Abstract
The signaling and adaptor protein Homer3 plays a role in controlling immune homeostasis and self-reactivity. Homer3 is recruited to the immune synapse (IS) following TCR ligation, although the mechanisms regulating this subcellular localization are unknown. We show that Homer3 specifically associates with a novel ubiquitin-like domain in the IκB kinase (IKK) β subunit of the IKK complex. Homer3 associates with IKKβ in T cells and colocalizes with the IKK complex at the IS. However, Homer3 is not required for IKK activation, as NF-κB signaling is intact in Homer3-deficient T cells. Instead, the IKK complex recruits Homer3 to the IS following TCR engagement, and we present evidence that this association regulates actin dynamics in T cells. These findings identify a novel interaction between two major signaling proteins and reveal an unexpected NF-κB–independent function for the IKK complex in regulating the subcellular localization of Homer3.
Signals that activate NF-κB converge at the NF-κB essential modulator (NEMO)-, IκB kinase (IKK)α- and IKKβ-containing IKK complex (1). We previously identified a novel domain in IKKβ (2) that resembles the ubiquitin-like domain that mediates interactions in a family of functionally divergent proteins (3). We named this the ubiquitin-like domain (ULD) (Supplemental Fig. 1) and found that mutation of the ULD blocked IKKβ activation (2). The IKK-related kinase TNFR-associated factor family member-associated NF-κB activator-binding kinase 1 also contains a ULD that interacts with its own catalytic domain (4), leading us to hypothesize that the IKKβ ULD functions as a novel IKKβ-specific interaction domain.
In seeking the function of the IKKβ ULD, we found that it associates with the signaling and adaptor protein Homer3. Homer3 belongs to a family of three genes (Homers 1–3) that function at the postsynaptic density in neurons (5). Each Homer has an N-terminal enabled/vasodilator-stimulated phosphoprotein homology 1 (EVH1) domain (Supplemental Fig. 1) that binds to PPxxF (x = any) motifs in a wide range of proteins (6). The Homers’ C-termini contain a coiled-coil (CC) domain encompassing two leucine zippers (LZs) that interact to form multimeric Homer complexes (5). Homer multimers form scaffolds that link distinct PPxxF motif-containing proteins bound by individual Homer EVH1 domains.
Homers 2 and 3 negatively regulate NFAT and control immune homeostasis and self-reactivity in vivo (7). Homer3 is recruited to the immune synapse (IS) following TCR ligation (8); however, the mechanisms regulating its subcellular localization are unknown. As TCR ligation also recruits the IKK complex to the IS (9), and Homer3 physically interacts with the IKKβ ULD, we questioned whether IKKβ and Homer3 functionally interact in T cells. We show that although Homer3 is dispensable for TCR-induced IKK activation, association with the IKK complex is essential for Homer3 localization at the IS. Furthermore, we provide evidence that Homer3 and the IKK complex regulate actin dynamics in T cells. Thus, in addition to activating NF-κB, the IKK complex plays an NF-κB–independent role as a scaffold that recruits an additional functionally relevant signaling and adaptor protein to the IS.
Materials and Methods
Abs and reagents
Anti-IKKα, IKKβ, NEMO, and Homer3 were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-Myc was from Millipore (Billerica, MA), and anti-FLAG was from Sigma-Aldrich (St. Louis, MO). Protein G-Sepharose beads were from Amersham Biosciences (Bucks, U.K.). For fluorescence staining, mouse anti-NEMO was from BD Biosciences (San Jose, CA). Anti-CD3 and anti-CD28 were from Drs. J. Riley and C. June at the University of Pennsylvania (Philadelphia, PA). The TCR cross-linking Ab C305 was from A. Weiss (University of California, San Diego, La Jolla, CA). Anti-rabbit AF546, anti-mouse AF647, and AF647 phalloidin were from Molecular Probes (Carlsbad, CA). FITC-labeled control and Homer3-specific small interfering RNA (siRNA) oligonucleotides were from Santa Cruz Biotechnology.
Cell culture
E6.1 Jurkat cells and HEK293 cells were from American Type Culture Collection (Manassas, VA). 3T8 and 8321 Jurkats were from Dr. A. Ting (Mount Sinai Medical Center, New York, NY). NEMO-reconstituted 8321N cells were described before (10).
