Abstract
Purpose of Review
Although latent HIV-1 infection in CD4+ T cells contributes to HIV persistence, there is mounting evidence that other viral reservoirs exist. Here, we review recent data suggesting that the infection of hematopoietic progenitor cells creates additional reservoirs for HIV in vivo.
Recent Findings
New studies suggest that some types of hematopoietic progenitor cells have the potential to generate reservoirs for HIV. This review focuses on two types that can be infected by HIV in vitro and in vivo: multipotent hematopoietic progenitor cells in the bone marrow and circulating mast cell progenitors. Of these two types, only CD34+ bone marrow cells have been shown to harbor latent provirus in HIV+ individuals with undetectable viral loads on HAART. Latent infection of these long-lived cell types may create a significant barrier to HIV eradication; indeed the potential infection of hematopoietic stem cells in particular could lead to an HIV reservoir that does not appreciably decay over the lifespan of the host.
Summary
To eradicate HIV infection, it will be necessary to purge all viral reservoirs in the host. The findings highlighted here suggest that multipotent hematopoietic progenitor cells and possibly tissue mast cells may constitute significant reservoirs for HIV that must be addressed in order to eliminate HIV infection. Future studies are needed to determine which types of CD34+ cells are infected in vivo and whether infected CD34+ cells contribute to residual viremia in people with undetectable viral loads on HAART.
Keywords: Hematopoietic progenitor cells, mast cells, HIV reservoirs, latent infection
Introduction
Reservoirs of latent HIV-1 infection represent a barrier to the eradication of the disease. Although latently infected resting CD4+ T cells are clearly an important viral reservoir, there is increasing evidence that resting T cells are not the only reservoir of HIV [1,2*,3*]. Recently, several studies have demonstrated that three subsets of hematopoietic precursor cells can become infected with HIV: multipotent hematopoietic progenitor cells (HPCs) [4**], mast cell progenitors [5], and monocytes (reviewed in [6]). As the infection of monocytes is important in the spread of HIV to the central nervous system, a topic addressed later this issue, these cells will not be discussed here. Instead, this review will focus on the infection of multipotent HPCs and progenitor mast cells, each of which has a unique potential to generate a long-lived reservoir of HIV. We will discuss the evidence for infection of multipotent HPCs and progenitor mast cells as well as the role that infection of these cells may play in HIV persistence.
HIV infection of multipotent HPCs
Studies of HIV infection in HPCs have focused on several cell populations. Many studies have examined infection in cells expressing CD34, a cell-surface marker found on many types of HPCs ranging from hematopoietic stem cells (HSCs) with extensive self-renewal capacity to progenitor cells committed to differentiation [7]. Other studies have examined the CD34+, CD133+ population, which is enriched for multipotent progenitor cells [7,8]. Finally, some studies have used in vitro colony-forming assays to focus on multipotent cells. Only multipotent cells – HSCs, multipotent progenitor cells (MPPs), and common myeloid progenitor cells (CMPs) – are capable of forming colonies with representatives from all myeloid lineages; thus colony-forming assays allow multipotent cells to be functionally defined [7]. Colony-forming assays do not allow lymphoid cells to grow, however, and thus HSCs and MPPs cannot be distinguished from CMPs with this assay. The surface markers and self-renewal capacity of CD34+ HPCs at different stages of differentiation are summarized in Figure 1.
Figure 1. Hematopoiesis.
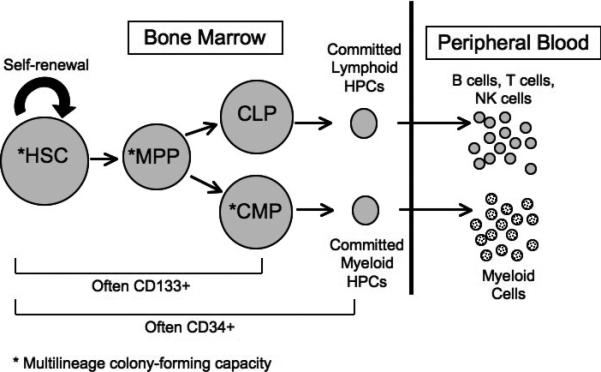
Hematopoietic stem cells (HSCs) with extensive self-renewal capacity give rise to multipotent progenitor cells (MPPs), which can in turn differentiate into committed lymphoid progenitors (CLPs) or committed myeloid progenitors (CMPs). These cells further differentiate into committed progenitors and eventually mature blood cells. *Indicates cells capable of forming multilineage colonies in methylcellulose media (methylcellulose does not support the growth of lymphoid cells).
