Abstract
Although it is known that injury enhances the regulatory activity of CD4+ regulatory T cells (Tregs), the cellular and molecular mechanisms responsible for injury-induced Treg activation remain unclear. This study was designed to investigate and compare injury-induced T cell receptor (TCR) signaling in Tregs, non-Tregs, and CD8+ T cells. Specifically, we used phospho-flow cytometry to measure the expression and phosphorylation of ZAP-70, PKC-θ, NFATc1, and GSK-3β in FoxP3+ Tregs vs. FoxP3-non-Tregs vs. CD8+ T cells. Groups of male, C57BL/6J mice underwent burn- or sham-injury and lymph nodes and spleens were harvested at early time points – 15, 30, 60, 120, and 240 minutes – to measure TCR signaling. As early as 15 minutes after burn injury, we observed a significant upregulation and phosphorylation of ZAP-70, PKC-θ, NFATc1, and GSK-3β in Tregs prepared from injury-site draining lymph nodes. Burn injury did not activate TCR signaling in Tregs from the spleen or in CD4+ non-Tregs and CD8+ T cells. In conclusion, the results of this study demonstrate that burn injury activates TCR signaling in Tregs, but not non-Tregs or CD8+ T cells. These findings suggest that injury provides an early TCR-activating signal to Tregs and supply new insights into how injury influences the adaptive immune system.
Keywords: Differential T cell signaling, Phospho-flow cytometry, Burn injury, TCR signaling, CD4+ regulatory T cells, T cell activation
Introduction
The Centers for Disease Control and Prevention (CDC) consistently identify in their yearly reports that ‘accidents/unintentional injuries’ are the leading causes of death in the United States in people up to the age of 44 (www.cdc.gov). While part of the mortality is attributable to the trauma, complications such as poor immune function and the development of a post-injury pro-inflammatory phenotype can also contribute to this statistic. An early and sustained inflammatory immune response is detectable in more than 90% of patients following trauma (1). This post-injury hyper-inflammatory state, the systemic inflammatory response syndrome (SIRS), depends on the severity of the initial insult and can lead to early multi-organ failure (MOF) (2). SIRS is usually accompanied by an increased anti-inflammatory response of the immune system, referred to as compensatory anti-inflammatory response syndrome (CARS) (3, 4). The development of SIRS and CARS following major injury predisposes the injured host to ‘second hits’ like nosocomial infections, ischemia, and surgical injury, which may lead to late MOF.
Recent studies to characterize the injury-induced changes in immune function show that severely injured patients exhibit an impaired adaptive immune response characterized by suppressed T-helper 1 (Th1)-type immunity (5, 6). Phenotypic changes in the innate immune system, like impaired function of neutrophils (7), reductions in circulating dendritic cells (8), and increased production of immune-suppressive mediators like PGE2 and nitric oxide (9) have been reported to occur in trauma patients. In addition, recent evidence suggests that CD4+ regulatory T cells (Tregs), which were originally identified as suppressors of CD4+ T cell activation (10), contribute to the counter-inflammatory reaction to severe injury (11). Tregs are identified by the forkhead transcription factor, FoxP3, which is expressed specifically in Tregs and is involved in Treg differentiation as well as function (12). It has been shown that Tregs block T cell proliferation by cell-contact mechanisms and suppress inflammatory responses by IL-10, IL-35 and TGF-β mediated mechanisms (13–16).
Of relevance to this study, it was reported that Treg activity is enhanced in cells derived from lymph nodes draining the injury site in burn-injured mice (5). In addition, it was shown by analyzing the expression pattern of the Ki-67 proliferation marker antigen that by 12 h after burn injury an early burst of proliferation can be seen in a small population of CD4+ T cells prepared from injury site draining lymph nodes (17). However, it remains unclear whether injury provides an early T cell receptor (TCR) activation signal to CD4+ or CD8+ T cells. In this study, we took advantage of phospho-flow cytometry as a tool to detect the expression and phosphorylation of the primary TCR signaling molecules: protein kinase C-θ (PKC-θ), ζ-associated protein of 70 kDa (ZAP-70), nuclear factor of activated T cells (NFATc1), and glycogen synthase kinase-3β (GSK-3β) in CD4+ and CD8+ T cells. The experiments were designed to compare and contrast TCR signaling in FoxP3+ CD4+ regulatory T cells, FoxP3− CD4+ T cells, and CD8+ T cells prepared from the lymph nodes or spleens of mice at early time points after burn injury. Our results show for the first time that injury induces an early TCR signaling as characterized by increased ZAP-70, PKC-θ, NFATc1, and GSK-3β expression and phosphorylation in injury-draining lymph node FoxP3+ CD4+ T cells (Tregs). In direct contrast, TCR signaling in non-Treg CD4+ T cells or in CD8+ T cells was not detected. We also provide comparative burn-induced kinetic data for Treg, non-Treg CD4+, and CD8+ TCR signaling responses in the spleen and lymph node. Taken together, the results presented here indicate that injury induces TCR signaling responses in Tregs, but not in CD4+ non-Tregs or CD8+ T cells. This general observation indicates that injury provides a localized and specific signal for rapid Treg activation.