Plasmids and transfection
IKK and Homer plasmids were described earlier (2, 11). Transfection protocols using FuGENE6 (Roche, Indianapolis, IN) were described previously (2).
Yeast two-hybrid screen and interaction analysis
Yeast two-hybrid (Y2H) was performed using the Matchmaker Two-Hybrid System 3 (Clontech, Mountain View, CA). DNA-binding domain (DB) constructs expressing IKKβ mutants and the ULD were cloned from human IKKβ cDNA (2). Activation domain (AD) constructs expressing Homer3 and its domains were cloned from pRK5/Myc-Homer3 (11). Yeast-expressing GAL4 DB-ULDs were mated with yeast containing a HeLa cDNA library. To retest the interaction, yeast-expressing DB, DB-ULD, or DB-IKKβΔULΔ were transformed with GAL4 AD or AD with full-length Homer3 (AD-H3). Growth was assessed on his-/ade- media containing β-gal. To determine the interaction region in Homer3, yeast-expressing DB or DB-ULD were transformed with GAL4 AD, AD-H3, AD-CC/LZ, or AD-EVH1.
Immunoblotting and immunoprecipitation
Cell lysis, protein assay, immunoblotting (IB), and immunoprecipitations (IPs) were performed as described before (12).
Confocal microscopy
Staining and quantitative analysis was performed using Volocity software (Improvision, Waltham, MA) as previously described (13). For conjugation, Jurkats were incubated for 10 min with Ab-coated Dynalbeads (Bangs Labs, Fishers, IN). At least 50 cells/slide from a minimum of two experiments were examined.
Transfection and analysis of F-actin content
Jurkats were transfected with FITC-control or FITC-Homer3 siRNA by nucleofection (Amaxa, Lonza, Walkersville, MD). After 28 h, cells were incubated with either PHA or anti-TCR (C305) prior to FACS staining with AF647-phalloidin. Mean fluorescence intensity (MFI) of phalloidin fluorophore was determined in FITC-positive cells using FlowJo software (Tree Star, Ashland, OR). All experiments were performed three times, and statistical significance was established using a paired two-tailed t test.
Results
Homer3 associates with the IKKβ ULD
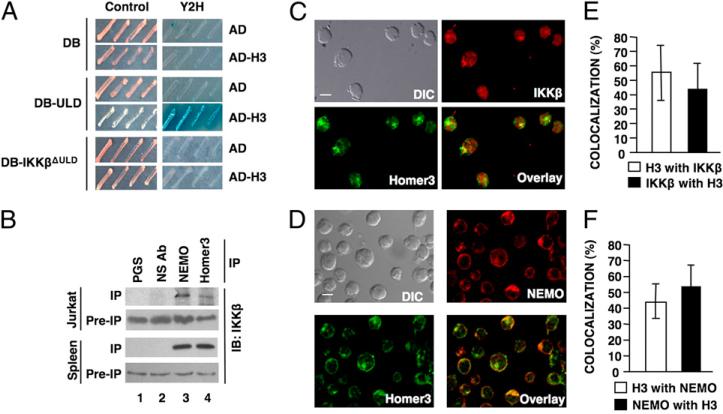
To identify IKKβ ULD-interacting proteins, we used the ULD in a Y2H screen. After screening ~5 million transformants, almost one quarter of the plasmids from positive clones (15 out of 62) encoded Homer3 (Supplemental Fig. 1). Retransformation of yeast recapitulated the Y2H interaction between Homer3 and the ULD, whereas an IKKβ mutant lacking the ULD (IKKβΔULΔ) did not associate (Fig. 1A).
FIGURE 1.
Homer3 associates with IKKβ. A, Y2H interaction analysis using the AD alone or AD-H3 with either the DNA-DB alone, DB-ULD, or DB-IKKβΔULΔ. Growth in the Y2H plate (right panels) indicates an interaction. B, IPs from E6.1 Jurkat T cell or mouse splenocyte extracts were performed using PGS alone, NS Ab, anti-NEMO, or anti-Homer3. IP and pre-IP extracts were subjected to IB using anti-IKKβ. DIC images and confocal micrographs of resting Jurkat E6.1 cells showing localization of IKKβ (C) or NEMO (D) in red with Homer3 in green. Overlay of Homer3 with IKKβ or NEMO is shown (scale bar, 5 μm; original magnification ×60). E and F, Quantitative analysis of the percentage of Homer3 colocalized with either IKKβ or NEMO and the percentage of IKKβ or NEMO with Homer3. DIC, differential interference contrast; NS Ab, nonspecific Ab; PGS, protein G-Sepharose.