Several studies have reported that a proportion of CD34+ cells express the HIV receptors CD4, CXCR4, and CCR5, making these cells potentially susceptible to HIV-1 infection (reviewed in [6]). Beginning more than twenty years ago, multiple studies suggested that infection in CD34+ cells was possible, though rare, both in vitro and in vivo [9-15]; however, these studies could not rule out contamination by other cell types. Furthermore, studies assessing HIV-1 infection of multipotent colony-forming or CD133+ HPCs failed to detect either HIV-1 infection or expression of any of the three main HIV receptors in these cells [16-19].
Based on these reports, the consensus has been that CD34+ cells are not an important target of HIV-1 infection and that HIV cannot infect multipotent HPCs at all. Recently, however, improved techniques have permitted reexamination of this topic, and this reexamination has unambiguously shown that a percentage of immature, multipotent HPCs are susceptible to HIV infection. First, a 2007 study investigated the ability of HIV-1 subtype C to infect multipotent CD34+ HPCs in vitro and in vivo [20**]. While the authors could not detect HIV-1 subtype B infection in HPCs capable of forming multilineage colonies, they found that several isolates of HIV-1C could infect multipotent cells. Furthermore, the authors were able to detect HIV proviruses in CD34+ cells from the peripheral blood of 12 out of 19 donors infected with HIV-1C; importantly, the level of HIV detected in 11/12 of these CD34+ samples was greater than the level observed in total peripheral blood mononuclear cells from the same patient, eliminating the potential for contamination that plagued earlier studies [20**]. The authors thus suggest that although HIV-1B may be unable to infect multipotent HPCs, HIV-1C faces no such barrier to infection.
While this study showed that HIV-1C infects multipotent HPCs, the ability of HIV-1B to infect multipotent HPCs remained ambiguous. The authors concluded that HIV-1B could not infect multipotent HPCs because they could not detect HIV DNA in multilineage colonies generated from HPCs exposed to HIV-1B isolates. However, the absence of HIV+ colonies could instead indicate that although HIV-1B can infect multipotent cells, the infection is cytotoxic either immediately or upon proliferation and differentiation of the cells, leading to cell death rather than infected colony formation.
We undertook a study to definitively assess whether HIV-1B could infect multipotent HPCs [4**]. Using a flow cytometric assay to detect the expression of HIV proteins in individual CD34+ cells after very short incubation periods (three days), we found that a variety of human immunodeficiency viruses, including several HIV-1B isolates, could infect CD34+ cells derived from bone marrow or umbilical cord blood [4**]. Because HIV-1B was cytopathic for the cells, however, the number of infected cells declined dramatically over time. We furthermore showed that exposure of CD34+ cells to a non-cytotoxic, GFP-expressing HIV-1B viral construct permitted the formation of multilineage colonies that were uniformly GFP+, demonstrating that HIV-1B envelopes can target multipotent HPCs in vitro [4**].
We next examined whether CD34+ HPCs could harbor latent as well as active HIV-1, a distinction that had not previously been assessed. As noted above, the initially robust infection in CD34+ HPCs declined over time until active infection could no longer be observed [4**]. If these cells were exposed to agents that stimulated myeloid differentiation, however, we observed a resurgence of viral gene expression [4**]. This observation was most notable with a dual-tropic virus that could efficiently spread the resurging infection to the differentiating myeloid cells [4**]. We also created a novel HIV latency probe that, in addition to expressing HIV proteins under the control of the viral LTR, expresses GFP under the control of the constitutively active spleen focus-forming virus (SFFV) promoter. When this construct was used to infect HPCs, we could visualize a population of GFP+, HIV Gag-, latently infected cells that was stable for at least 20 days in culture [4**]. Together, these data demonstrate that latent HIV-1 infection of HPCs is possible in vitro.