Materials and Methods
Animals
Male C57BL/6J mice were purchased from The Jackson Laboratories (Bar Harbor, ME). All mice were maintained in our full-barrier animal facility under controlled temperature, humidity and a 12-hour light/dark regimen. Mice were acclimatized for at least 1 week prior to use with a diet of standard chow and water ad libitum. Experiments were performed on mice aged 6–8 weeks. All animal protocols were approved by the Harvard Medical School standing committee on animal research (Boston, MA) and were found to be in accordance with the guidelines of the National Institutes of Health (NIH; Bethesda, MD).
Reagents
Cells were prepared in culture medium (C5); RPMI 1640 was supplemented with 5% heat-inactivated FCS, 1 mM glutamine, 10 mM HEPES, 100 μM nonessential amino acids, penicillin/streptomycin/fungiozone, and 2.5 × 10−5 M 2-Mercaptoethanol, all purchased from Gibco-Invitrogen (Grand Island, NY, USA). Cells were fixed and permeabilized for FACS stains using phosphate-buffered saline with paraformaldehyde (PFA) and methanol purchased from Sigma Chemical Company (St. Louis, MO). Non-specific binding was prevented by using Fc-block reagent, anti-CD16/32 mAb from BD Biosciences (San Diego, CA, USA). Anti-CD4 and anti-CD8 mAb were purchased from BD Biosciences (San Jose, CA, USA). Anti-FoxP3 mAb, purchased from eBioscience (SanDiego, CA, USA), was used to stain cells for intracellular expression of the transcription factor FoxP3. Primary antibodies, source rabbit, to stain intracellular signaling molecules included the following: Anti-ZAP-70 [99F2], anti-p-ZAP-70 [Tyr 493], anti-p-GSK-3β [Ser 9] (all purchased from Cell Signaling Technology, Danvers, MA, USA), and anti-PKC-θ [C-18], anti-p-PKC-θ [Thr 538], anti-NFATc1 [H-110], anti-p-NFATc1 [Ser 259], anti-GSK-3β [H-76] (all purchased from Santa Cruz Biotechnology, Santa Cruz, CA, USA). Detection of rabbit antibodies was performed by secondary incubation with Alexa Fluor 555 conjugated F(ab′)2 fragment of goat anti-rabbit IgG, purchased from Invitrogen (Eugene, OR, USA).
Mouse Injury Model
The mouse burn injury protocol was performed as described (18). Briefly, mice were anesthestized by i.p. injection with ketamine (125 mg/kg) and xylazine (10 mg/kg). The dorsal fur was shaved and mice were placed in a plastic mold that exposes only 25% of their total body surface area (TBSA). Thermal injury was induced by immersing the exposed part of the dorsum to 90° C for 9 sec in a water bath. This approach causes a full-thickness anesthetic burn injury. Sham treated mice underwent the same procedure, but were exposed to water at 24° C for 9 sec. All animals were resuscitated with an i.p. injection of 1 ml of 0.9% pyrogen-free saline. The mortality from burn injury was <5%.
Lymph Node and Spleen Cell Preparation
Groups of male, C57BL6/J mice (n = 3) underwent burn injury. At 15 min, 30 min, 1 hour, 2 hours, or 4 hours post-injury, mice were sacrificed by CO2 asphyxiation and the draining lymph nodes (axillary, brachial, inguinal) and the spleens were harvested. Sham mice (n=3) were sacrificed at 30 min. The lymph nodes and spleens were immediately minced using a sterile wire mesh and cell suspensions were prepared in C5 medium. Red blood cells in spleen cell preparations were lysed using an RBC lysis buffer and cell preparations were washed twice by centrifugation (200 × g, 10 min) in C5 medium, then strained to remove debris. Cells were plated in tissue-culture treated round-bottom 96-well plates at 5 × 105 cells/well and immediately fixed by adding 0.5 % PFA buffer.
Phospho-Flow Cytometry
Following 10 min fixation in 0.5% PFA, cells were centrifuged to pellet and then permeabilized by adding ice-cold methanol. Cells were washed by centrifugation and then incubated with Fc-block reagent. Fixed and permeablized cells were stained with APC-labeled anti-CD4, PE-Cy5-labeled anti-CD8, and FITC-labeled anti-FoxP3. Cells were incubated with one of the following rabbit anti-mouse primary antibodies: anti-ZAP-70, anti-p-ZAP-70, anti-PKC-θ, anti-p-PKC-θ, anti-NFATc1, anti-p-NFATc1, anti-GSK-3β, anti-p-GSK-3β. The Alexa555-labeled secondary goat anti-rabbit IgG (F(ab′)2 fragment) was added as a last step in the staining procedure. Negative controls for signaling molecule detection were generated by performing the staining procedure without the primary Ab. Stained samples were fixed in 0.3% PFA buffer, washed and reconstituted in PBS for flow cytometry analysis on a FACSCalibur instrument (Becton-Dickinson, San Jose, CA, USA). Data analysis was conducted using the accompanying CELLQuestPro software program. Mean fluorescence intensity (MFI) for intracellular signaling molecules was detected and the relative fluorescence intensity (RFI) was calculated as MFI minus MFI of negative control.