Homers have been extensively characterized in neurons (5), and Homer3 is expressed in T cells, where it plays a role in TCR-induced activation (7, 8). As TCR ligation activates NF-κB (14), we questioned whether Homer3 associates with the IKK complex in T cells and found that IP of Homer3 pulled down IKKβ from Jurkat T cells and primary mouse splenocytes (Fig. 1B). Furthermore, most Homer3 coeluted with the high m.w. IKK complex (1) by size-exclusion chromatography (Supplemental Fig. 2). These findings substantiate our Y2H analysis and provide evidence that Homer3 associates with the endogenous IKK complex in T cells.
Consistent with these observations, confocal imaging demonstrated Homer3 colocalized with IKKβ and NEMO in Jurkat cells (Fig. 1C, 1D). Quantitation revealed similar levels of colocalization between Homer3 and IKKβ (Fig. 1E) or NEMO (Fig. 1F), demonstrating that a portion of cellular Homer3 colocalizes with the IKK complex. Supporting these results, anion exchange chromatography yielded fractions containing Homer3, IKKα, IKKβ, and NEMO and separate fractions containing Homer3, but lacking the IKK complex subunits (Supplemental Fig. 2). Thus, at least two pools of Homer3 exist in T cells: one localized with a subset of IKK complexes and one that is separate from IKKβ and NEMO.
The Homer3 CC/LZ interacts with the IKKβ ULD
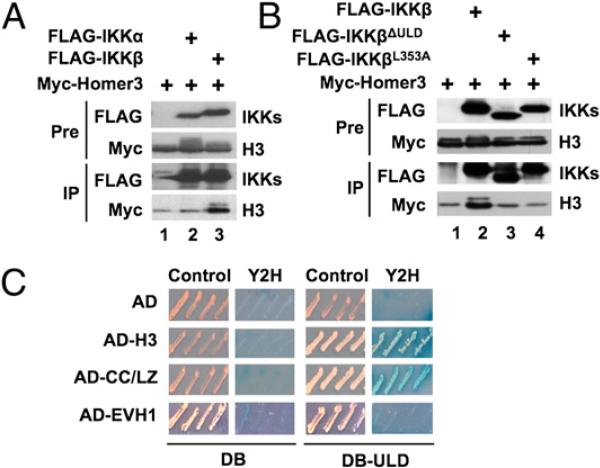
Overexpression of Myc-Homer3 and FLAG-IKKβ in HEK293 followed by anti-FLAG IP confirmed that Homer3 associates with IKKβ (Fig. 2A; lane 3), and reciprocal IP of Xpress-Homer3 also pulled down FLAG-IKKβ (Supplemental Fig. 3). Consistent with our previous finding that IKKα does not contain a ULD (2), Myc-Homer3 did not associate with FLAG-IKKα (Fig. 2A; lane 2). Myc-Homer3 did not bind to FLAG-IKKβΔULΔ or IKKβ containing a point mutation (FLAG-IKKβL353A)(Fig. 2B) within the putative hydrophobic patch (Supplemental Fig. 1), supporting a critical role for the ULD in this interaction. Notably, the Homer3 band upshifted when Homer3 was coexpressed with IKKβ or IKKα (Fig. 2A, 2B), and we found that this was due to phosphorylation (Supplemental Fig. 4), suggesting that Homer3 can be phosphorylated in an IKK-dependent manner.
FIGURE 2.
The Homer3 CC/LZ domain associates with the IKKβ ULD. HEK293 cells were transfected with Myc-Homer3 together with FLAG-IKKα or FLAG-IKKβ (A) or FLAG-IKKβ, FLAG-IKKβΔULΔ, or FLAG-IKKβL353A (B), and extracts were subjected to IP with anti-FLAG. IP and pre-IP extracts were subjected to IB using anti-Myc or anti-FLAG. C, Y2H analysis was performed using AD alone or AD-H3), the CC/LZ (AD-CC/LZ) or EVH1 domain (AD-EVH1), and either DB alone or DB-ULD. Growth in the Y2H plate indicates an interaction.