Finally, our study assessed the infection of CD34+ bone marrow HPCs in HIV-infected patients. In a sample of HIV+ individuals with high viral loads (>50,000 copies HIV-1 RNA/mL), we could directly detect HIV Gag expression in CD34+, CD133+ cells from a subset of donors [4**]. In the remaining donors in this high viral load cohort, we observed HIV Gag expression when we stimulated the CD34+ cells with cytokines to induce myeloid differentiation, thus providing evidence that latent HIV infection occurs in CD34+ cells in vivo [4**]. As expected, some CD34-depleted bone marrow cells also expressed Gag initially. However, these cells rapidly died under our culture conditions, which were optimized for differentiating CD34+ cells. The ability to specifically propagate cells derived from CD34+ cells substantially reduced the possibility that contaminating cell types were confounding our results[4**].
We also looked for HIV-1 proviral DNA in CD34+ cells from a group of HIV-positive individuals on HARRT with clinically undetectable (<50 copies/mL) viral loads. As expected, these individuals had no evidence of active infection in their CD34+ cells [4**]. However, we detected HIV-1 DNA in CD34+ cells from more than 40% of these donors; in all cases, we could not detect comparable amounts of HIV DNA from bone marrow depleted for CD34, indicating that infected CD34- bone marrow cells do not persist at similar levels in people on HAART [4**]. The sensitivity of this assay was low – we could detect HIV genomes in CD34+ cells only if at least 1 in 10,000 cells harbored an HIV genome. This limit of detection is higher than the frequency of integrated genomes that have been observed in the resting CD4+ T cell reservoir in some patients [21]; thus, it is possible that other patients in our cohort harbor HIV-infected CD34+ cells at a lower frequency. The ability of CD34+ cells to harbor latent HIV in vivo, even in patients undergoing successful HAART treatment, demonstrates that CD34+ cells can act as a long-lived reservoir of HIV. Additional studies are now needed to determine which type of CD34+ cells harbor HIV genomes in vivo and whether latently infected CD34+ cells contribute to residual viremia in patients on HAART.
HIV infection and hematologic abnormalities
In addition to creating a latent viral reservoir, HIV infection of CD34+ cells might lead to HPC death and hematopoietic abnormalities. Consistent with this possibility, many studies have reported hematopoietic defects associated with HIV [22-30], including the depletion of CD34+ bone marrow cells [31,32]. The existence of HIV-associated hematologic abnormalities is well known and has been attributed to a variety of factors, including altered stromal cytokine production [32] and use of specific antiretrovirals [33]. Recently, however, Redd and colleagues were able to show a direct association between infection of CD34+ cells and anemia in their cohort [20**]. This evidence suggests that in addition to creating a latent reservoir of virus, HIV-1 infection of HPCs can cause HPC death and hematopoietic defects.
A model for infection of multipotent HPCs
Although HIV-1 infection of hematopoietic stem cells has not been directly assessed, the presence of infected CD34+ cells even in individuals on successful HAART treatment suggests that at least some infected CD34+ cells are sufficiently long-lived to create a significant barrier to HIV eradication. Based on this finding and the studies described above, we have developed the following model describing HIV infection of HPCs in vivo (Figure 2). When multipotent HPCs become infected with HIV, there are two possible outcomes: either an active infection leads to HPC death, contributing to the HIV-associated hematologic abnormalities described above, or a latent infection occurs. If latent infection occurs in a cell with self-renewal capacity, continued self-renewal can generate a long-lived reservoir of latent HIV. If a latently infected daughter cell is stimulated to differentiate, however, the latent virus reactivates, leading to the death of the cell and further contributing to hematologic dysfunction. In this model, viral reactivation also results in virion release, contributing to the low-level viremia observed even in HAART-treated patients.
Figure 2. Model of HIV-1 infection in HPCs.
