Abstract
Background
For non-small cell lung cancer (NSCLC) patients with pN2 status, the use of postoperative radiotherapy (PORT) remains controversial. Here, we investigated the association between different clinicopathological features and postoperative therapy and local control and survival in patients with resected pN2 NSCLC.
Methods
We retrospectively analyzed 83 patients with pN2 NSCLC who underwent resection at Vanderbilt University Medical Center between 1994 and 2004. The relationship between 10 prognostic factors—gender, age at diagnosis, histology, tumor size, number of nodal stations involved, positive node number, surgical margin, extracapsular extension (ECE), and use of postoperative chemotherapy and PORT—and 2-year local recurrence-free survival (LRFS), distant recurrence-free survival (DRFS), recurrence-free survival (RFS), and overall survival (OS) rates was evaluated. Univariate and multivariate analyses were conducted using the Kaplan–Meier method and Cox proportional hazards ratios, respectively.
Results
On univariate analysis, PORT was significantly associated with greater LRFS, RFS, and OS rates, whereas chemotherapy was associated with a trend toward a higher OS rate. Negative surgical margins were predictive of a higher OS rate, and negative ECE was associated with higher LRFS and RFS rates. On multivariate analysis, only PORT and negative ECE were associated with a higher LRFS rate. On subgroup analysis, in negative ECE patients, PORT was significantly associated with a higher OS rate.
Conclusions
PORT is associated with a higher OS rate for patients with resected pN2 NSCLC with negative ECE but not with positive ECE. The absence of ECE may serve as a useful prognostic variable in the selection of pN2 NSCLC patients for PORT and warrants further investigation in randomized clinical trials.
Keywords: Adjuvant radiotherapy, Non-small cell lung cancer, pN2, Prognostic factors, Extracapsular extension
Introduction
Lung cancer is the most prevalent cancer for both men and women, and remains the leading cause of cancer-related mortality in the U.S., with 160,390 estimated deaths in 2007 [1]. Approximately 85% of all lung cancers are non-small cell lung cancer (NSCLC), which includes squamous cell carcinoma, adenocarcinoma, and large-cell carcinoma. Surgery is the treatment of choice for patients with early-stage NSCLC, whereas radiation is the most effective single treatment modality for unresectable patients. For the subgroup of patients with completely resected stage IIIA pN2 (defined by ipsilateral mediastinal or subcarinal lymph node involvement) NSCLC, several recent, large trials have confirmed the benefit of postoperative chemotherapy [2, 3]. Importantly, up to 50% of these patients fail locoregionally even after postoperative chemotherapy, with most of these recurrences at the bronchial stump line [4 – 6].
There has thus been an interest in the use of postoperative radiotherapy (PORT) to improve outcomes for these patients. However, the value of PORT in NSCLC is still controversial, with some studies suggesting a positive benefit and others suggesting that it does not prolong life and only increases toxicity [7]. PORT was shown, in several randomized trials in the 1980s and 1990s, to provide superior locoregional control [8–11]. However, enthusiasm for PORT dramatically waned after the 1998 PORT Meta-Analysis Trialists Group reported a 7% absolute greater mortality associated with PORT [12]. That study analyzed patient data from nine prospective randomized trials of patients with resected NSCLC in which PORT was compared with surgery alone, and strongly suggested that PORT had a detrimental effect on survival for patients with pN0 or pN1 disease, but remained equivocal for patients with pN2 disease. Overall, the 2-year survival rate was 48% in patients receiving PORT, compared with 55% for patients treated with surgery alone, with an inverse relationship between survival decrement and increasing nodal status. The apparent paradox between better local control and a detriment in terms of survival for the use of PORT in patients with completely resected NSCLC may have been a result of the high toxicity associated with PORT, particularly in regard to cardiopulmonary complications. In the PORT meta-analysis study, 15% of the deaths with PORT and 9% of the deaths with surgery alone were coded as intercurrent and, respectively, 4% and 2% were coded as treatment related, suggestive of the toxic effects of PORT used in the studies [13]. Indeed, the studies included in the meta-analysis used relatively large radiation fields, in contrast to the more conformal computed tomography (CT)-based treatment fields used today. The meta-analysis also included trials in which a majority of the patients were treated with cobalt machines, which are no longer standard of care. In addition, unpublished data were included in the analysis, and one third of the participants included in the analysis came from one randomized trial using excessive radiation doses. Despite these detrimental factors, there was a trend, although not statistically significant, toward longer survival for patients with pN2 status.
Since the publication of the PORT meta-analysis, there was a sustained decline in the use of PORT for NSCLC between 1992 and 2002, as reported by an analysis of the Surveillance, Epidemiology, and End Results (SEER) database [14]. Of particular interest, PORT use in patients with N2 disease dropped from 65% in 1995 to 37% in 2002.
A number of recent studies have suggested that PORT may be of benefit for patients with completely resected pN2 NSCLC. An analysis of the SEER database for patients with resected NSCLC between 1998 and 2002 showed that, although PORT had a detrimental effect on survival for patients with pN0 or pN1 disease, it was associated with longer survival for patients with pN2 disease [15]. In addition, a subgroup analysis of the Adjuvant Navelbine International Trialist Association (ANITA) trial, which compared adjuvant cisplatin plus vinorelbine with observation in patients with completely resected stage IB–IIIA NSCLC, showed that PORT led to longer overall survival (OS) in patients with resected pN2 NSCLC, both in the chemotherapy arm and in the observation arm [16].