All primary antibodies used in this study were tested in vitro prior to use for in vivo studies. For these tests, whole spleen cell suspensions were cultured at 37° C, 5 % CO2 at 5 × 105 cells per well in a round-bottom 96-well plate. After 1 hour pre-incubation, anti-CD3ε mAb was added at different concentrations (0, 0.01, 0.1, or 1 μg/ml) for different incubation times (30 min, 1, 2, 4 hours). Cells were processed for intracellular TCR signaling molecule staining and analyzed by flow cytometry as described above.
Statistical Analysis
The Kruskal-Wallis ANOVA on ranks test was followed by the nonparametric Student-Newman-Keuls posttest for multiple comparisons (SigmaPlot 11.0, Systat Software, San Jose, CA, USA) to determine statistical significance with P<0.05 considered as significant. The data are plotted as Mean values ± SEM.
Results
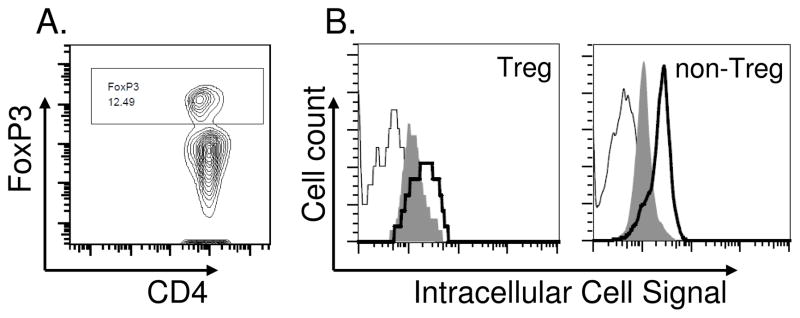
Phospho-flow cytometry, rather than Western Blotting, was used to analyze the expression and phosphorylation of the TCR signaling molecules; ZAP-70, PKC-θ, NFATc1, and GSK-3β in Tregs vs. non-Tregs following injury. FACS gating for FoxP3+ CD4+ T cells allowed us to identify Tregs in heterogeneous cell populations (Fig. 1). Intracellular staining for each of the TCR signaling molecules was performed by using antibodies specific for the non-phosphorylated or phosphorylated epitopes. This enabled us to analyze changes in the overall expression levels as well as their phosphorylation in CD4+ and CD8+ T cells. All antibodies used in this study were first tested in vitro to assure that they could effectively detect anti-CD3ε antibody induced TCR signaling in Tregs, non-Tregs, and CD8+ T cells. A representative in vitro test for anti-CD3ε mAb-induced PKC-θ signaling in FoxP3+ and FoxP3− CD4+ T cells is shown in Figure 1. In this study, spleen cell were stimulated with 1 μg/ml of anti-CD3ε mAb for 1 hour and prepared for FACS stains. We show the gating strategy used for identifying FoxP3+ and FoxP3− CD4+ T cells and the histograms represent the level of phospho-PKC-θ staining in Tregs and non-Tregs. These data illustrate our ability to detect TCR signaling molecule phosphorylation and expression in TCR-activated CD4+ Tregs and non-Tregs. We performed identical type in vitro testing experiments for other antibodies used in the study to confirm that all TCR signaling molecule specific antibodies could effectively detect anti-CD3ε stimulated signaling responses in CD4+ and CD8+ mouse T cells (data not shown).
Figure 1. Analysis of differential signaling in Tregs vs. non-Tregs using phospho-flow cytometry.
Phospho-flow cytometry was used to analyze differential intracellular signaling in CD4+FoxP3+ regulatory T cells (Tregs) vs. CD4+ FoxP3− non-regulatory T cells (non-Tregs). The intracellular transcription factor, FoxP3, was used to gate for Tregs to distinguish between Tregs and non-Tregs (A). Representative histograms (B) show the level of phosphorylated PKC-θ in Tregs and non-Tregs at 1 hour after stimulation in vitro (negative control solid line [=background]; stimulation with 0 μg/ml anti-CD3ε Ab grey filling [=no stimulation, constitutive phosphorylation]; stimulation with 1 μg/ml anti-CD3ε Ab solid bold line [=stimulation, induced phosphorylation]). We used this identical approach to test all signaling molecule specific antibodies for their ability to detect CD3-induced signaling responses in Tregs and non-Tregs. We found that all antibodies used in this study were able to detect CD3-induced increases in intracellular expression of un-phosphorylated and phosphorylated TCR signaling molecules in Tregs, non-Tregs, and CD8+ T cells.