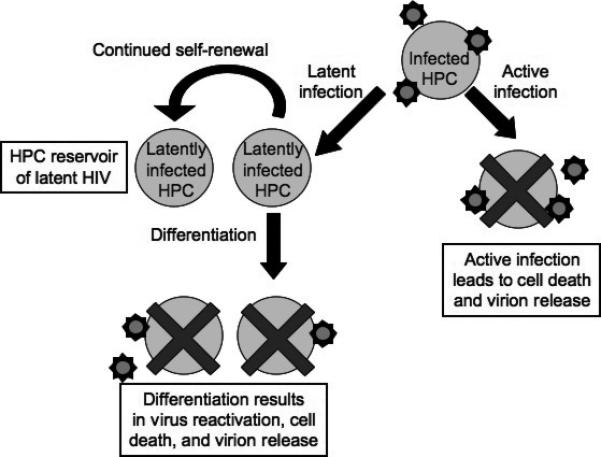
Multipotent HPCs can become infected with HIV-1, leading either to active infection, cell death, and virion release, or to latent infection. Latently infected HPCs with self-renewal capacity will then continue to self-renew, generating an expanded reservoir of latent HIV-1 in these cells. If the host cell is stimulated to differentiate, the latent virus reactivates, leading to cell death and virion release.
This model would seem to suggest that defective HIV-1 proviruses incapable of reactivation should be detectable in multiple hematopoietic lineages that cannot be infected by HIV-1. The presence of such defective proviruses in CD8+ T lymphocytes and granulocytes has been observed in one patient [34]; however, a second study detected only minimal integration of HIV genomes in naïve CD8+ cells from patients and could not rule out contamination by other cell types [35]. Additional studies are required to determine whether defective HIV-1 genomes can be observed in CD8+ T cells and other hematopoietic lineages in multiple patients.
Mast cell progenitor infection
In addition to multipotent HPCs, committed mast cell progenitors (prMCs) are also susceptible to HIV infection [5]. Mast cells are tissue-resident immune cells important in allergy, inflammation, and helminthic infection [36,37]. Although HIV-1 cannot infect mature mast cells [38*], HIV-1 can infect prMCs in peripheral blood [5]. As prMCs eventually migrate to tissues, where they survive for at least ten months [39,40], infection of prMCs could create a relatively short-term reservoir of HIV-1 infection (Figure 3).
Figure 3. Current model of HIV-1 infection in mast cell progenitors (prMC) and the creation of a stable reservoir of infected tissue mast cells.
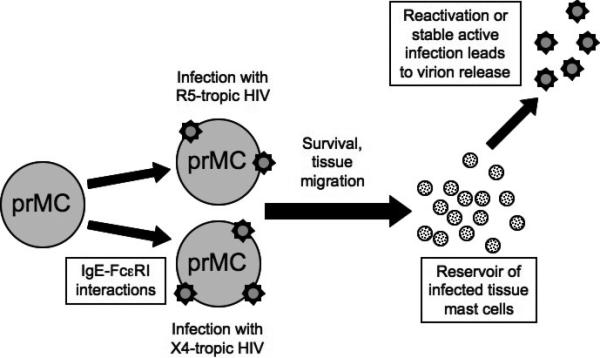
Circulating mast cell progenitors can become infected with CCR5-tropic HIV-1 or, following interactions with IgE, CXCR4-tropic HIV-1 as well. The progenitor mast cells then mature into tissue mast cells that are resistant to new HIV-1 infection. These mast cells may harbor latent HIV-1 that can later be reactivated, or they may be able to continuously produce HIV-1 due to the virus's reduced cytotoxicity in this cell type. In either case, a viral reservoir in tissue mast cells is created and can contribute to viral persistence.
Recently, placental tissue mast cells were shown to harbor latent HIV in vivo in pregnant, HAART-treated women with detectable viral loads [38*], suggesting that infection of prMCs creates a reservoir in mast cells of patients with ongoing viral replication. However, a recent study could not detect active HIV-1 replication in tissue mast cells from ten donors [41*], suggesting that actively infected mast cells are rapidly cleared in successfully treated patients even though HIV-1 is not rapidly cytopathic in these cells [38*]. At present, long-term significance of the possible mast cell reservoir is unclear, and it is not known whether a mast cell reservoir exists in HAART-treated patients with undetectable viral loads. Additional studies of mast cell infection to determine whether there is a reservoir of latently infected mast cells in patients with undetectable viral loads would clarify the role of these cells in HIV persistence.
While prMCs were originally thought to be susceptible only to CCR5-tropic HIV-1, a recent report demonstrated that prMCs are also susceptible to CXCR4-tropic HIV infection in the presence of IgE [42**]. Interactions between IgE and FcεRI on the mast cell progenitor surface lead to a dramatic increase in CXCR4 expression and susceptibility to CXCR4-tropic HIV infection [42**]. Thus, if mast cells do constitute a reservoir of HIV in HAART-treated patients, they could harbor both CCR5- and CXCR4-tropic HIV-1. The putative role of mast cells in HIV persistence is shown in Figure 3.
