Abstract
Background:
p16 INK4a (p16) is a well-recognized surrogate molecular marker for human papilloma virus (HPV) related squamous dysplasia. Our hypothesis is that the invasive interventions and related morbidities could be avoided by objective stratification of positive cytologic interpretations by p16 immunostaining of cell block sections of cytology specimens.
Materials and Methods:
Nuclear immunoreactivity for p16 was evaluated in cell block sections in 133 adequate cases [20 negative for intraepithelial lesion or malignancy, 28 high-grade squamous intraepithelial lesion (HSIL), 50 low-grade squamous intraepithelial lesion (LSIL), 21 atypical squamous cells, cannot exclude HSIL (ASC-H), and 14 atypical squamous cells of undetermined significance (ASCUS)] and analyzed with cervical biopsy results.
Results:
(a) HSIL cytology (28): 21 (75%) were p16 positive (11 biopsies available — 92% were positive for cervical intraepithelial neoplasia (CIN) 1 and above) and 7 (25%) were p16 negative (3 biopsies available — all showed only HPV with small atypical parakeratotic cells). (b) LSIL cytology (50): 13 (26%) cases were p16 positive (12 biopsies available — all were CIN1 or above) and 37 (74%) were p16 negative (12 biopsies available — all negative for dysplasia. However, 9 (75%) of these biopsies showed HPV). (c) ASC-H cytology (21): 14 (67%) were p16 positive (6 biopsies available — 5 showed CIN 3/Carcinoma in situ/Ca and 1 showed CIN 1 with possibility of under-sampling. Cytomorphologic re-review favored HSIL) and 7 (33%) were p16 negative (5 biopsies available — 3 negative for dysplasia. Remaining 2 cases — 1 positive for CIN 3 and 1 showed CIN 1 with scant ASC-H cells on cytomorphologic re-review with possibility under-sampling in cytology specimen). (d) ASCUS cytology (14): All (100%) were p16 negative on cell block sections of cervical cytology specimen. HPV testing performed in last 6 months in 7 cases was positive in 3 (43%) cases.
Conclusion:
p16 immunostaining on cell block sections of cervical cytology specimens showed distinct correlation patterns with biopsy results. Reflex p16 immunostaining of cell blocks based on the algorithmic approach to be evaluated by a multiinstitutional comprehensive prospective study is proposed.
Keywords: Cervical cancer, cervical cytology, immunohistochemistry, p16, Pap test, reflex testing, screening, squamous intraepithelial neoplasia
INTRODUCTION
Globally, after breast cancer, carcinoma of cervix is the second most frequent cancer in women.[1] Detection of cervical premalignant lesions is a crucial component to reduce the associated morbidity and mortality. Over the years, cervical cytology (Pap test) has proven to be a very effective screening tool to achieve this goal, reducing the incidence from 14.8 per 100,000 in 1975 to 6.5 per 100,000 in 2006 in the United States[2] and trends show a significant fall in incidence of 3.5% every year for the last ten years, until 2006.[3] However, it is desirable to increase the specificity of this screening test. Any management algorithm that results in fewer false positives would be beneficial in preventing the complications of over-treatment.[4]
p16 INK4a (p16), as a surrogate molecular marker of Human Papilloma Virus (HPV) related squamous dysplasia, has been relatively well established in cervical biopsy specimens[5–8] with excellent inter- and intraobserver reproducibility.[7] Its application to cervical cytology specimens has also been evaluated. However, the main challenge in our experience in applying p16 to cytology preparations is the inherent difficulty in interpretation of diagnostic nuclear immunoreactivity for p16 in whole cells with nucleus enveloped by surrounding cytoplasm due to the obscuring cytoplasmic immunoreactivity. As a result, reproducible and objective interpretation of p16 immunoreactivity in cytologic preparations especially with relatively few diagnostic intact cells is inherently suboptimal.[9,10] Performing immunohistochemistry (IHC) on cell block sections of cytologic specimens could circumvent this limitation. However, preparation of cell blocks from liquid-based cytology (LBC) specimens with singly scattered cells may not produce cell block sections with reproducible cellularity.[11]
We applied a protocol specially standardized to produce cell blocks from cytology specimens with single scattered cells such as in LBC specimens.[11] This protocol included a centrifugation step to align the dispersed solitary and small groups of cells in LBC specimens along the flat cutting surface of the cell block. A visible dark colored marker was included to monitor the depth of section cutting. By utilizing this protocol, the cell block sections showed a reproducible adequate cellularity. In this study, we evaluated application of p16 immunoreactivity in cell block sections prepared by this method from the residual SurePath™ specimen (TriPath Imaging, Burlington, NC) (SP).