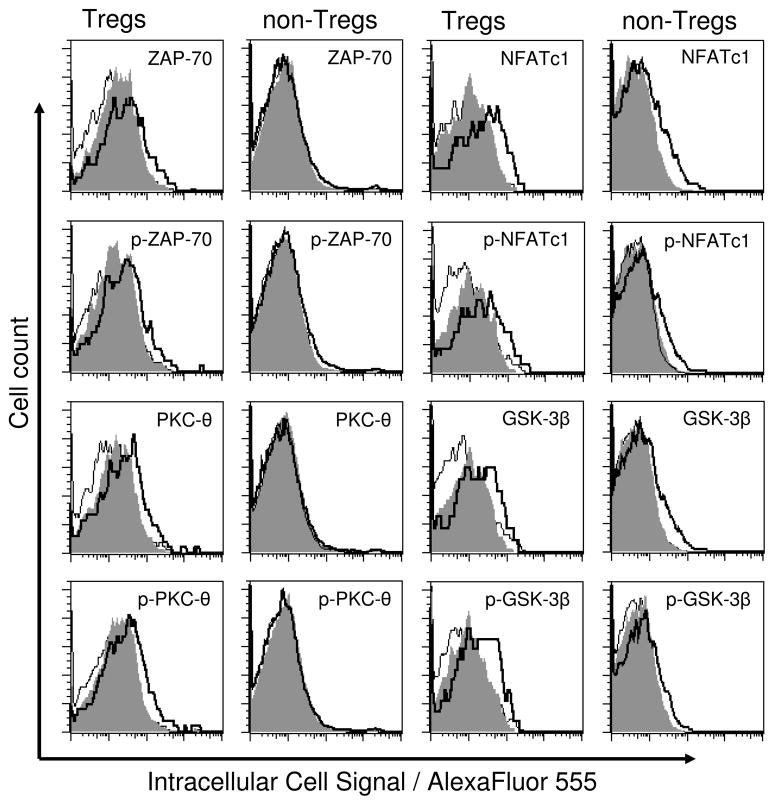
Following sham or burn injury, cell suspensions were prepared from draining lymph nodes or spleens for FACS analysis. All cell suspensions were FACS stained for CD4, CD8, and FoxP3 at time points after injury. The representative histograms in Figure 2 show staining for p-ZAP-70, p-PKC-θ, p-NFATc1, and p-GSK-3β in Tregs (CD4+ FoxP3+ T cells) or non Tregs (CD4+ FoxP3− T cells) from the draining lymph nodes of sham- or burn-injured mice. These histograms illustrate the relative levels of TCR signaling responses in Tregs vs. non-Tregs at 1 hour after injury and demonstrate our ability to detect TCR signaling in in vivo activated T cells. During the course of performing these experiments, we consistently observed differences in FACS autofluorescence levels between Tregs and non-Tregs. Therefore, to account for this difference in auto-fluorescence, we calculated the relative fluorescence intensity (RFI) from the mean fluorescence intensity (MFI) values for signaling molecule detection by the following formula: RFI = MFI of sample – MFI of negative staining control. This allowed us to better analyze FACS data and to directly compare injury-induced expression and phosphorylation of ZAP-70, PKC-θ, NFATc1, and GSK-3β in Tregs and non-Tregs without interference from auto-fluorescence. We performed statistical analysis on the RFI values and statistical significance is indicated in the data plots. Of note, phospho-flow cytometry allowed us to directly compare a very small population of cells (Tregs) to large populations of cells (non-Tregs and CD8+ T cells). In contrast to large cell populations, biologically significant changes in the expression and phosphorylation of signaling molecules in Tregs may show no statistical significance due to the impact of flow cytometry associated measurement variability in this small population.
Figure 2. Differential signaling in Tregs vs. non-Tregs derived from sham treated animals and animals following injury.
Using phospho-flow cytometry, intracellular signaling was analyzed in CD4+ FoxP3+ regulatory T cells (Tregs) vs. CD4+ FoxP3− non-regulatory T cells (non Tregs). These representative histograms display ZAP-70, PKC-θ, NFATc1, and GSK-3β protein expression levels as well as their phosphorylation (p-ZAP-70, p-PKC-θ, p-NFATc1, and p-GSK-3β) in Tregs vs. non-Tregs prepared from the draining lumph nodes of mice at 1 hour after sham or burn injury (negative control solid line, sham injury grey filling, burn injury solid bold line).