Do multipotent HPC or mast cell reservoirs contribute to HIV persistence?
Although the role of HPCs in HIV persistence has not yet been directly evaluated, recent data on the structure of the residual viral population is consistent with the possibility that infection of HPCs generates a long-lived viral reservoir in many patients. A substantial body of evidence indicates that not all of the residual viremia in many HIV-infected individuals on successful HAART therapy originates from the resting CD4+ T cell reservoir [1,3*] or indeed from any peripheral blood cells [2*], though there is evidence that plasma viral sequences are more closely related to sequences in peripheral blood monocytes than to sequences in CD4+ T cells [43*]. In addition, residual virus does not undergo significant evolution during effective treatment, thus suggesting that continuing active replication is not a major source of residual viremia [44]. Together, these data suggest that a major source of residual viremia in treated patients may be neither latently infected CD4+ T cells nor continued active replication, but instead a heretofore uncharacterized reservoir of latent HIV.
There is also evidence that residual plasma viremia is largely homogenous, with just one or two viral clones accounting for most residual viremia in most patients on HAART [1] or most rebounding virus during treatment interruptions [45]. These data suggest that the major source of persistent HIV production is either a single cell, such as an actively infected mast cell that resists HIV-induced cell death, or a clonal population of cells originating from a single infected cell, such as a group of HPCs derived from the infection of a self-renewing HSC. Studies comparing proviral sequences in CD34+ cells and mast cells to residual plasma sequences would reveal whether either of these explanations is accurate.
Finally, a recent analysis of the decay kinetics of HIV reservoirs in HAART-treated patients suggests the existence of two long-term viral reservoirs: one with a half-life of 9-15 months, approximately consistent with the previously reported half-life of the resting T cell reservoir (6-44 months [46-49]), and a second reservoir with no appreciable decay over at least seven years [50*]. The indefinite half-life of this latter reservoir is consistent with the exceedingly long lifespan of a long lived precursor cell, potentially even HSCs. More research will be needed to determine which type of CD34+ cells harbor latent forms of HIV in vivo and what the half-life of this reservoir is.
Conclusion
Although for many years it was unclear whether multipotent HPCs could become infected with HIV-1, recent studies show that CD34+ cells support both active and latent infection in vitro and in vivo. Infection of these cells likely contributes to the anemia and hematopoietic dysfunction associated with HIV and furthermore creates a long-lived, stable viral reservoir that may contribute to residual plasma viremia in HAART-treated patients. This reservoir may seriously impede disease eradication, and thus an effort to better understand this viral reservoir is required.
Infection of progenitor mast cells may also generate an HIV reservoir in tissue mast cells; however, the longevity of this reservoir and its importance in HAART-treated patients remains to be investigated. A study of viral sanctuaries during HAART in a rhesus macaque model for HIV has revealed the presence of HIV DNA in both the bone marrow and the gastrointestinal tract, a region in which mast cells (as well as a variety of lymphoid cells) are abundant [38,51*]. Further study of this model to determine the particular cell types harboring HIV DNA in each anatomical location, as well as the contribution that each cell type makes to residual plasma viremia during HAART, could enhance our understanding of these potentially important viral reservoirs. As evidence mounts that the CD4+ T cell reservoir is not the sole source of persistent viremia during therapy, the role of additional reservoirs in HIV persistence must be thoroughly evaluated.
Acknowledgments
Lucy McNamara is a recipient of an NSF pre-doctoral fellowship and a Bernard Maas Fellowship. We thank Adewunmi Onafuwa-Nuga for critical reading of the manuscript.