To determine how Homer3 associates with the ULD, we performed Y2H analysis using the separate Homer3 domains (Supplemental Fig. 1) and found that the ULD interacted with full-length Homer3 and the CC/LZ, but not the EVH1 domain (Fig. 2C). Thus, Homer3 specifically associates with the ULD in IKKβ via its CC/LZ domain (Supplemental Fig. 5).
Homer3 colocalizes with IKK at the IS
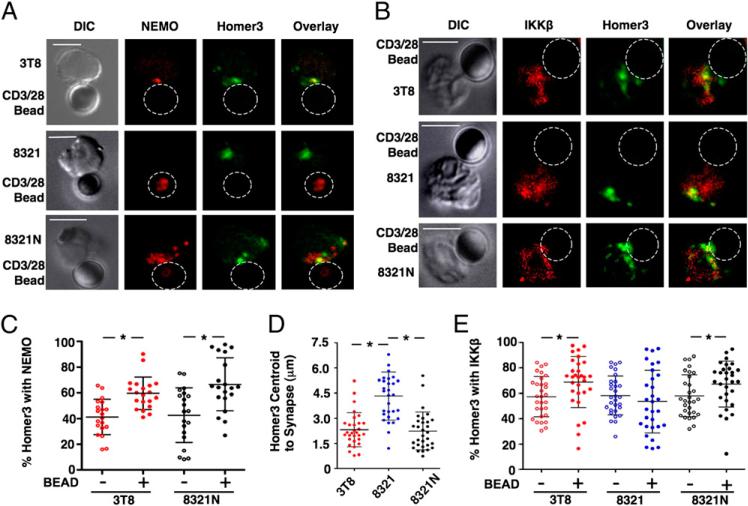
The CC/LZ domain of Homer3 is required for its localization to the IS following TCR engagement (8). Because Homer3 interacts with IKKβ via its CC/LZ (Fig. 2C, Supplemental Fig. 5), we asked if the presence of Homer3 at the IS correlates with its colocalization with the IKK complex. Confocal imaging showed NEMO and IKKβ at the contact area between 3T8 Jurkat T cells and anti-CD3/CD28–coated beads indicating IKK complex localization at the synapse (Fig. 3A, 3B; top panels). Homer3 was also detected at the interface, and overlay of fluorescence revealed extensive colocalization of Homer3 and NEMO or IKKβ (Fig. 3A, 3B; top panels). Identical results were obtained using clone E6.1 Jurkat cells (Supplemental Fig. 6). Quantitation demonstrated that the amount of NEMO with Homer3 was unchanged following conjugation (Supplemental Fig. 6). In contrast, the amount of Homer3 with NEMO at the IS in 3T8 (Fig. 3C) and E6.1 cells (Supplemental Fig. 6) was significantly higher than in unconjugated cells, suggesting that the amount of IKK-associated Homer3 increases following T cell activation.
FIGURE 3.
The IKK complex recruits Homer3 to the immune synapse. 3T8, 8321, and 8321N cell conjugates with anti-CD3–and anti-CD28–coated beads were stained for Homer3 (green) and either NEMO (A) or IKKβ (B) that appear red. Homer3 colocalized with either NEMO (A) or IKKβ (B) is yellow in overlays (scale bar, 5 μm; original magnification ×60). Quantitation of the percentage of Homer3 with NEMO (C) or IKKβ (E) in the absence (–) or presence (+) of beads. D, The distance from the synapse to the Homer3 centroid in the conjugates in A and B were calculated. *p < 0.05.
As recruitment to the IS activates the IKK complex (9), we questioned whether Homer3 regulates NF-κB activation. Overexpressed Homer3 but not Homer1 or Homer2 modestly activated NF-κB via IKKβ, suggesting that Homer3 specifically regulates IKKβ activity (Supplemental Fig. 7). However, our prior analysis of Homer3-depleted Jurkat cells showed normal NF-κB signaling in response to PMA and ionomycin (7). Consistent with this, NF-κB activation was intact in T cells from Homer3–/– and Homer2/3 double-deficient mice (Supplemental Fig. 8). Consequently, although overexpressed Homer3 activates NF-κB, Homers are not required for NF-κB activation.
