferric iron to its ferrous form induces Fenton-like chemistry from the interaction of the ferrous iron with the peroxide linkage of the artemisinin leading to the reduction and cleavage of the peroxo-bridge. This correlates with the biological activity observed for this complex, since this latter reduction would generate free radicals that would be expected to cause intracellular damage. We propose the coupled electrochemical/chemical processes also shown in Scheme 1 are consistent with our observations (where arto and artR represent the artemisinin component of the conjugate in the oxidized and reduced form, respectively). This scheme illustrates an initial reduction of conjugate bound Fe3+, followed by dissociation of the labile Fe2+ and/or intramolecular redox reaction with the artemisinin component and regeneration of Fe3+ with subsequent electrochemical reduction and dissociation of Fe2+. The free Feaq2+ can generate toxic reactive oxygen species by Fenton-type reactions as discussed earlier.
Figure 3.

Cyclic Voltammograms of Fe-loaded mycobactin J, artemisinin, and the Fe-loaded mycobactin-artemisinin conjugate. Conditions: glassy carbon working electrode, Pt wire auxiliary electrode, Ag/AgCl reference electrode, scan rate = 10 mV/s, [NaClO4] = 100 mM as background electrolyte in 95% EtOH. [Fe-mycobactin J] = 3 mM, Ecp = −856 mV vs. NHE (7 μA/mM), [artemisinin] = 3mM, Ecp = −1063 mV vs. NHE (9 μA/mM), [Fe-mycobactin-artemisinin conjugate] = 1.625 mM, Ecp = −955 mV vs. NHE (18 μA/mM), Ecp = −1090 mV vs. NHE (19 μA/mM).
Scheme 1.
Proposed reaction for the mycobactin-artemisinin conjugate 3.
To verify that conjugate 3 fueled the formation of hydroxyl radicals in Mtb, we exposed washed drug-treated cells to the dye hydroxyphenyl fluorescein, which is oxidized by hydroxyl radicals to yield a fluorescent derivative.26 Conjugate 3 resulted in hydroxyl radical formation within 3 h of treatment at 1X and 10X MIC (Figure 4). Rifampicin, a transcriptional inhibitor and a bactericidal anti-TB drug,27 showed more than two-fold lower levels of hydroxyl radical release (Figure 4).
Figure 4.
Treatment of M. tuberculosis with mycobactin-artemisinin conjugate 3 generates reactive oxygen species. H37Rv was treated with rifampicin (0.1 μg/mL and 1 μg/mL) or the mycobactin-artemisinin conjugate (3, 0.5 μg/mL and 5 μg/mL) at 1× and 10× the MIC. Following 3 h incubation, reactive oxygen species were measured with HPF. M. tuberculosis treated with DMSO was used as a vehicle control. Shown is a representative graph from at least three independent experiments performed in triplicate. The means ± standard deviations are displayed.
In conclusion, we have demonstrated that a conjugate of the antimalarial agent and a mycobacterial siderophore analog retains the antimalarial activity while also inducing selective potent activity against Mtb. In contrast to other siderophore-based Trojan horse antibiotics,16,28,29 the unique activity of 3 depends on active participation of the microbe selective siderophore and the chemistry of both the iron and drug (artemisinin) being transported.
Supplementary Material
Figure 2.
Protected mycobactin conjugate (6) and (DFO) (7) and triacetyl protected DFO (8) conjugates.
Acknowledgments
M.J.M. and M.T.F. gratefully acknowledge support from the US National Institutes of Health (grant numbers R01 AI054193 and AI055035). A.L.C. thanks the NSF (CHE 0809466) for financial support. This research was supported in part by the Intramural Research Program of the NIH, NIAID.