Funding for this work was received from the National Institutes of Health and the Burroughs Wellcome Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bailey J, Sedaghat A, Kieffer T, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol. 2006;80(13):6441–6457. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *2.Brennan T, Woods J, Sedaghat A, et al. Analysis of human immunodeficiency virus type 1 viremia and provirus in resting CD4+ T cells reveals a novel source of residual viremia in patients on antiretroviral therapy. J Virol. 2009;83(17):8470–8481. doi: 10.1128/JVI.02568-08. [This paper demonstrated that the majority of plasma viral clones in HAART-treated patients are not closely related to those found in CD4+ T cells or other peripheral blood cells, thus suggesting that residual viremia comes predominantly from a non-circulating reservoir.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *3.Sahu G, Paar D, Frost S, et al. Low-level plasma HIVs in patients on prolonged suppressive highly active antiretroviral therapy are produced mostly by cells other than CD4 T-cells. J Med Virol. 2009;81(1):9–15. doi: 10.1002/jmv.21366. [This paper demonstrates that not only are residual plasma HIV sequences in HAART treated patients unlikely to be produced by CD4 T cells, rebounding virus in patients who stop HAART therapy comes from the same source as the residual plasma virus.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- **4.Carter C, Onafuwa-Nuga A, McNamara L, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16:446–451. doi: 10.1038/nm.2109. [This paper showed that CD34+ bone marrow cells with multilineage colony-forming potential can be both actively and latently infected by HIV-1. Active and latent infection of CD34+ cells in HIV+ individuals was also detected, and HIV-1 genomes were found in the CD34+ cells of a substantial proportion of patients with well-controlled viremia on HAART.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannert N, Farzan M, Friend D, et al. Human mast cell progenitors can be infected by macrophagetropic human immunodeficiency virus type 1 and retain virus with maturation in vitro. J Virol. 2001;75(22):10808–10814. doi: 10.1128/JVI.75.22.10808-10814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexaki A, Wigdahl B. HIV-1 infection of bone marrow hematopoietic progenitor cells and their role in trafficking and viral dissemination. PLoS Pathog. 2008;4(12):e1000215. doi: 10.1371/journal.ppat.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Civin C, Gore S. Antigenic analysis of hematopoiesis: a review. Journal of Hematotherapy. 1993;2(2):137–144. doi: 10.1089/scd.1.1993.2.137. [DOI] [PubMed] [Google Scholar]
- 8.Yin A, Miraglia S, Zanjani E, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90(12):5002–5012. [PubMed] [Google Scholar]
- 9.Folks T, Kessler S, Orenstein J, et al. Infection and replication of HIV-1 in purified progenitor cells of normal human bone marrow. Science. 1988;242(4880):919–922. doi: 10.1126/science.2460922. [DOI] [PubMed] [Google Scholar]
- 10.Stanley S, Kessler S, Justement J, et al. CD34+ bone marrow cells are infected with HIV in a subset of seropositive individuals. J Immunol. 1992;149(2):689–697. [PubMed] [Google Scholar]
- 11.Slobold K, Bennett T, Freiden P, et al. Mobilization of CD34+ progenitor cells by granulocyte colony-stimulating factor in human immunodeficiency virus type 1-infected adults. Blood. 1996;88(9):3329–3335. [PubMed] [Google Scholar]
- 12.Von Laer D, Hufert F, Fenner T, et al. CD34+ hematopoietic progenitor cells are not a major reservoir of the human immunodeficiency virus. Blood. 1990;76(7):1281–1286. [PubMed] [Google Scholar]
- 13.Davis B, Schwartz D, Marx J, et al. Absent or rare human immunodeficiency virus infection of bone marrow stem/progenitor cells in vivo. J Virol. 1991;65(4):1985–1990. doi: 10.1128/jvi.65.4.1985-1990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zauli G, Re M, Davis B, et al. Impaired in vitro growth of purified (CD34+) hematopoietic progenitors in human immunodeficiency virus-1 seropositive thrombocytopenic individuals. Blood. 1992;79(10):2680–2687. [PubMed] [Google Scholar]
- 15.Neal T, Holland H, Baum C, et al. CD34+ progenitor cells from asymptomatic patients are not a major reservoir for human immunodeficiency virus-1. Blood. 1995;86(5):1749–1756. [PubMed] [Google Scholar]