Our hypothesis is that ancillary reflex application of p16 immunostaining on cell block sections of cytology specimens could subcategorize atypical squamous cells of undetermined significance (ASCUS) and low-grade squamous intraepithelial lesion (LSIL) into cases with and without dysplasia. Similarly, atypical squamous cells, cannot exclude HSIL (ASC-H) and high-grade squamous intraepithelial lesion (HSIL) could be confirmed for dysplasia with increased objectivity. This approach would avoid invasive interventions and prevent potentially increased morbidities by utilizing objective options at the minimally invasive cervical cytology stage. Developing and adopting appropriate algorithms based on results of larger, comprehensive, multiinstitutional trial with reflex testing (p16 and other markers such as HPV L1 capsid protein[12] and Ki67)[13] on specially prepared cell blocks of cervical cytology specimens may be indicated.
MATERIALS AND METHODS
In this study, performed after approval from institutional review board (IRB), cell blocks were made from LBC specimens obtained from patients ranging 18 to 70 years of age. Out of approximately 120,000 gynecologic cytology specimens examined over a 2-year period, residual LBC specimens with positive cytopathologic interpretations (ASCUS and above) were available in 114 cases (in addition to 20 negative cases) for cell block preparation. The limiting factors controlling this final number included: (a) adequate cellularity of LBC, (b) availability of residual LBC specimen subject to reflex testing such as HPV test, (c) unavoidable logistic complexities related to selection of specimen for the study including multiplicity of cytopathologists-cytotechnologists involved with initial cytopathologic evaluation, (d) selection of representative numbers in each category of positive squamous lesions, and (e) practical limitations related to procurement of the residual specimen prior to its final disposal. These factors could not be controlled reproducibly to achieve inclusion of all cases based on limitations related to expedited IRB in smaller studies such as the present study.
Twenty cell blocks were prepared from cases with negative results and showing an uneventful follow-up pattern based on consecutive negative cervical cytologies and/or biopsies for one year. A total of 114 cell blocks were prepared from specimens with positive cytopathologic interpretations for squamous epithelial lesions (ASCUS and above). We used a standardized protocol for cell block preparation of specimens with singly scattered individual cells or small groups of cells using HistoGel™.[11] The protocol achieves alignment and concentration of the cells in the sample along the cutting surface of the cell block. It also includes AV-marker which serves to visualize the level at which the cells are concentrated. It allows selection of the sections from the plane of the cell block with the highest concentration of cells by the technologist during section cutting. For actual methodology, please refer to recently published video article which describes the technical details and is available as free publication in open access.[11]
Three unstained, serial, cell block sections (one for hematoxylin and eosin (H & E) staining, one for p16 immunostaining, and one spare for elective immunostaining) were cut from each cell block. H & E stained sections were evaluated morphologically for cellularity. Cell blocks with sections showing more than 100 cells (either singly scattered or in cohesive groups) or showing any number of epithelial cells with morphological atypia were considered adequate. One (out of total 134) cell block was relatively hypocellular without morphological atypia and was not included in the study. The cytopathologic interpretations of 133 cases evaluated by p16 immunostaining of cell block sections included 20 negative for intraepithelial lesion or malignancy (NILM), 28 HSIL, 50 LSIL, 21 ASC-H, and 14 ASCUS [Figure 1]. One section from each of finally selected 133 cell blocks was immunostained for p16 [Table 1] with appropriate positive and negative controls for each batch.
Figure 1.