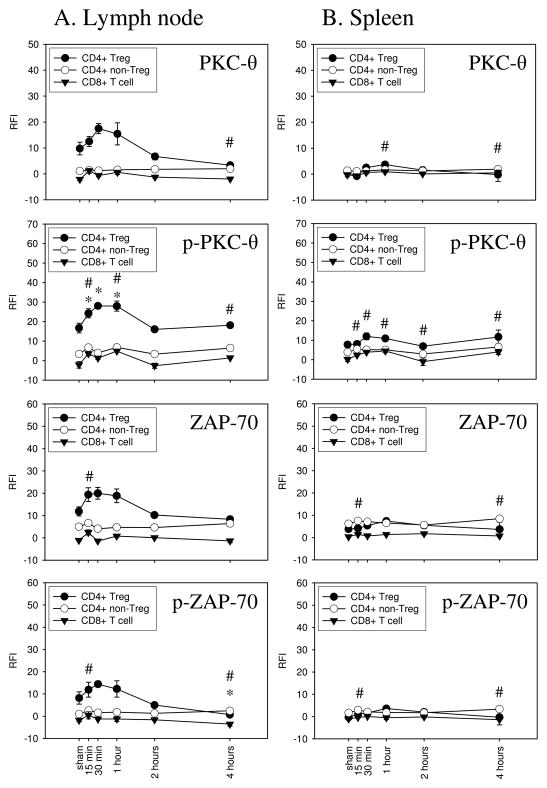
We observed that burn injury induced a biologically significant increase in the expression and phosphorylation of PKC-θ in Tregs, but not in CD4+ non-Tregs or CD8+ T cells that were prepared from injury-draining lymph nodes (Fig. 3, column A). As early as 15 min after injury, the elevated RFI for labeled p-PKC-θ in Tregs was statistically significantly higher as compared to sham-treated animals (24.24±2.32 vs. 16.73±2.50). Although we detected a slight increase in PKC-θ phosphorylation in CD4+ non-Tregs, Tregs showed an obvious and biologically significant time-dependent increase in p-PKC-θ up to one hour after burn injury. By 2 hours after burn injury, p-PKC-θ dropped to the baseline level. Interestingly, PKC-θ protein expression in Tregs increased with similar kinetics to p-PKC-θ. In contrast to Tregs from the draining lymph node, splenic Tregs did not show an augmented expression and phosphorylation of PKC-θ following injury (Fig. 3, column B). The expression and phosphorylation of ZAP-70 was also induced in Tregs by burn injury. As early as 15 min after injury, ZAP-70 expression and phosphorylation increased in draining lymph node Tregs. In contrast, CD4+ non-Tregs from the draining lymph node as well as Tregs and non-Tregs derived from the spleen did not show increased ZAP-70 expression or phosphorylation.
Figure 3. Injury induces PKC-θ and ZAP-70 signaling in Tregs, but not in non-Tregs or CD8+ T cells in draining lymph nodes.
Using phospho-flow cytometry, PKC-θ and ZAP-70 signaling was analyzed in Tregs, CD4+ non-Tregs, and CD8+ T cells following sham or burn injury. Cells were harvested from lymph nodes (column A) or spleens (column B) from sham- or burn-injured mice. The results are plotted as relative fluorescence intensity (RFI) calculated as described in the Materials and Methods. Burn injury induced an early time dependent upregulation and phosphorylation of PKC-θ and ZAP-70 in lymph node Tregs, but not in non-Tregs or in CD8+ T cells. Levels of p-PKC-θ were significantly higher at 15 min, 30 min, and 1 hour in lymph node Tregs from burn mice. We did not detect significant changes in PKC-θ and ZAP-70 expression or phosphorylation in splenic Tregs. Data shown is from one of two independent experiments. n=3 mice per group, mean ± SEM, #p<0.05 non-Tregs vs. sham, *p<0.05 Tregs vs. sham.
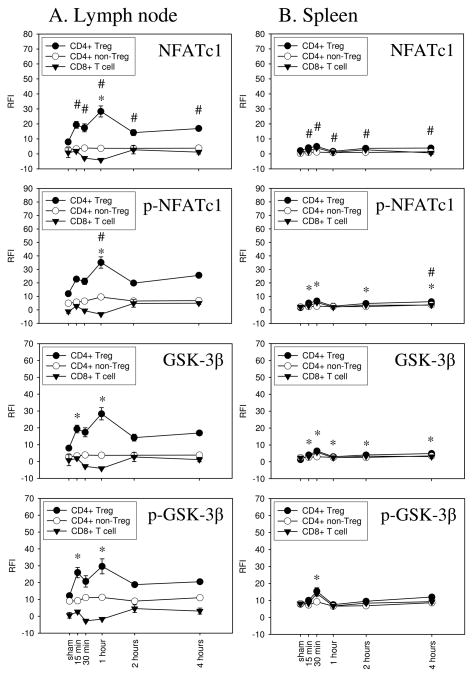
In addition to the TCR and CD28 dependent signaling pathways involving PKC-θ and ZAP-70, we investigated injury-induced changes in Ca2+/calcineurin regulated NFATc1 signaling in Tregs, CD4+ non-Tregs, and CD8+ T cells. We also measured changes in its regulatory counterplayer, the NFAT kinase GSK-3β. In comparison to sham treatment, burn injury induced a biologically significant increase in NFATc1 phosphorylation as early as 15 minutes in Tregs from the draining lymph node (22.77±1.83 vs. 11.99±1.71 RFI). Though the phosphorylation level declined slightly at 30 minutes, it then peaked at 1 hour after injury (35.12±4.18). NFATc1 protein expression followed similar kinetics to p-NFATc1 in lymph node Tregs showing an early peak at 15 minutes and peak expression at 1 hour after burn injury. Injury-induced increased NFATc1 expression and phosphorylation was not detected in lymph node CD4+ non-Tregs or CD8+ T cells. Furthermore, changes in NFATc1 activation were not observed in splenic Tregs, non-Tregs, and CD8+ T cells. Not surprisingly, the NFATc1 counterplayer, GSK-3β, showed similar phosphorylation and expression as NFATc1. We detected increased GSK-3β phosphorylation and expression by 15 minutes after burn injury in lymph node Tregs and it peaked at 1 hour after injury. Again, we did not detect biologically significant changes in GSK-3β phosphorylation or expression in non-Tregs or CD8+ T cells derived from the draining lymph nodes and burn injury did not induce GSK-3β phosphorylation or expression in splenic Tregs, CD4+ non-Tregs, or CD8 + T cells.