IKK recruits Homer3 to the IS
As Homer3 and IKK colocalize at the IS following T cell activation, and Homer3 is not upstream of IKK activation (Supplemental Fig. 8), we asked whether the IKK complex directly recruits Homer3 to the IS. IKK recruitment to the IS requires NEMO (9), so we employed NEMO-deficient 8321 Jurkat cells (15) and NEMO-reconstituted 8321N cells (Supplemental Fig. 9) (10). Confocal analysis revealed no differences in colocalization of Homer3 with IKKβ in either cell type compared with the parental 3T8 line (Supplemental Fig. 9), suggesting that the constitutive interaction of Homer3 and IKKβ in resting cells is unaffected by the absence or presence of NEMO. However, recruitment of Homer3 to the IS was clearly NEMO dependent, as NEMO and Homer3 colocalized at the IS in 3T8 and 8321N cells following conjugation with anti-CD3/CD28 beads but did not colocalize at the synapse in 8321 cells (Fig. 3A). Notably, the amount of Homer3 colocalized with NEMO at the IS was increased in 8321N cells similar to the 3T8 parental line (Fig. 3C).
Analysis of IKKβ and Homer3 colocalization at the IS revealed that this was NEMO dependent, as IKKβ colocalized with Homer3 at the IS in 3T8 and 8321N cells but not in 8321 cells (Fig. 3B). In 8321 cells lacking NEMO, a portion of IKKβ was colocalized with Homer3 distant from the interface (Fig. 3B). Furthermore, measurement of the distance from the IS to the geometric center of the sum of regions stained for Homer3 (centroid) revealed no differences between 3T8 and 8321N cells, whereas this distance was significantly increased in 8321 cells (Fig. 3D). Together, these results suggest that IKKβ associates with Homer3, but cannot recruit Homer3 to the IS in the absence of NEMO. Consistent with this model, the amount of IKKβ associated with Homer3 was similar between resting and conjugated cells in all three lines (Supplemental Fig. 10), and the amount of Homer3 with IKKβ in 8321 cells was unchanged following conjugation (Fig. 3E). In contrast, the amount of Homer3 with IKKβ was increased in conjugates with either 3T8 or 8321N cells (Fig. 3E), suggesting again that the IKK complex recruits additional Homer3 to the synapse following TCR engagement.
Homer3 and NEMO regulate F-actin dynamics in T cells
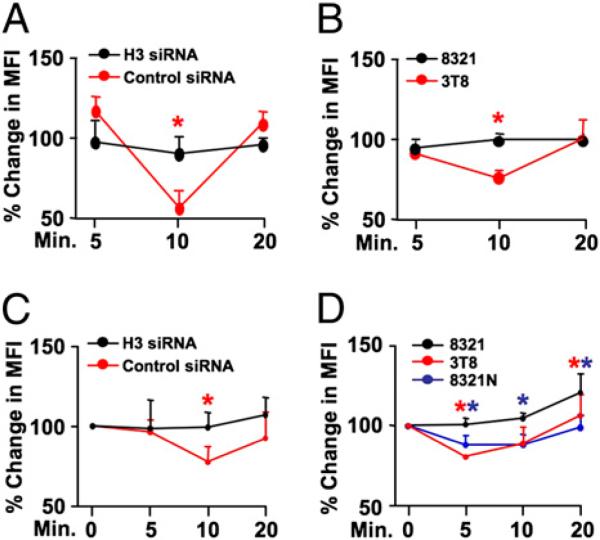
Homers regulate actin content in neurons (5, 16), and we questioned whether Homer3 plays a similar role in T cells. Jurkats transfected with control- or Homer3-siRNA (Supplemental Fig. 11) were stimulated using the TCR cross-linking IgM Ab C305 and stained for FACS using AF647-labeled phalloidin that binds F-actin. Consistent with previous studies in T cells (17), a transient decrease in total cellular phalloidin MFI due to F-actin organization occurred in control siRNA-treated Jurkats postactivation (Fig. 4A, Supplemental Fig. 11). In contrast, phalloidin MFI did not change in cells receiving Homer3 siRNA. Notably, a similar transient decrease in phalloidin MFI occurred in C305-stimulated 3T8 Jurkats but not in the NEMO-deficient 8321 cells (Fig. 4B). When Jurkats were stimulated with PHA, a transient decrease in total cellular phalloidin MFI occurred in control siRNA-treated Jurkats but not in Homer3 siRNA-treated cells (Fig. 4C). Furthermore, when we stained PHA-activated 3T8, 8321, and 8321N Jurkats for F-actin, the MFI transiently decreased in 3T8 and 8321N cells but not in 8321 cells lacking NEMO (Fig. 4D). These findings, therefore, demonstrate that Homer3 and NEMO regulate F-actin content following T cell activation.