Taken as a whole, patients with pN2, stage IIIA NSCLC have a poor long-term OS rate, estimated at 24% in the analysis of the SEER database [17]. However, patients with pN2, stage IIIA NSCLC are a heterogeneous group with different clinicopathologic features that can range from microscopic, single-station mediastinal nodal involvement to bulky, multistation, fixed mediastinal nodal disease. Thus, there may be significant variability in survival among this heterogeneous group. Indeed, the 5-year survival rate is 5%– 8% for multistation bulky disease and 35% for single-station microscopic disease [18]. Given the heterogeneity of the pN2 NSCLC population, we evaluated the prognostic significance of different clinicopathologic features and postoperative therapy on local recurrence-free survival (LRFS), distant recurrence-free survival (DRFS), recurrence-free survival (RFS), and OS. These features included patient gender, age, histology, tumor size, number of nodal stations involved, number of positive nodes, surgical margin status, extracapsular extension (ECE) status, the use of PORT, and the use of postoperative chemotherapy.
Materials and Methods
Patients and Study Design
Between July 1994 and December 2004, 104 consecutive patients diagnosed with NSCLC and pN2 status were treated with surgical resection at Vanderbilt University Medical Center. Among those patients, 21 had neoadjuvant treatment prior to surgery, consisting of chemotherapy (70%) or chemoradiotherapy (30%). The remainder of the patients (n = 83) had no further therapy (39%), postoperative chemotherapy (14%), or postoperative radiotherapy with (28%) or without (19%) concurrent chemotherapy.
Inclusion in this retrospective analysis required the following: resection consisting of a lobectomy or pneumonectomy, pathological confirmation of pN2 NSCLC, and operative reports and imaging studies (CT scan or positron emission tomography [PET] scan) available for review. Patients who received neoadjuvant chemotherapy/radiation therapy or who had a simultaneous or sequential secondary primary lung cancer or other cancer were excluded from the study. We required that all patients have complete information on tumor size, tumor location, extent of disease/lymph node involvement, surgical margin status, ECE status, and cause of death if applicable. Patients who did not meet one of these criteria were excluded. As a result of our selection criteria, this analysis included a total of 83 patients. The Vanderbilt University institutional review board approved this study.
Workup, Pathology Features, and Adjuvant Treatment
The preoperative workup included standard biochemical tests, pulmonary function tests, a ventilation/perfusion scan, and a chest x-ray. In addition, all patients had a preoperative chest CT. Of the 83 patients, 53 (64%) also had a preoperative mediastinoscopy and 54 (65%) had a PET scan. All patients had pathological confirmation of NSCLC based on biopsy. The histological cancer type was squamous cell carcinoma in 38% of the patients, adenocarcinoma in 51% of the patients, and large-cell carcinoma in 11% of the patients. Complete resection with negative surgical margins was achieved in 71 (∼85%) patients. In the other 12 (15%) patients, surgical margins were microscopically positive.
Pathologic features, including specific histology, tumor size, number of involved nodal stations, number of positive nodes, surgical margin status, and ECE status, were assessed for each patient. Lymphatic invasion was found in 39, vascular invasion was found in 66, and perineural invasion was found in 80 patients. Systematic mediastinal lymph node dissection, consisting of at least three nodal stations, was performed in all patients. Pathology reports after surgery showed that 60% of patients had > 1 involved nodal station, 80% had more than one positive lymph node, and 17% of patients had a positive ECE status.
Adjuvant chemotherapy delivered with or without radiation therapy consisted of either cisplatin or carboplatin and paclitaxel. The median thoracic radiation dose was 54 Gy (range, 50–60 Gy). All 39 patients were treated with CT-based planning according to departmental guidelines. Radiotherapy consisted of a three-field technique (posterior and two lateral fields). Individual variation in field design was based on patient characteristics and physician preference. Radiotherapy for all patients was delivered using a linear accelerator with effective photon energies ≥6 MV and customized complex blocking.
Follow-Up
The median follow-up time for all patients was 64 months (range, 2–172 months). CT imaging documenting the site of recurrence was available for all patients. Follow-up information was obtained from patient chart records, Department of Radiation Oncology records, pathology reports, and radiology reports. Follow-up information was also obtained from the Vanderbilt-Ingram Cancer Center Tumor Registry.
Statistical Analysis
We collected data on the following patient characteristics: gender, age at diagnosis, histology, tumor size, number of nodal stations involved, number of positive nodes, surgical margin status, and ECE status. We also recorded the use of adjuvant treatment, including no adjuvant treatment, PORT, postoperative chemotherapy, and postoperative chemoradiotherapy. A χ2 test was used to determine the distribution of patient characteristics within each treatment group. We collected data on the method of surgical resection (lobectomy versus pneumonectomy), presence or absence of mediastinoscopy, radiation treatment approach, treatment field, fractionation schedule, total dosage, chemotherapeutic regimen, and schedule of chemotherapy and radiation delivered. Categorical variables analyzed included: patient gender (male versus female), age at diagnosis (≤60 years versus >60 years), histology (squamous cell carcinoma versus other histology), tumor size (≤40 mm versus >40 mm), number of nodal stations involved (one or fewer versus more than one), number of positive nodes (one or fewer versus more than one), surgical margin status (negative versus positive), ECE status (negative versus positive), chemotherapy (no versus yes), and radiotherapy (no versus yes).
LRFS, DRFS, RFS, and OS were the primary endpoints. The LRFS time was defined as the time from diagnosis until the presence of recurrence in the lung or regional lymph nodes as confirmed by pathology or imaging. The DRFS time was defined as the time from diagnosis until the presence of recurrence in sites other than the lung or regional lymph nodes as confirmed by pathology or imaging. The RFS time was defined as the time from diagnosis until the presence of local or distant recurrence as confirmed by pathology or imaging or death attributable to the patient's lung cancer. The OS time was defined as the time from diagnosis until death. LRFS, DRFS, RFS, and OS rates were calculated by the Kaplan–Meier method. To determine prognostic value, study variables were compared with the survival measures using log-rank tests. The Pearson χ2 test was used to determine unadjusted associations between categorical variables of interest and LRFS, DRFS, RFS, and OS rates. Multivariable Cox proportional hazard models were used to calculate adjusted hazard ratios (HRs) and their 95% confidence intervals (CIs) relating to the variables as described. The R statistical package (version 2.6.1) was used for all analyses.