- 16.Chelucci C, Hassan H, Locardi C, et al. In vitro human immunodeficiency virus-1 infection of purified hematopoietic progenitors in single-cell culture. Blood. 1995;85(5):1181–1187. [PubMed] [Google Scholar]
- 17.Shen H, Cheng T, Preffer F, et al. Intrinsic human immunodeficiency virus type 1 resistance of hematopoietic stem cells despite coreceptor expression. J Virol. 1999;73(1):728–737. doi: 10.1128/jvi.73.1.728-737.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hariharan D, Li Y, Campbell D, et al. Human immunodeficiency virus infection of human placental cord blood CD34+ AC133+ stem cells and their progeny. AIDS Research and Human Retroviruses. 1999;15(17):1545–1552. doi: 10.1089/088922299309838. [DOI] [PubMed] [Google Scholar]
- 19.Weichold F, Zella D, Barabitskaja O, et al. Neither human immunodeficiency virus-1 (HIV-1) nor HIV-2 infects most-primitive human hematopoietic stem cells as assessed in long-term bone marrow cultures. Blood. 1998;91(3):907–915. [PubMed] [Google Scholar]
- **20.Redd A, Avalos A, Essex M. Infection of hematopoietic progenitor cells by HIV-1 subtype C, and its association with anemia in southern Africa. Blood. 2007;110(9):3143–3149. doi: 10.1182/blood-2007-04-086314. [This study showed that HIV-1 subtype C but not HIV-1 subtype B could infect multipotent colony forming HPCs and furthermore detected HIV-1 infection of CD34+ cells from a majority of HIV-1 subtype C infected patients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chun T, Stuyver L, Mizell S, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci. 1997;94(24):13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adetifa I, Temiye E, Akinsulie A, et al. Haematological abnormalities associated with paediatric HIV/AIDS in Lagos. Annals of Tropical Paediatrics. 2006;26:121–125. doi: 10.1179/146532806X107467. [DOI] [PubMed] [Google Scholar]
- 23.Calis J, Phiri K, Faragher E, et al. Severe anemia in Malawian children. N Engl J Med. 2008;358(9):888–899. doi: 10.1056/NEJMoa072727. [DOI] [PubMed] [Google Scholar]
- 24.Costantini A, Giuliodoro S, Mancini S, et al. Impaired in-vitro growth of megakaryocytic colonies derived from CD34 cells of HIV-1-infected patients with active viral replication. AIDS. 2006;20(13):1713–1720. doi: 10.1097/01.aids.0000242817.88086.8c. [DOI] [PubMed] [Google Scholar]
- 25.Dikshit B, Wanchu A, Sachdeva R, et al. Profile of hematological abnormalities of Indian HIV infected individuals. BMC Blood Disorders. 2009;9:5. doi: 10.1186/1471-2326-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isgrò A, Aiuti A, Leti W, et al. Immunodysregulation of HIV disease at bone marrow level. Autoimmunity Reviews. 2005;4(8):486–490. doi: 10.1016/j.autrev.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Meira D, Lorand-Metze I, Toro A, et al. Bone marrow features in children with HIV infection and peripheral blood cytopenias. Journal of Tropical Pediatrics. 2005;51(2):114–119. doi: 10.1093/tropej/fmh096. [DOI] [PubMed] [Google Scholar]
- 28.Mlisana K, Auld S, Grobler A, et al. Anaemia in acute HIV-1 subtype C infection. PLoS ONE. 2008;3(2):e1626. doi: 10.1371/journal.pone.0001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moses A, Nelson J, Bagby G. The influence of human immunodeficiency virus-1 on hematopoiesis. Blood. 1998;91(5):1479–1495. [PubMed] [Google Scholar]
- 30.Redd A, Avalos A, Phiri K, Essex M. Effects of HIV type 1 infection on hematopoiesis in Botswana. AIDS Research and Human Retroviruses. 2007;23(8):996–1003. doi: 10.1089/aid.2006.0283. [DOI] [PubMed] [Google Scholar]
- 31.Banda N, Simon G, Sipple J, et al. Depletion of CD34 CD4 cells in bone marrow from HIV-1-infected individuals. Biol Blood Marrow Transplant. 1999;5:162–172. doi: 10.1053/bbmt.1999.v5.pm10392962. [DOI] [PubMed] [Google Scholar]
- 32.Isgrò A, Leti W, De Santis W, et al. Altered clonogenic capability and stromal cell function characterize bone marrow of HIV-infected subjects with low CD4+ T cell counts despite viral suppression during HAART. Clin Infect Dis. 2008;46:1902–1910. doi: 10.1086/588480. [DOI] [PubMed] [Google Scholar]
- 33.Carr A, Cooper D. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–1430. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 34.Kaneda T, Murakami T, Hagiwara T, et al. Defective HIV-1 provirus found in peripheral T lymphocytes and granulocytes in an AIDS patient imply viral infection of progenitor cells. AIDS. 2001;15(7):939–940. doi: 10.1097/00002030-200105040-00017. [DOI] [PubMed] [Google Scholar]