FIGURE 4.
Homer3 and NEMO regulate F-actin content in T cells. A, 3T8 Jurkat cells transfected with control (red) or Homer3 siRNA (black) were incubated with the TCR cross-linking IgM Ab C305 (0.25 μg/ml) for the times indicated and then stained for F-actin using AF647-linked phalloidin. B, 3T8 (red) and 8321 (black) Jurkats incubated for the times indicated with C305 were stained with AF647-phalloidin. For A and B, the percentage change in MFI was determined relative to control IgM-stimulated cells. C, 3T8 cells transfected with control (red) or Homer3 siRNA (black) were stimulated with PHA (1 μg/ml) for the times shown, then AF647-phalloidin stained and the percentage change in MFI was determined relative to unstimulated cells. D, 3T8 (red), 8321 (black), and 8321N (blue) Jurkats incubated for the times indicated with PHA were stained for F-actin using AF647-phalloidin. *p < 0.05.
Discussion
We demonstrate that the signaling and adaptor protein Homer3 binds to the ULD of IKKβ. The mode of interaction is atypical of Homers, which normally bind via their EVH1 domains to PPxxF motifs in target proteins. The ULD does not contain a PPxxF sequence (Supplemental Fig. 1), and the CC/LZ of Homer3 is required for interaction with the ULD (Supplemental Fig. 5). A previous study showed that the Homer2 CC/LZ interacts with a ULD in a protein named 2B28 (18), suggesting that the CC/LZ domain contains a novel motif that binds ULDs. Importantly, our findings provide compelling evidence that in addition to its role in multimerization, the CC/LZ is also a binding region for Homer-interacting proteins.
Consistent with our previous study (7), NF-κB activation was intact in Homer3-deficient T cells, indicating that NF-κB signaling is not dependent upon Homer3. However, we demonstrate that the subcellular localization of Homer3 to the IS requires IKK-mediated recruitment. Specifically, we show that NEMO, essential for IKK recruitment to the IS following T cell activation (9), is required to localize Homer3 to the IS. The C terminus of Homer3 is critical for its localization to the IS (8), and we have shown that the CC/LZ associates with the IKKβ ULD. Hence, we conclude that IKKβ-associated Homer3 localizes to the IS via NEMO-dependent recruitment of IKKβ. Notably, such interactions will leave the EVH1 domain of Homer3 free to associate with other potential binding partners (Supplemental Fig. 5).
Homer3 negatively regulates NFAT (7); however, NFAT activation is normal in NEMO-deficient Jurkat cells (15), suggesting that Homer3 recruitment to the IS is not needed for NFAT signaling. We identified two pools of Homer3 in T cells, only one of which was complexed with IKK proteins. Thus, it is possible that IKKβ-independent Homer3 regulates NFAT signaling, whereas IKKβ-associated Homer3 performs a separate function. Consistent with this model, we have been unable to detect an interaction between the IKK complex and NFAT in T cells (not shown). Homers regulate actin content at neuronal synapses (5, 16), and we found diminished F-actin reorganization following activation of Homer3 siRNA-transfected Jurkats. Importantly, alterations in F-actin content were also abrogated in the absence of NEMO, suggesting that the intact IKK complex and Homer3 are required to regulate signal-induced F-actin restructuring. As the Homers’ EVH1 domain facilitates actin dynamics (16), and through interaction with IKKβ at the IS this domain will remain accessible, it may interact with regulators of actin dynamics or other proteins involved in the formation and function of the IS.
In summary, we have identified a novel interaction between IKKβ and Homer3. Although Homers do not regulate NF-κB activation, recruitment of Homer3 to the IS absolutely requires the IKK complex. Thus, in addition to activating NF-κB, the IKK complex functionally regulates the subcellular localization of Homer3.
Supplementary Material
Acknowledgments
We thank G. Huang and M. Dehoff for expert assistance and L. King for reading the manuscript.
This work was supported by National Institutes of Health Grants HL080612 (to M.J.M.), AI067946 and AI079731 (to J.S.O.), T32-AI055428 (to L.A.S.), and T32-CA09140 (to K.A.M.).