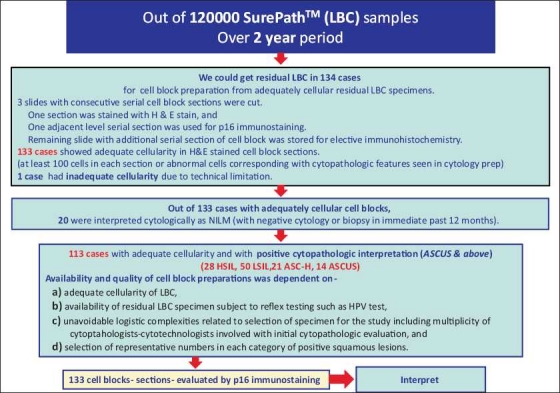
Selection and evaluation of 20 negative and 113 abnormal cases included in the study.
Table 1.
Immunostaining of cell block sections with p16

The p16 immunoreactivity patterns included: (a) none, (b) cytoplasmic alone, (c) nuclear alone, and (d) nuclear with cytoplasmic. Only nuclear and nuclear with cytoplasmic immunostaining were considered positive. None or only cytoplasmic immunostaining was considered negative.[10,14–16]
Cell block sections prepared from 20 cases with negative cytologic interpretation showed lack of nuclear (with or without cytoplasmic) immunoreactivity for p16 in squamous cells. Based on the previous study[14] and many published studies,[9,17–19] the p16 antibody clone used in this study (E6H4) showed excellent results.
The follow-up cervical biopsy results were available in 49 cases (15 out of 28 HSIL, 10 out of 21 ASC-H, and 24 out of 50 LSIL). Biopsy results were not available/performed in all 14 cases with ASCUS. The cervical biopsy was considered positive if it was reported as CIN1 or greater (confirmed with p16 IHC of biopsy sections in equivocal cases). Negative for dysplasia with or without HPV cytopathic effects were included for this study in the negative category.
The specimens were available for cell block preparation in 134 cases, because HPV DNA testing (HPVT) was not requested on the residual cervical cytology specimen. In cases with ASCUS interpretations, results of HPVT performed in last 6 months could be obtained in seven cases.
RESULTS
Correlation of p16 immunostaining on cell block sections of LBC specimens with biopsy results is shown in Table 2. The morphology of p16 immunostained cells in cell block sections in various groups is illustrated in Figures 2–4. Trouble shooting of false-positive and false-negative cases predominantly revealed sampling and biopsy interpretation issues. Cytoplasmic immunoreactivity alone was observed in scant cells in two cases.
Table 2.
Correlation of p16 immunostaining on cell block sections of LBC specimens with biopsy results
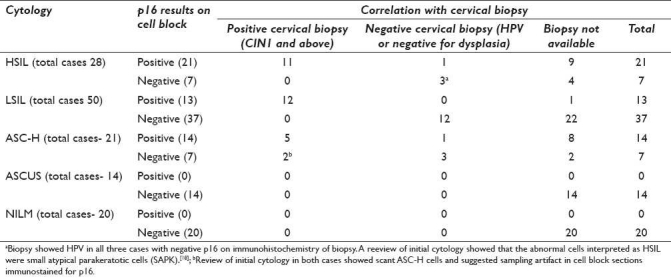
Figure 2.
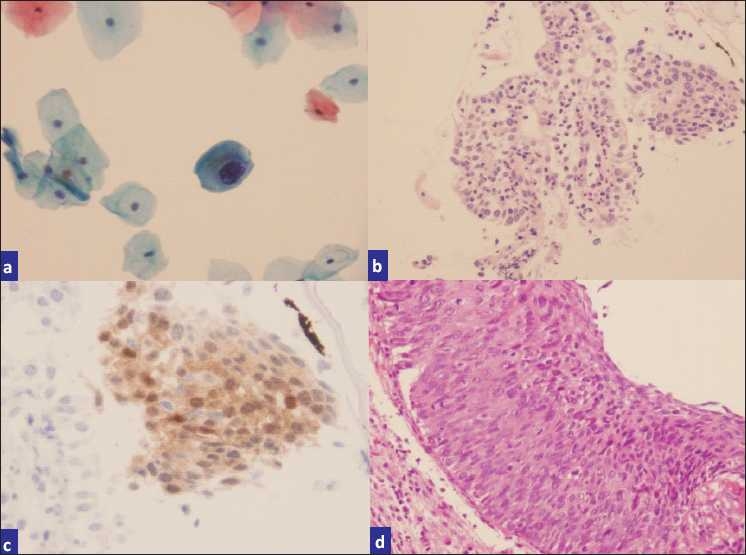
a) Pap smear interpreted as LSIL, b) H and E cell block sections, c) p16-stained cell block sections, d) biopsy showing CIN II-III.