Results
As a result of the selection criteria, the analysis included a total of 83 patients, the characteristics of which are shown in Table 1. The median age at diagnosis of the patients in the analysis was 61 years (range, 35–85 years), with the majority of patients aged >60 (60.2%) and male (57.8%). The histological cancer type was predominantly adenocarcinoma (51%) or squamous cell carcinoma (38%), with only a minority of patients presenting with large-cell carcinoma (11%). Complete resection with negative surgical margins was achieved in most patients (85%). In the remainder of the patients, surgical margins were microscopically positive. Pathology reports after surgery also showed that a majority of the patients had more than one involved nodal station (60.2%) and more than one positive lymph node (79.5%), whereas 14.5% of the patients had positive surgical margins and 16.9% had a positive ECE status. With regard to adjuvant treatment, less than two thirds of the patients received either PORT alone (19.3%), postoperative chemotherapy alone (14.5%), or a combination of PORT and chemotherapy (27.7%), whereas 38.9% had no adjuvant treatment. On association analysis, there was a statistically significant difference in the treatment groups for patients aged ≤60 compared with patients aged >60 (p = .016). The distribution of adjuvant treatment was not statistically different for the other patient characteristics analyzed.
Table 1. Patient characteristics.
| Characteristic | Total n = 83 (%) |
Treatment group | p-value | |||
|---|---|---|---|---|---|---|
| Surgery only n = 32 (38.9%) |
Surgery + radiotherapy n = 16 (19.3%) |
Surgery + chemotherapy n = 12 (14.5%) |
Surgery + chemotherapy + radiotherapy n = 23 (27.7%) |
|||
| Gender | ||||||
| Female | 35 (42.2) | 12 (37.5) | 6 (37.5) | 6 (50) | 11 (47.8) | .799 |
| Male | 48 (57.8) | 20 (62.5) | 10 (62.5) | 6 (50) | 12 (52.2) | |
| Age at diagnosis | ||||||
| ≤60 yrs | 33 (39.8) | 6 (18.75) | 8 (50) | 6 (50) | 13 (56.5) | .016 |
| >60 yrs | 50 (60.2) | 26 (81.25) | 8 (50) | 6 (50) | 10 (43.5) | |
| Histology | ||||||
| SCC | 30 (36.1) | 12 (37.5) | 4 (25) | 4 (33.3) | 10 (43.5) | .707 |
| Other (ADC,…) | 53 (63.9) | 20 (62.5) | 12 (75) | 8 (66.7) | 13 (56.5) | |
| Tumor size | ||||||
| ≤40 | 45 (54.2) | 13 (40.63) | 11 (68.7) | 8 (66.67) | 13 (56.5) | .221 |
| >40 | 38 (45.8) | 19 (59.38) | 5 (31.3) | 4 (33.33) | 10 (43.5) | |
| n of nodal stations involved | ||||||
| ≤1 | 33 (39.8) | 15 (46.9) | 7 (43.7) | 2 (16.7) | 9 (39.1) | .337 |
| >1 | 50 (60.2) | 17 (53.1) | 9 (56.3) | 10 (83.3) | 14 (60.9) | |
| n of positive nodes | ||||||
| ≤1 | 17 (20.5) | 8 (25) | 5 (31.3) | 0 | 4 (17.4) | .172 |
| >1 | 66 (79.5) | 24 (75) | 11 (68.7) | 12 (100) | 19 (82.6) | |
| Surgical margins | ||||||
| Negative | 71 (85.5) | 25 (78.1) | 14 (87.5) | 11 (91.7) | 21 (91.3) | .583 |
| Positive | 12 (14.5) | 7 (21.9) | 2 (12.5) | 1 (8.3) | 2 (8.7) | |
| ECE | ||||||
| Negative | 69 (83.1) | 26 (81.2) | 14 (87.50) | 9 (75.00) | 20 (87) | .566 |
| Positive | 14 (16.9) | 6 (18.8) | 2 (12.50) | 3 (25.00) | 3 (13) | |
Abbreviations: ADC, adenocarcinoma; ECE, extracapsular extension; SCC, squamous cell carcinoma.
Of the 83 patients, 19 (23%) had locoregional recurrence as the first site of failure, including those with a simultaneous distant metastasis (n = 4). Locoregional failures were defined as recurrences in lymph node areas (N1, N2, N3) or at the bronchial margin of resection. Recurrences beyond these sites were considered distant metastases. Twenty-seven patients (33%) had distant metastasis as the first site of recurrence, and all were exempt from local recurrence. The distribution of distant recurrences was as follows: brain, n = 11; bone, n = 12; and liver, n = 4.
Univariate analyses were performed to determine whether clinicopathologic features, the use of PORT, and the use of postoperative chemotherapy were potentially associated with local control and survival. The results of all univariate analyses are listed in Table 2. On univariate analysis, for all patients, the use of PORT was significantly associated with higher LRFS (p <.001), RFS (p =.013), and OS (p =.002) rates but not DRFS rate (p =.569). Negative surgical margins were also predictive of a higher OS rate (p = .016) but not higher LRFS (p = .753), DRFS (p = .171), or RFS (p = .458) rates. In contrast, negative ECE status was significantly associated with higher LRFS (p < .001) and RFS (p = .004) rates but not OS (p = .357) or DRFS (p = .517) rates. Female gender was associated with a higher DRFS rate (p = .027), but not LRFS (p = .876), RFS (p = .504), or OS (p = .379) rates. Although not significant, patients presenting with squamous cell carcinoma histology had a trend toward a lower DRFS rate than those with adenocarcinomas. There was also a trend toward a higher OS rate with postoperative chemotherapy (p = .055). Age at diagnosis, tumor size, number of nodal stations involved, and number of positive nodes were not prognostic. A Kaplan–Meier plot of the association between PORT and LRFS duration is presented in Figure 1, demonstrating a longer LRFS time in patients treated with PORT.