Abbreviations used in this paper
- AD
activation domain
- AD-H3
activation domain with full-length Homer3
- CC
coiled-coil
- DB
binding domain
- DIC
differential interference contrast
- EVH1
enabled/vasodilator-stimulated phosphoprotein homology 1
- IB
immunoblotting
- IKK
IκB kinase
- IP
immunoprecipitation
- IS
immune synapse
- LZ
leucine zipper
- MFI
mean fluorescence intensity
- NEMO
NF-κB essential modulator
- NS Ab
nonspecific Ab
- PGS
protein G-Sepharose
- siRNA
small interfering RNA
- ULD
ubiquitin-like domain
- Y2H
yeast two-hybrid
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 2.May MJ, Larsen SE, Shim JH, Madge LA, Ghosh S. A novel ubiquitin-like domain in IkappaB kinase beta is required for functional activity of the kinase. J. Biol. Chem. 2004;279:45528–45539. doi: 10.1074/jbc.M408579200. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann-Petersen R, Gordon C. Integral UBL domain proteins: a family of proteasome interacting proteins. Semin. Cell Dev. Biol. 2004;15:247–259. doi: 10.1016/j.semcdb.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda F, Hecker CM, Rozenknop A, Nordmeier RD, Rogov V, Hofmann K, Akira S, Dötsch V, Dikic I. Involvement of the ubiquitin-like domain of TBK1/IKK-i kinases in regulation of IFN-inducible genes. EMBO J. 2007;26:3451–3462. doi: 10.1038/sj.emboj.7601773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 7.Huang GN, Huso DL, Bouyain S, Tu J, McCorkell KA, May MJ, Zhu Y, Lutz M, Collins S, Dehoff M, et al. NFAT binding and regulation of T cell activation by the cytoplasmic scaffolding Homer proteins. Science. 2008;319:476–481. doi: 10.1126/science.1151227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishiguro K, Xavier R. Homer-3 regulates activation of serum response element in T cells via its EVH1 domain. Blood. 2004;103:2248–2256. doi: 10.1182/blood-2003-08-2671. [DOI] [PubMed] [Google Scholar]
- 9.Weil R, Schwamborn K, Alcover A, Bessia C, Di Bartolo V, Israël A. Induction of the NF-kappaB cascade by recruitment of the scaffold molecule NEMO to the T cell receptor. Immunity. 2003;18:13–26. doi: 10.1016/s1074-7613(02)00506-x. [DOI] [PubMed] [Google Scholar]
- 10.Hanson EP, Monaco-Shawver L, Solt LA, Madge LA, Banerjee PP, May MJ, Orange JS. Hypomorphic nuclear factor-kappaB essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J. Allergy Clin. Immunol. 2008;122:1169–1177. doi: 10.1016/j.jaci.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 12.Solt LA, Madge LA, May MJ. NEMO binding domains of both IKKalpha and IKKbeta regulate IKK complex assembly and classical NF-kappaB activation. J. Biol. Chem. 2009;284:27596–27608. doi: 10.1074/jbc.M109.047563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee PP, Pandey R, Zheng R, Suhoski MM, Monaco-Shawver L, Orange JS. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J. Exp. Med. 2007;204:2305–2320. doi: 10.1084/jem.20061893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 15.He KL, Ting AT. Essential role for IKKgamma/NEMO in TCR-induced IL-2 expression in Jurkat T cells. Eur. J. Immunol. 2003;33:1917–1924. doi: 10.1002/eji.200323650. [DOI] [PubMed] [Google Scholar]
- 16.Usui S, Konno D, Hori K, Maruoka H, Okabe S, Fujikado T, Tano Y, Sobue K. Synaptic targeting of PSD-Zip45 (Homer 1c) and its involvement in the synaptic accumulation of F-actin. J. Biol. Chem. 2003;278:10619–10628. doi: 10.1074/jbc.M210802200. [DOI] [PubMed] [Google Scholar]
- 17.Demetriou M, Granovsky M, Quaggin S, Dennis JW. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature. 2001;409:733–739. doi: 10.1038/35055582. [DOI] [PubMed] [Google Scholar]
- 18.Ishibashi T, Ogawa S, Hashiguchi Y, Inoue Y, Udo H, Ohzono H, Kato A, Minakami R, Sugiyama H. A novel protein specifically interacting with Homer2 regulates ubiquitin-proteasome systems. J. Biochem. 2005;137:617–623. doi: 10.1093/jb/mvi074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.