Discussion
Severe injury suppresses Th1-type immunity, promotes Th2-type cytokine production by T cells, and increases Treg function (5, 19). Tregs have also been shown to contribute to the counter-inflammatory response to injury, as measured by the enhanced inflammatory behavior of innate immune cell responses in Treg-deficient mice in burn-injured mice (11, 17, 20). The mechanisms responsible for T cell activation following injury have not yet been identified. Furthermore, little is known about the location, kinetics, and which T cell subsets are activated by injury. For these reasons, we initiated this study to begin addressing these fundamental questions. We used TCR signaling responses as an approach to directly compare TCR activation in CD4+ and CD8+ T cells prepared from the injury-site draining lymph nodes and spleen at early time points after burn injury. Our data indicate that CD4+ Tregs are the primary T cell subset that is activated by burn injury. We did not observe biologically significant TCR signaling responses by CD4+ non-Tregs or CD8+ T cells at time points up to 4 hours after burn injury. Moreover, we show that Tregs in injury-site draining lymph nodes displayed an early TCR signaling response, while splenic Tregs did not show injury-induced TCR signaling. To our knowledge, this is the first study to demonstrate that burn injury triggers TCR signaling in Tregs. In total, the findings present direct evidence that injury provides an early TCR-specific signal to CD4+ Tregs and supports the concept that Tregs might respond to injury-specific antigens.
The phospho-flow cytometry method used in these experiments made this study feasible for a number of reasons. First, it is important to point out that signaling events occurring in response to TCR ligation have been mostly studied by Western blotting using T cell lines or tumor cells (21). We reasoned it would be impossible to study early injury-induced TCR signaling events in T cells by Western blotting due to the time required to prepare purified T cell subsets from the lymph nodes and spleens of sham- or burn-injured mice. Furthermore, purifying mouse Tregs is complicated by the inadequacy of CD25 as a cell surface marker for Tregs (22). In contrast, we believed that phospho-flow cytometry would allow us to simultaneously analyze intracellular signaling molecule expression and phosphorylation in specific T cell subsets (21, 23). In a direct comparison with Western Blotting, it was shown that phospho-flow cytometry provides similar qualitative results, however, was characterized as providing more flexibility, higher efficiency, and the capacity to perform large screening experiments (21). The authors showed a strong correlation between Western Blotting and phospho-flow cytometry, analyzing the phosphorylation of ERK, p38, and JNK in Jurkat T cells as well as Stat1, Stat5, and Stat6 in a monocytic lymphoma cell line (21). Because of this, we worked to optimize this method for studying TCR signaling events in mouse CD4+ and CD8+ T cells. The optimization of the procedure was facilitated by performing a series of in vitro anti-CD3ε antibody induced flow staining studies that tested cell fixation approaches and different TCR signaling molecule specific antibodies. We found experimental conditions that allowed us to detect CD3 signaling responses in CD4+ Tregs, non-Tregs, and CD8+ T cells with significant sensitivity for 4 different and major TCR signaling molecules. Using our optimized phospho-flow cytometry, we produced data to indicate for the first time differential injury-specific signaling response by Tregs, mainly in the draining lymph nodes. We show, that the TCR- and CD28-dependent signaling molecules ZAP-70 and PKC-θ are transiently upregulated and phosphorylated in Tregs, but not in non-Tregs or CD8+ T cells. The TCR signaling molecules, NFATc1 and GSK-3β, showed a similar response. Furthermore, we demonstrate the advantage of using phospho-flow cytometry to study in vivo T cell activation by identifying the early kinetics and tissue location of Treg activation in peripheral lymphoid compartments. The restricted activation of Tregs in the draining lymph nodes of burn-injured mice suggests that Tregs might be activated by self antigens being flushed into lymph nodes as free antigens or carried there by antigen presenting cells.