Table 2. Results of univariate analysis of prognostic factors.
| Factor | n of patients | 2-Yr OS | 2-Yr RFS | 2-Yr LRFS | 2-Yr DRFS | ||||
|---|---|---|---|---|---|---|---|---|---|
| % (95% CI) |
p-value | % (95% CI) |
p-value | % (95% CI) |
p-value | % (95% CI) |
p-value | ||
| Gender | |||||||||
| Female | 35 | 38 (22–55) | .38 | 30 (12–49) | .504 | NA | .876 | 33 (12–54) | .027 |
| Male | 48 | 27 (15–40) | 35 (19–52) | 57 (37–76) | 55 (35–75) | ||||
| Age at diagnosis | |||||||||
| ≤60 yrs | 33 | 27 (15–40) | .182 | 35 (19–52) | .959 | 45 (27–64) | .023 | 54 (34–75) | .125 |
| >60 yrs | 50 | 38 (21–55) | 31 (11–50) | NA | 36 (16–56) | ||||
| Histology | |||||||||
| SCC | 30 | 30 (18–43) | .658 | 32 (17–47) | 60 (43–77) | .746 | 38 (21–55) | .059 | |
| Other (ADC,…) | 53 | 33 (16–50) | 34 (12–57) | .559 | NA | 58 (31–85) | |||
| Tumor size | |||||||||
| ≤40 mm | 45 | 26 (12–40) | .435 | 26 (8–44) | .506 | NA | .055 | 36 (12–60) | .788 |
| >40 mm | 38 | 36 (22–51) | 39 (21–56) | 66 (47–85) | 52 (33–70) | ||||
| n of nodal stations involved | |||||||||
| ≤1 | 33 | 33 (19–46) | .612 | 29 (14–44) | .246 | NA | .09 | 43 (25–61) | .359 |
| >1 | 50 | 30 (14–46) | 42 (20–64) | 67 (42–93) | 52 (28–76) | ||||
| n of positive nodes | |||||||||
| ≤1 | 17 | 32 (21–44) | .884 | 36 (22–50) | .816 | NA | .396 | 52 (35–68) | .555 |
| >1 | 66 | NA | 23 (0–49) | 58 (21–96) | NA | ||||
| Surgical margins | |||||||||
| Negative | 71 | 37 (26–49) | .016 | 37 (24–50) | .458 | 59 (45–73) | .753 | 51 (36–66) | .171 |
| Positive | 12 | 18 (8–27) | NA | NA | NA | ||||
| ECE | |||||||||
| Negative | 69 | 35 (23–47) | .357 | 42 (27–57) | .003 | 71 (56–86) | <.001 | 49 (33–64) | .517 |
| Positive | 14 | 22 (0–47) | 15 (4–30) | NA | NA | ||||
| Chemotherapy | |||||||||
| No | 48 | 27 (14–40) | .055 | 33 (15–50) | .233 | 55 (36–73) | .162 | 49 (28–71) | .686 |
| Yes | 35 | 38 (21–54) | 33 (14–51) | NA | 41 (21–61) | ||||
| Radiotherapy | |||||||||
| No | 44 | 21 (9–33) | .002 | NA | .013 | NA | <.001 | NA | .569 |
| Yes | 39 | 43 (28–59) | 47 (29–65) | 77 (60–94) | 51 (33–69) | ||||
p-value for each variable was calculated using the log-rank test, limited to patients for whom data were available.
Abbreviations: ADC, adenocarcinoma; CI, confidence interval; DRFS, distant recurrence-free survival; ECE, extracapsular extension; LRFS, local recurrence-free survival; NA, not available; OS, overall survival; RFS, recurrence-free survival; SCC, squamous cell carcinoma.
Figure 1.
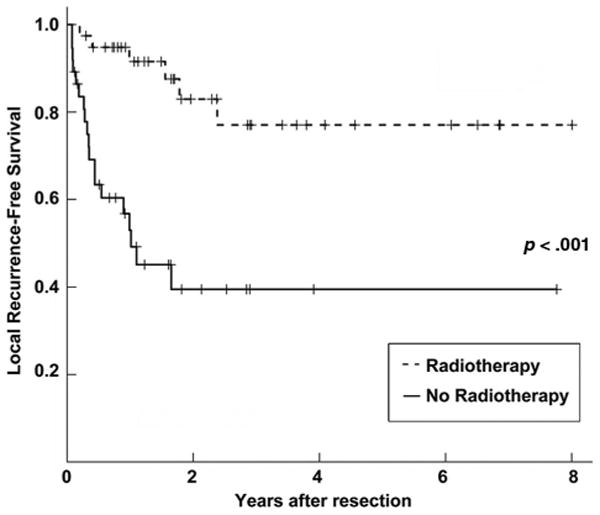
Kaplan–Meier plot of local recurrence-free survival for all patients stratified by postoperative radiotherapy (PORT) use. The solid line represents patients who did not receive PORT, and the dashed line represents patients who received PORT.
We next performed a multivariate analysis to determine independent prognostic factors with respect to local control and survival, based on the results of our univariate analyses. As shown in Table 3, the use of PORT (HR, 0.254; 95% CI, 0.087– 0.741; p = .012) and negative ECE status (HR, 0.311; 95% CI, 0.118–0.799; p = .016) were independent predictors of a higher LRFS rate (Table 3) but not DRFS, RFS, or OS rates. Postoperative chemotherapy, age at diagnosis, and sex were not prognostic on multivariate analysis. Subset analysis was then performed for all patients based on ECE status. The use of PORT was associated with a statistically significant higher OS rate with respect to patients with a negative ECE status (HR, 0.518; 95% CI, 0.276–0.971; p = 0.040) but not those with a positive ECE status (HR, 2.052; 95% CI, 0.495–8.507; p = 0.322) (Table 4). Postoperative chemotherapy, age at diagnosis, and sex were not prognostic with respect to ECE status. Kaplan–Meier plots of the association between PORT and OS according to ECE status are shown in Figures 2 and 3, demonstrating a longer OS time with PORT only in the subset of patients with a negative ECE status.