Footnotes
Supporting Information Available: Experimental procedures, syntheses of conjugate 3 and control compounds as well as details of all microbiological and biological studies. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Hurtley S, Ash C, Roberts L. Science. 2010;328:841. doi: 10.1126/science.328.5980.841. [DOI] [PubMed] [Google Scholar]
- 2.Snider DE, Jr, Raviglione M, Kochi A. In: Global Burden of Tuberculosis, Tuberculosis: Pathogenesis, Protection, and Control. Bloom Barry., editor. ASM Press; Washington, D.C: 1994. p. 3. Also see: WHO, Web Site, http://www.who.int/gtb/publications/factsheet/index.htm and links for an overview of tuberculosis worldwide. [Google Scholar]
- 3.Dorman SE, Chaisson RE. Nature Medicine. 2007;13:295–298. doi: 10.1038/nm0307-295. [DOI] [PubMed] [Google Scholar]
- 4.Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, Langhorne MH, Anderson SW, Towell JA, Yuan Y, McMurray DN, Kreiswirth BN, Barry CE, Baker WR. Nature. 2000;405:962–966. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK. Antimicrob Agents Chemother. 2009;53:3720–3725. doi: 10.1128/AAC.00106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makarov V, Manin G, Mikusova K, Möllmann U, et al. Science. 2009;324:801–804. doi: 10.1126/science.1171583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harper C. Nature Medicine. 2007;13:309–312. doi: 10.1038/nm0307-309. [DOI] [PubMed] [Google Scholar]
- 8.Winstanley PA, Ward SA, Snow RW. Microbes Infect. 2002;4:157–164. doi: 10.1016/s1286-4579(01)01523-4. [DOI] [PubMed] [Google Scholar]
- 9.Devdutt C, Abhishek G, Partha PS, Nabin CB, Paruchuri GR. Chem Soc Rev. 2010;39:435–454. [Google Scholar]
- 10.Richmond S, Keasling JD. Nature. 2006;440:940–943. [Google Scholar]
- 11.Posner GH, O’Neill PM. Acc Chem Res. 2004;37:397–404. doi: 10.1021/ar020227u. [DOI] [PubMed] [Google Scholar]
- 12.Rosenthal AS, Chen X, Jun O, Liu JO, West DC, Hergenrother PJ, Shapiro TA, Posner GH. J Med Chem. 2009;52:1198–1203. doi: 10.1021/jm801484v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai H, Sasaki T, Singh NP. Expert Opin Ther Targets. 2005;9:995–1007. doi: 10.1517/14728222.9.5.995. [DOI] [PubMed] [Google Scholar]
- 14.Oh O, Kim BJ, Singh NP, Lai H, Tomikazu Sasaki T. Cancer Lett. 2009;274:33–39. doi: 10.1016/j.canlet.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 15.Ratledge C. Tuberculosis. 2004;84:110–130. doi: 10.1016/j.tube.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Roosenberg JM, II, Lin YM, Lu Y, Miller MJ. Curr Med Chem. 2000;7:159–197. doi: 10.2174/0929867003375353. [DOI] [PubMed] [Google Scholar]
- 17.Vergne AF, Walz AJ, Miller MJ. Nat Prod Rep. 2000;17:99–116. doi: 10.1039/a809397k. [DOI] [PubMed] [Google Scholar]
- 18.For a discussion on the potential of bioreduction to generate reactive intermediates in the development of alternative antituberculosis agents based on aromatic nitro reduction, see: Goldman RC. Future Med Chem. 2010;2:1253. doi: 10.4155/fmc.10.215.
- 19.For relevant strains and assays see: Jeon CY, Hwang SH, Min JH, Prevots DR, Goldfeder LC, Lee H, Eum SY, Jeon DS, Kang HS, Kim JH, Kim BJ, Kim DY, Holland SM, Park SK, Cho SN, Barry CE, 3rd, Via LE. Clin Infect Dis. 2008;46:42–49. doi: 10.1086/524017.
- 20.Xu Y, Miller MJ. J Org Chem. 1998;63:4314–4322. [Google Scholar]
- 21.Kim BJ, Sasaki T. J Org Chem. 2004;69:3242–3244. doi: 10.1021/jo0498765. [DOI] [PubMed] [Google Scholar]
- 22.Mabeza GF, Biemba G, Gordeuk VR. Acta Haematol. 1996;95:78–86. doi: 10.1159/000203953. [DOI] [PubMed] [Google Scholar]
- 23.Schumann G, Möllmann U. Antimicrob Agents Chemother. 2001;45:1317–1322. doi: 10.1128/AAC.45.5.1317-1322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crumbliss A, Harrington JM. Adv Inorg Chem. 2009;61:179–250. [Google Scholar]
- 25.Zhang F, Gosser DK, Meshnick SR. Biochemical Pharmacol. 1992;43:1805–1809. doi: 10.1016/0006-2952(92)90713-s. [DOI] [PubMed] [Google Scholar]
- 26.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 27.Buda NR, Lee RE, Meibohm B. Current Med Chem. 2008;15:809–825. doi: 10.2174/092986708783955509. [DOI] [PubMed] [Google Scholar]
- 28.Hider RC, Kong X. Natural Prod Rep. 2010;27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 29.Miethke M, Marahiel MA. Microb Mol Biol Rev. 2007;71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.