In comparison to conventional CD4+ T effector cells, Tregs show defects in certain signaling pathways, like the PI3-kinase pathway (24), the Ras/MAPK pathway (25, 26), and in the capacity to phopsphorylate AKT and PLCγ1 (22, 27). Nevertheless, Tregs have the capacity to respond rapidly to environmental changes. This general observation was used by Matarese and coworkers to formulate the ‘on/off hypothesis’ (28). In comparison to conventional CD4+ T cells, Tregs are described to have a higher metabolic rate, a higher sensitivity to environmental stimuli and a higher rapidity of response (28). It is thought that Tregs are more sensitive to environmental changes due to their expression of a wider repertoire of receptors than are normally expressed on CD4+ T cells (28). These include, for example, constitutive expression of the IL-2 receptor (CD25), the ATP converting enzymes (CD39 and CD73), and the hyaluronan binding protein (CD44) to name a few (29–31). Because of the diversity of innate immune receptors expressed on Tregs, it remains controversial whether antigen is needed to activate Tregs. For example, it was recently shown by using antigen-specific Tregs from TCR transgenic mice that Tregs can suppress CD4+ T cells proliferation independently of TCR activation (32). In contrast, other reports have shown that TCR triggering is required for Tregs to exert their suppressive effects, but that their suppressor activity is nonspecific and does not require re-engagement of their TCR (33–35). Although the intention of this study was not to determine whether antigen-driven TCR signaling is required for injury-induced Treg activation, the rapid activation of TCR/CD28 triggered intracellular signaling molecules like ZAP-70, PKC-θ, NFATc1, and GSK-3β imply that some type of a TCR-specific antigen might be involved in the injury-induced activation of Tregs.
In conclusion, this study shows that injury induces TCR signaling in Tregs. In addition, we document the kinetics and localization of TCR signaling responses in Tregs following injury. Lymph node Tregs responded as early as 15 minutes after burn injury, which suggests that a population of Tregs may have specificity for injury-induced self antigens or injury-induced TCR activating factors. Importantly, we did not detect any TCR signaling responses by CD4+ non-Tregs or CD8+ T cells. Therefore, the early T cell response to burn injury in mice appears to be mediated exclusively by CD4+ Tregs. This information provides new and significant insight into how injury activates that adaptive immune system. Future studies will address the specificity and nature of the Treg response to injury. The identification of how Tregs are activated by injury will provide insights into the potential for using Tregs as a clinical target for modulating the host response to traumatic injury.
Figure 4. Injury induces NFATc1 and GSK-3β signaling in lymph node Tregs, but not in CD4+ non-Tregs or CD8+ T cells.
The expression and phosphorylation of NFATc1 and GSK-3β were analyzed in Tregs, non-Tregs, and CD8+ T cells by phospho-flow cytometry. Mice underwent sham or burn injury and the draining lymph nodes or the spleen were harvested at 15 min, 30 min, 1 hour, 2 hours, or 4 hours. Gating for FoxP3+ CD4+ T cells was used to distinguish the expression and phosphorylation of NFATc1 and GSK-3β in Tregs vs. non-Tregs. Data is presented as relative fluorescence intensity (RFI). Injury induced a significant increase in the expression and phosphorylation of NFATc1 and GSK-3β in Tregs, but not in non-Tregs or CD8+ T cells, derived from the draining lymph nodes (column A). In contrast, no marked increase in the expression and phosphorylation of these signaling molecules were detected in splenic Tregs, non-Tregs, or CD8+ T cells (column B). Data shown is from one of two independent experiments. n=3 mice per group, mean ± SEM, #p<0.05 non-Tregs vs. sham, *p<0.05 Tregs vs. sham.
Acknowledgments
This study was supported by grant funding from the National Institutes of Health 5R01GM035633-22 and 2RO1GM57664-09.
Abbreviations
- GSK-3β
glycogen synthase kinase-3β
- MFI
mean fluorescence intensity
- NFATc1
nuclear factor of activated T cells
- non-Treg
CD4+FoxP3− non-regulatory T cell
- PKC-θ
Protein Kinase C-θ
- RFI
relative fluorescence intensity
- TCR
T cell receptor
- Treg
CD4+FoxP3+ regulatory T cell
- WT
wild-type
- ZAP-70
ζ-associated protein of 70 kDa
Footnotes
Potential conflict of interest:
Nothing to report
References
- 1.Hoover L, Bochicchio GV, Napolitano LM, Joshi M, Bochicchio K, Meyer W, Scalea TM. Systemic inflammatory response syndrome and nosocomial infection in trauma. J Trauma. 2006;61:310–316. doi: 10.1097/01.ta.0000229052.75460.c2. [DOI] [PubMed] [Google Scholar]
- 2.Sauaia A, Moore FA, Moore EE, Lezotte DC. Early risk factors for postinjury multiple organ failure. World J Surg. 1996;20:392–400. doi: 10.1007/s002689900062. [DOI] [PubMed] [Google Scholar]
- 3.Mannick JA. 15th Annual Semmelweis Lecture Surgical Infection Society-Europe. Injury-induced immune dysfunction: is the lymphocyte irrelevant? Surg Infect (Larchmt ) 2002;3:297–302. doi: 10.1089/109629602762539526. [DOI] [PubMed] [Google Scholar]