Table 3. Independent factors predictive of local recurrence-free survival from non-small cell lung cancer.
| Factor | Hazard ratio (95% CI) |
p-value |
|---|---|---|
| Radiotherapy versus no radiotherapy | 0.254 (0.087–0.741) | .012 |
| Negative ECE versus positive ECE | 0.311 (0.118–0.799) | .016 |
| Chemotherapy versus no chemotherapy | 0.900 (0.372–2.177) | .815 |
| Age at diagnosis | 0.988 (0.941–1.038) | .642 |
| Female versus male | 0.883 (0.364–2.139) | .782 |
p-values were derived from the Cox proportional-hazards model, with simultaneous inclusion of all factors shown. Abbreviations: CI, confidence interval; ECE, extracapsular extension.
Table 4. Independent factors predictive of overall survival in patients with non-small cell lung cancer according to ECE status.
| Factor | Negative ECE | Positive ECE | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) |
p-value | Hazard ratio (95% CI) |
p-value | |
| Radiotherapy versus no radiotherapy | 0.518 (0.276–0.971) | .04 | 2.052 (0.495–8.507) | .322 |
| Chemotherapy versus no chemotherapy | 0.554 (0.295–1.041) | .067 | 0.932 (0.228–3.803) | .921 |
| Age at diagnosis | 1.025 (0.995–1.054) | .099 | 1.010 (0.925–1.101) | .831 |
| Female versus male | 0.837 (0.448–1.564) | .578 | 0.436 (0.102–1.861) | .262 |
p-values were derived from the Cox proportional-hazards model, with simultaneous inclusion of all factors shown. Abbreviations: CI, confidence interval; ECE, extracapsular extension.
Figure 2.
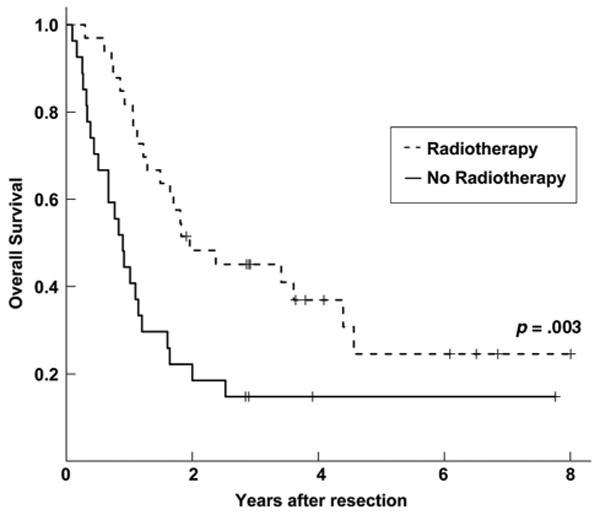
Kaplan–Meier plot of overall survival for extracapsular extension negative patients stratified by postoperative radiotherapy (PORT) use. The solid line represents patients who did not receive PORT, and the dashed line represents patients who received PORT.
Figure 3.
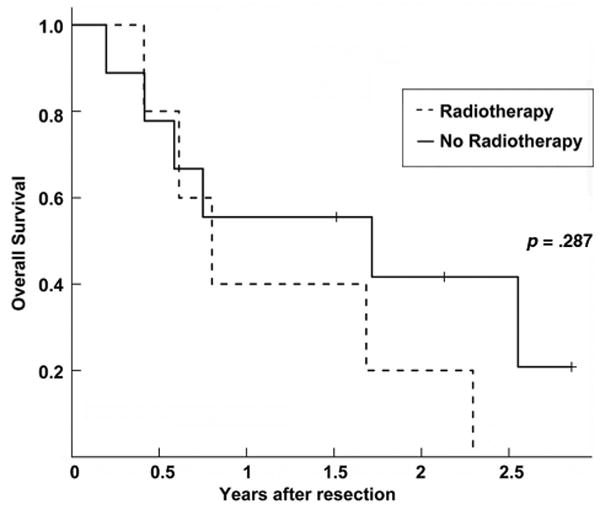
Kaplan–Meier plot of overall survival for extracapsular extension positive patients stratified by postoperative radiotherapy (PORT) use. The solid line represents patients who did not receive PORT, and the dashed line represents patients who received PORT.
Discussion
Patients with N2 NSCLC have a poor outcome even when surgical resection is complete. Those patients are recognized as a very heterogeneous population with multiple subgroups, ranging from microscopic N2 to bulky multistation nodal involvement. Favorable N2 disease is characterized by pathologic features such as a single lymph node or single-station involvement and microscopic and/or intracapsular metastases, but the 5-year survival rate remains low, at 35%. In contrast, multistation, bulky N2 disease has a dramatic 5% 5-year survival rate. In this study, we investigated the prognostic significance of different clinicopathologic features (including histology, tumor size, number of nodal stations involved, number of positive nodes, surgical margin status, and ECE status) and postoperative therapy in patients with resected NSCLC with pN2 status. Interestingly, our results suggest that PORT leads to longer OS times for patients with resected pN2 NSCLC with a negative ECE status but not with a positive ECE status.