- 35.Brenchley J, Hill B, Ambrozak D, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol. 2004;78(3):1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casale T, Wood D, Richerson H, et al. Direct evidence of a role for mast cells in the pathogenesis of antigen-induced bronchoconstriction. J Clin Invest. 1987;80(5):1507–1511. doi: 10.1172/JCI113234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lantz C, Boesiger J, Song C, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- *38.Sundstrom J, Ellis J, Hair G, et al. Human tissue mast cells are an inducible reservoir of persistent HIV infection. Blood. 2007;109(12):5293–5300. doi: 10.1182/blood-2006-11-058438. [This study finds that placental tissue mast cells from HAART-treated, HIV-infected women are latently infected by HIV.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padawer J. Mast cells: extended lifespan and lack of granule turnover under normal in vivo conditions. Experimental and Molecular Pathology. 1974;20:269–280. doi: 10.1016/0014-4800(74)90059-8. [DOI] [PubMed] [Google Scholar]
- 40.Gurish M, Boyce J. Mast cell growth, differentiation, and death. Clinical Reviews in Allergy and Immunology. 2002;22(2):107–118. doi: 10.1385/CRIAI:22:2:107. [DOI] [PubMed] [Google Scholar]
- *41.Nelson A, Auerbach A, Man Y. Failure to detect active virus replication in mast cells at various tissue sites of HIV patients by immunohistochemistry. Int J Biol Sci. 2009;5:603–610. doi: 10.7150/ijbs.5.603. [This study was unable to confirm HIV-1 protein expression in tissue mast cells, suggesting either that mast cells are not a significant reservoir of HIV-1 or that they harbor predominantly latent virus.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- **42.Sundstrom J, Hair G, Ansari A, et al. IgE-FcεRI interactions determine HIV coreceptor usage and susceptibility to infection during ontogeny of mast cells. J Immunol. 2009;182:6401–6409. doi: 10.4049/jimmunol.0801481. [This study shows that IgE-FcεRI interactions greatly increase CXCR4 expression in mast cell progenitors, permitting infection of these cells by CXCR4-tropic HIV-1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *43.Lopez C, Vazquez M, Hill M, et al. Characterization of HIV-1 RNA forms in the plasma of patients undergoing successful HAART. Arch Virol. 2010;155:895–903. doi: 10.1007/s00705-010-0659-3. [This study showed that viral sequences in the plasma were more closely related to those in monocytes than to those in CD4+ T cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kieffer T, Finucane M, Nettles R, et al. Genotypic analysis of HIV-1 drug resistance at the limit of detection: virus production without evolution in treated adults with undetectable HIV loads. J Infect Dis. 2004;189:1452–1465. doi: 10.1086/382488. [DOI] [PubMed] [Google Scholar]
- 45.Joos B, Fischer M, Kuster H, et al. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci. 2008;105(43):16725–16730. doi: 10.1073/pnas.0804192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siliciano J, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9(6):727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 47.Finzi D, Blankson J, Siliciano J, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5(5):512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Ramratnam B, Tenner-Racz K, et al. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N Engl J Med. 1999;340(21):1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 49.Ramratnam B, Mittler J, Zhang L, et al. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged anti-retroviral therapy. Nat Med. 2000;6(1):82–85. doi: 10.1038/71577. [DOI] [PubMed] [Google Scholar]
- *50.Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci. 2008;105(10):3879–3884. doi: 10.1073/pnas.0800050105. [This study calculated that there is a reservoir of virus that contributes to viremia and did not detectably decay over the 7-year period of the study.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *51.North T, Higgins J, Deere J, et al. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J Virol. 2010;84(6):2913–2922. doi: 10.1128/JVI.02356-09. [This study found SHIV DNA and RNA in diverse anatomical locations in HAART-treated macaques, including the bone marrow and gastrointestinal tract.] [DOI] [PMC free article] [PubMed] [Google Scholar]