- 4.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 5.Ni Choileain N, MacConmara M, Zang Y, Murphy TJ, Mannick JA, Lederer JA. Enhanced regulatory T cell activity is an element of the host response to injury. J Immunol. 2006;176:225–236. doi: 10.4049/jimmunol.176.1.225. [DOI] [PubMed] [Google Scholar]
- 6.Kelly JL, O’Suilleabhain CB, Soberg CC, Mannick JA, Lederer JA. Severe injury triggers antigen-specific T-helper cell dysfunction. Shock. 1999;12:39–45. doi: 10.1097/00024382-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Calum H, Moser C, Jensen PO, Christophersen L, Maling DS, van GM, Bjarnsholt T, Hougen HP, Givskov M, Jacobsen GK, Hoiby N. Thermal injury induces impaired function in polymorphonuclear neutrophil granulocytes and reduced control of burn wound infection. Clin Exp Immunol. 2009;156:102–110. doi: 10.1111/j.1365-2249.2008.03861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Arpa N, ccardo-Palumbo A, Amato G, D’Amelio L, Pileri D, Cataldo V, Mogavero R, Lombardo C, Napoli B, Conte F. Circulating dendritic cells following burn. Burns. 2009;35:513–518. doi: 10.1016/j.burns.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 9.Alexander M, Chaudry IH, Schwacha MG. Relationships between burn size, immunosuppression, and macrophage hyperactivity in a murine model of thermal injury. Cell Immunol. 2002;220:63–69. doi: 10.1016/s0008-8749(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 11.Murphy TJ, Ni CN, Zang Y, Mannick JA, Lederer JA. CD4+CD25+ regulatory T cells control innate immune reactivity after injury. J Immunol. 2005;174:2957–2963. doi: 10.4049/jimmunol.174.5.2957. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105–1111. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, Weiner HL. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:249–256. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]
- 16.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 17.Purcell EM, Dolan SM, Kriynovich S, Mannick JA, Lederer JA. Burn injury induces an early activation response by lymph node CD4+ T cells. Shock. 2006;25:135–140. doi: 10.1097/01.shk.0000190824.51653.32. [DOI] [PubMed] [Google Scholar]
- 18.Fujimi S, Lapchak PH, Zang Y, MacConmara MP, Maung AA, Delisle AJ, Mannick JA, Lederer JA. Murine dendritic cell antigen-presenting cell function is not altered by burn injury. J Leukoc Biol. 2009;85:862–870. doi: 10.1189/jlb.0408257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lederer JA, Rodrick ML, Mannick JA. The effects of injury on the adaptive immune response. Shock. 1999;11:153–159. doi: 10.1097/00024382-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 20.MacConmara MP, Maung AA, Fujimi S, McKenna AM, Delisle A, Lapchak PH, Rogers S, Lederer JA, Mannick JA. Increased CD4+ CD25+ T regulatory cell activity in trauma patients depresses protective Th1 immunity. Ann Surg. 2006;244:514–523. doi: 10.1097/01.sla.0000239031.06906.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 22.Crellin NK, Garcia RV, Levings MK. Flow cytometry-based methods for studying signaling in human CD4+CD25+FOXP3+ T regulatory cells. J Immunol Methods. 2007;324:92–104. doi: 10.1016/j.jim.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Chow S, Patel H, Hedley DW. Measurement of MAP kinase activation by flow cytometry using phospho-specific antibodies to MEK and ERK: potential for pharmacodynamic monitoring of signal transduction inhibitors. Cytometry. 2001;46:72–78. doi: 10.1002/cyto.1067. [DOI] [PubMed] [Google Scholar]
- 24.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109:2014–2022. doi: 10.1182/blood-2006-07-035279. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Godfrey WR, Porter SB, Ge Y, June CH, Blazar BR, Boussiotis VA. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood. 2005;106:3068–3073. doi: 10.1182/blood-2005-04-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsang JY, Camara NO, Eren E, Schneider H, Rudd C, Lombardi G, Lechler R. Altered proximal T cell receptor (TCR) signaling in human CD4+CD25+ regulatory T cells. J Leukoc Biol. 2006;80:145–151. doi: 10.1189/jlb.0605344. [DOI] [PubMed] [Google Scholar]
- 27.Hickman SP, Yang J, Thomas RM, Wells AD, Turka LA. Defective activation of protein kinase C and Ras-ERK pathways limits IL-2 production and proliferation by CD4+CD25+ regulatory T cells. J Immunol. 2006;177:2186–2194. doi: 10.4049/jimmunol.177.4.2186. [DOI] [PubMed] [Google Scholar]
- 28.Matarese G, De RV, La CA. Regulatory CD4 T cells: sensing the environment. Trends Immunol. 2008;29:12–17. doi: 10.1016/j.it.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–747. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- 32.Szymczak-Workman AL, Workman CJ, Vignali DA. Cutting edge: regulatory T cells do not require stimulation through their TCR to suppress. J Immunol. 2009;182:5188–5192. doi: 10.4049/jimmunol.0803123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med %20. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maggi E, Cosmi L, Liotta F, Romagnani P, Romagnani S, Annunziato F. Thymic regulatory T cells. Autoimmun Rev. 2005;4:579–586. doi: 10.1016/j.autrev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]