Because the current study is retrospective, there are some limitations. Indeed, our study is based on a relatively small data set from a single institution and thus has the potential for not having general representation. Although pathology specimens were not available for review at the time of the retrospective analysis, strict standards are maintained at our institution ensuring that pathology specimens are analyzed and entered into electronic medical records accurately. Yet we hope that this study will be a reminder for pathologists of the importance of specifically looking for ECE and reporting its positive or negative status systematically, especially at institutions where there is no standard for reporting pathologic findings. Nevertheless, our findings of a benefit of PORT in terms of OS are consistent with several recent retrospective analyses suggesting a longer OS duration with PORT for patients with resected pN2 NSCLC. An analysis of the SEER database for patients with resected NSCLC between 1998 and 2002 showed that PORT was associated with a longer OS time for patients with pN2 disease (HR, 0.855; p = .008) [15]. Furthermore, a subgroup analysis of the ANITA trial, which compared adjuvant cisplatin plus vinorelbine with observation in patients with completely resected stage IB–IIIA NSCLC, showed that PORT led to a longer OS time in patients with resected pN2 NSCLC both in the chemotherapy arm (median, 47.4 months versus 23.8 months) and in the observation arm (median, 22.7 months versus 12.7 months) [16].
Thus far, there has been no randomized study demonstrating an OS benefit with PORT for patients with resected pN2 NSCLC, although several trials have shown a local control benefit. Among the earliest positive studies, the Lung Cancer Study Group randomized patients with completely resected stage II–III NSCLC to PORT versus observation [8]. They found a lower rate of local recurrence as the first site of relapse, 3% versus 41% (p < .001), and a lower overall local recurrence rate for pN2 disease, 29% versus 57% (p = .03), in favor of PORT. Later, a Medical Research Council Trial and Cancer and Leukemia Group B trial both found a trend toward a lower local recurrence rate with PORT than with observation for patients with resected pN2 NSCLC [11]. In contrast to these positive results, the 1998 PORT metaanalysis, which analyzed nine prospective randomized trials, demonstrated that PORT was detrimental in terms of survival for pN0 or pN1 disease but was equivocal for patients with pN2 disease [12]. However, the meta-analysis remains controversial because most of the included studies had limitations, such as suboptimal, outdated radiotherapy volumes (large fields), dose regimens (large fractions), and radiation techniques (including cobalt machines) that are not part of currently accepted practices [7]. In our study, all patients were treated with linear accelerators as well as with CT-based conformal treatment planning, which can “shape” the radiation better around the target and thus minimize toxicity to normal tissues, including the heart and lungs, to which many of the deaths in the PORT meta-analysis study were attributed. More recently, a Beijing trial randomized 366 patients with resected pN1–N2 NSCLC to PORT versus observation. Interestingly, they used linear accelerators and treatment planning that share similarities with our study, and found lower local recurrence rate, 13% versus 33% (p < .01), in favor of PORT [9]. These results are consistent with our findings, and combined with those of the SEER database analysis and ANITA subgroup analysis suggest that the role of PORT for patients with resected pN2 NSCLC should be further investigated in a randomized clinical trial in an era of superior radiotherapy delivery techniques.
Clinicopathological features in resected pN2 disease associated with a favorable prognosis in our study included negative surgical margins, negative ECE status, and female gender on univariate analysis. Age at diagnosis, tumor size, histology, number of involved nodal stations, and total number of positive nodes showed no prognostic significance. On multivariate analysis, only negative ECE status and the use of PORT were associated with a favorable prognosis. Previous studies have shown that negative margins [19 –21], negative ECE status [22–25], smaller tumor size [20, 22, 25–27], squamous cell histology [24, 25, 28], fewer involved mediastinal nodal stations [19, 25], and fewer involved N2 nodes [20, 26] are associated with a better prognosis. Differences in the prognostic significance of clinicopathological features for resected pN2 NSCLC in our study compared with other studies may also be attributed to differences in adjuvant treatment for studies analyzed by univariate analysis. Of note, negative surgical margins were predictive of a higher OS rate but not LRFS, DRFS, or RFS rate in our univariate analyses. We suspect that local control was more dependent on the presence of “bulky” N2 disease than the presence of positive surgical margins. In addition, the small sample size and low number of events in that specific subgroup could be limiting factors for LRFS, DRFS, and RFS analyses.
The main objective of PORT in patients with a high risk for local recurrence is to eradicate residual microscopic disease, such as at the resection margin or in mediastinal node areas. In these situations, the superior local control provided by PORT should translate into a survival benefit, as seen in other tumor sites such as the breast [29]. A recent analysis showed that the use of PORT was associated with a longer OS time for patients with multinodal resected pN2 disease [30]. Consistently, a retrospective analysis from the Mayo Clinic demonstrated that PORT was more effective for patients with pN2 NSCLC with a high risk for local recurrence, although ECE status was not analyzed in that study [21]. As we know, nodal ECE is an adverse risk factor in a number of cancers treated with radiation therapy, including head and neck cancer [31] and breast cancer [32, 33]. Indeed, it is usually associated with the risk for local recurrence and thus the rationale (and potential benefit) for the use of PORT. In daily practice, many radiation oncologists extrapolate the findings from other cancer sites and use ECE as an additional indicator of greater local risk in lung cancer patients with mediastinal disease. Although a positive ECE status is considered an adverse local risk factor, and as such an indication for PORT, our results suggest that PORT paradoxically leads to longer OS times for patients with resected pN2 NSCLC with a negative ECE status but not with a positive ECE status. We speculate that a positive ECE status may be an indicator for a higher risk for clinically occult distant metastatic disease in NSCLC. Thus, only patients with pN2 NSCLC with a negative ECE status benefit from PORT. Although our univariate analysis did not show an association between a negative ECE status and a higher DRFS rate, perhaps as a result of confounding variables or the limited sample size, a positive ECE status has been associated with a greater risk for distant failure in breast cancer [33] and rectal cancer [34] patients. To the best of our knowledge, no evidence suggesting that a positive ECE status is associated with occult distant metastatic disease in lung cancer has been reported in the literature so far. In a recent retrospective study, Lee et al. [35] reported that ECE had a negative impact on OS in lung cancer patients who underwent surgical resection without any adjuvant treatment. Unfortunately, that study did not provide details concerning the pattern of recurrence and thus provides no information on local control versus distant failure based on ECE status. Based on our experience, we suggest that the definitive value of ECE as a predictive factor for the use of PORT should be examined in a large, prospective randomized trial in lung cancer patients presenting with pN2 disease. We recommend that pathologic features such as ECE be evaluated in all patients that are enrolled in such a trial. In addition, the statistical design should provide enough power to stratify patients according to number of positive lymph nodes, number of involved nodal stations, and ECE status with respect to the use of PORT.
Of note, recent studies have shown a benefit for the use of adjuvant chemotherapy using a variety of regimens [2, 3, 36]. In the ANITA subgroup analysis, patients with pN2 disease had a 47.4% survival rate at 5 years with adjuvant chemotherapy plus PORT, compared with a 34% survival rate in patients treated with adjuvant chemotherapy, 21.3% in patients treated with adjuvant PORT, and 16.6% in patients treated with surgery only. Adjuvant chemotherapy is now considered the standard of care for patients with resected pN2 NSCLC. The limited benefit of chemotherapy in our study may be a result of the limited sample size or confounding variables such as differences in timing of delivery of chemotherapy and the concurrent use of radiotherapy. Nevertheless, larger studies are needed to determine how the use of chemotherapy impacts the prognosis of patients with pN2 NSCLC treated with PORT with respect to ECE status. Indeed, if chemotherapy is able to control metastatic disease such that the primary tumor site becomes the primary location for disease recurrence, PORT may also be beneficial for patients with a positive ECE status.
In summary, our study suggests that PORT results in a higher OS rate for patients with resected pN2 NSCLC with a negative ECE status but not with a positive ECE status. This study raises the question of the utility of selecting subgroups of patients with pN2 NSCLC for treatment with PORT. We suggest that the absence of ECE may be used as a prognostic variable in the decision to use PORT for resected pN2 NSCLC patients and that it deserves further investigation in future randomized clinical trials.
Acknowledgments
Authors Luigi Moretti and David S. Yu contributed equally to this manuscript.
Footnotes
Disclosures: Luigi Moretti: None; Davis S. Yu: None; Heidi Chen: None; David P. Carbone: None; David H. Johnson: None; Vicki L. Keedy: None; Joe B. Putnam, Jr.: None; Alan B. Sandler: None; Yu Shyr: None; Bo Lu: None.
Section editors Cesare Gridelli and Lecia Sequist have disclosed no financial relationships relevant to the content of this article.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias.
Author Contributions:
Conception/Design: Bo Lu, Luigi Moretti
Administrative support: Bo Lu, David Carbone, David Johnson
Provision of study material or patients: Joe B. Putnam, Jr., Alan Sandler, David Carbone, David Johnson, Vicki Keedy
Collection and/or assembly of data: Luigi Moretti, David Yu
Data analysis and interpretation: Bo Lu, Yu Shyr, Luigi Moretti, David Yu, Heidi Chen
Manuscript writing: Luigi Moretti, David Yu, Vicki Keedy
Final approval of manuscript: Bo Lu, Luigi Moretti, David Yu, Vicki Keedy
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 3.Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): A randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 4.Dautzenberg B, Arriagada R, Chammard AB, et al. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Groupe d'Etude et de Traitement des Cancers Bronchiques. Cancer. 1999;86:265–273. doi: 10.1002/(sici)1097-0142(19990715)86:2<265::aid-cncr10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 5.Kelsey CR, Light KL, Marks LB. Patterns of failure after resection of non-small-cell lung cancer: Implications for postoperative radiation therapy volumes. Int J Radiat Oncol Biol Phys. 2006;65:1097–1105. doi: 10.1016/j.ijrobp.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Rosell R, Gómez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med. 1994;330:153–158. doi: 10.1056/NEJM199401203300301. [DOI] [PubMed] [Google Scholar]
- 7.Erman M, Moretti L, Soria JC, et al. Adjuvant chemotherapy and radiotherapy in non-small cell lung cancer. Semin Radiat Oncol. 2004;14:315–321. doi: 10.1016/j.semradonc.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Effects of postoperative mediastinal radiation on completely resected stage II and stage III epidermoid cancer of the lung. The Lung Cancer Study Group. N Engl J Med. 1986;315:1377–1381. doi: 10.1056/NEJM198611273152202. [DOI] [PubMed] [Google Scholar]
- 9.Feng QF, Wang M, Wang LJ, et al. A study of postoperative radiotherapy in patients with non-small-cell lung cancer: A randomized trial. Int J Radiat Oncol Biol Phys. 2000;47:925–929. doi: 10.1016/s0360-3016(00)00509-5. [DOI] [PubMed] [Google Scholar]
- 10.Mayer R, Smolle-Juettner FM, Szolar D, et al. Postoperative radiotherapy in radically resected non-small cell lung cancer. Chest. 1997;112:954–959. doi: 10.1378/chest.112.4.954. [DOI] [PubMed] [Google Scholar]
- 11.Stephens RJ, Girling DJ, Bleehen NM, et al. The role of post-operative radiotherapy in non-small-cell lung cancer: A multicentre randomised trial in patients with pathologically staged T1–2, N1–2, M0 disease Medical Research Council Lung Cancer Working Party. Br J Cancer. 1996;74:632–639. doi: 10.1038/bjc.1996.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.PORT Meta-Analysis Trialists Group Postoperative radiotherapy in non-small-cell lung cancer: Systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet. 1998;352:257–263. [PubMed] [Google Scholar]
- 13.Munro AJ. What now for postoperative radiotherapy for lung cancer? Lancet. 1998;352:250–251. doi: 10.1016/S0140-6736(98)22030-7. [DOI] [PubMed] [Google Scholar]
- 14.Bekelman JE, Rosenzweig KE, Bach PB, et al. Trends in the use of postoperative radiotherapy for resected non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66:492–499. doi: 10.1016/j.ijrobp.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 15.Lally BE, Zelterman D, Colasanto JM, et al. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2006;24:2998–3006. doi: 10.1200/JCO.2005.04.6110. [DOI] [PubMed] [Google Scholar]
- 16.Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: The adjuvant Navelbine International Trialist Association (ANITA) randomized trial. Int J Radiat Oncol Biol Phys. 2008;72:695–701. doi: 10.1016/j.ijrobp.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 17.Ravdin PM, Davis G. Prognosis of patients with resected non-small cell lung cancer: Impact of clinical and pathologic variables. Lung Cancer. 2006;52:207–212. doi: 10.1016/j.lungcan.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Andre F, Grunenwald D, Pignon JP, et al. Survival of patients with resected N2 non-small-cell lung cancer: Evidence for a subclassification and implications. J Clin Oncol. 2000;18:2981–2989. doi: 10.1200/JCO.2000.18.16.2981. [DOI] [PubMed] [Google Scholar]
- 19.Miller DL, McManus KG, Allen MS, et al. Results of surgical resection in patients with N2 non-small cell lung cancer. Ann Thorac Surg. 1994;57:1095–1100. doi: 10.1016/0003-4975(94)91335-8. discussion 1100–1101. [DOI] [PubMed] [Google Scholar]
- 20.Mountain CF. Surgery for stage IIIa-N2 non-small cell lung cancer. Cancer. 1994;73:2589–2598. doi: 10.1002/1097-0142(19940515)73:10<2589::aid-cncr2820731021>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.Sawyer TE, Bonner JA, Gould PM, et al. The impact of surgical adjuvant thoracic radiation therapy for patients with nonsmall cell lung carcinoma with ipsilateral mediastinal lymph node involvement. Cancer. 1997;80:1399–1408. doi: 10.1002/(sici)1097-0142(19971015)80:8<1399::aid-cncr6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 22.Ishida T, Tateishi M, Kaneko S, et al. Surgical treatment of patients with nonsmall-cell lung cancer and mediastinal lymph node involvement. J Surg Oncol. 1990;43:161–166. doi: 10.1002/jso.2930430308. [DOI] [PubMed] [Google Scholar]
- 23.Roeslin N, Warter A, Gasser B, et al. Non-aplastic N2 operated bronchial cancers. Multifactorial analysis of the prognosis. Ann Chir. 1991;45:673–678. In French. [PubMed] [Google Scholar]
- 24.van Klaveren RJ, Festen J, Otten HJ, et al. Prognosis of unsuspected but completely resectable N2 non-small cell lung cancer. Ann Thorac Surg. 1993;56:300–304. doi: 10.1016/0003-4975(93)91164-i. [DOI] [PubMed] [Google Scholar]
- 25.Vansteenkiste JF, De Leyn PR, Deneffe GJ, et al. Survival and prognostic factors in resected N2 non-small cell lung cancer: A study of 140 cases. Leuven Lung Cancer Group. Ann Thorac Surg. 1997;63:1441–1450. doi: 10.1016/s0003-4975(97)00314-7. [DOI] [PubMed] [Google Scholar]
- 26.Martini N, Flehinger BJ. The role of surgery in N2 lung cancer. Surg Clin North Am. 1987;67:1037–1049. doi: 10.1016/s0039-6109(16)44341-0. [DOI] [PubMed] [Google Scholar]
- 27.Régnard JF, Magdeleinat P, Azoulay D, et al. Results of resection for bronchogenic carcinoma with mediastinal lymph node metastases in selected patients. Eur J Cardiothorac Surg. 1991;5:583–586. doi: 10.1016/1010-7940(91)90224-8. discussion 587. [DOI] [PubMed] [Google Scholar]
- 28.Goldstraw P, Mannam GC, Kaplan DK, et al. Surgical management of non-small-cell lung cancer with ipsilateral mediastinal node metastasis (N2 disease) J Thorac Cardiovasc Surg. 1994;107:19–27. discussion 27–28. [PubMed] [Google Scholar]
- 29.Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 30.Matsuguma H, Nakahara R, Ishikawa Y, et al. Postoperative radiotherapy for patients with completely resected pathological stage IIIA-N2 non-small cell lung cancer: Focusing on an effect of the number of mediastinal lymph node stations involved. Interact Cardiovasc Thorac Surg. 2008;7:573–577. doi: 10.1510/icvts.2007.174342. [DOI] [PubMed] [Google Scholar]
- 31.Huang DT, Johnson CR, Schmidt-Ullrich R, et al. Postoperative radiotherapy in head and neck carcinoma with extracapsular lymph node extension and/or positive resection margins: A comparative study. Int J Radiat Oncol Biol Phys. 1992;23:737–742. doi: 10.1016/0360-3016(92)90646-y. [DOI] [PubMed] [Google Scholar]
- 32.Huang EH, Tucker SL, Strom EA, et al. Predictors of locoregional recurrence in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, mastectomy, and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:351–357. doi: 10.1016/j.ijrobp.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 33.Neri A, Marrelli D, Roviello F, et al. Prognostic value of extracapsular extension of axillary lymph node metastases in T1 to T3 breast cancer. Ann Surg Oncol. 2005;12:246–253. doi: 10.1245/ASO.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Heide J, Krull A, Berger J. Extracapsular spread of nodal metastasis as a prognostic factor in rectal cancer. Int J Radiat Oncol Biol Phys. 2004;58:773–778. doi: 10.1016/S0360-3016(03)01616-X. [DOI] [PubMed] [Google Scholar]
- 35.Lee YC, Wu CT, Kuo SW, et al. Significance of extranodal extension of regional lymph nodes in surgically resected non-small cell lung cancer. Chest. 2007;131:993–999. doi: 10.1378/chest.06-1810. [DOI] [PubMed] [Google Scholar]
- 36.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]