Figure 4.
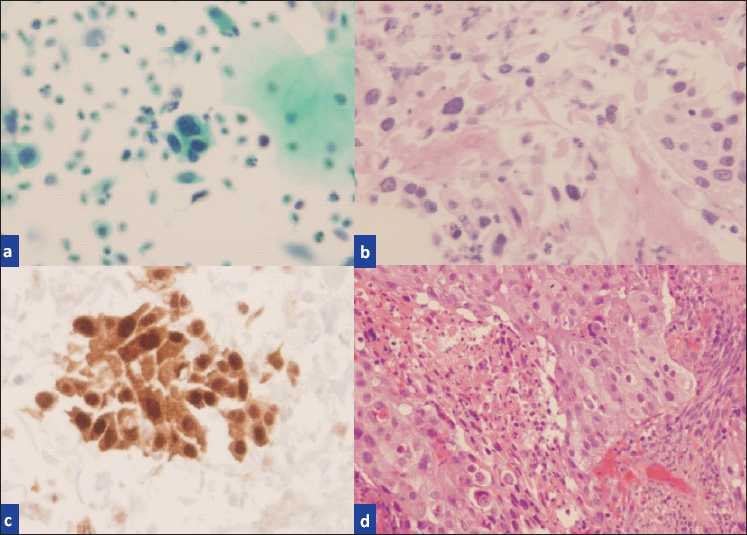
a) Pap smear interpreted HSIL, b) H and E cell block section containing “microbiopsies”, c) p16-stained cell block section showing true nuclear positivity, d) biopsy showing invasive squamous cell carcinoma.
Figure 3.
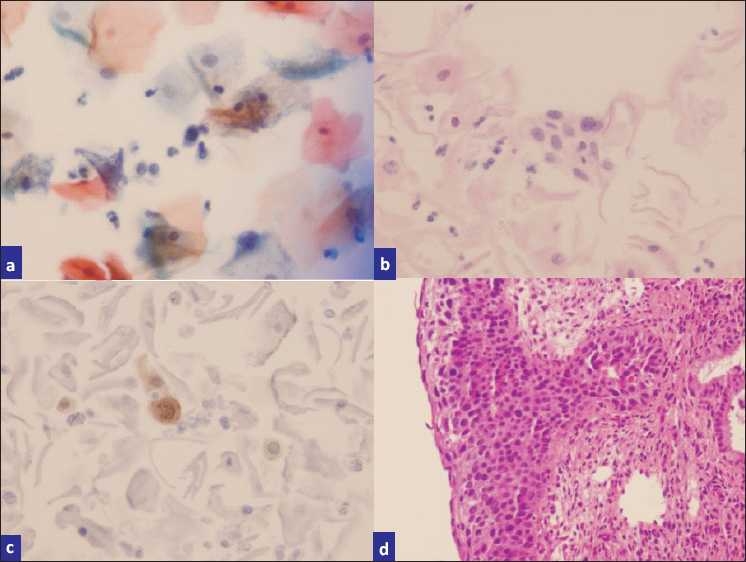
a) ASC-H (rare single cells with hyperchromatic nuclei and high N:C ratios), b) H and E stained cell block sections, c) p16-stained sections highlighting scattered high-grade cells, d) biopsy showing CIN III with extensive endocervical glandular involvement.
-
Twenty-eight cases with HSIL cytology
Twenty-one (75%) cases showed positive results with p16. Out of these, 12 had follow-up biopsies. Eleven (92%) biopsies were positive. The 75% (9/12) of these cases showed CIN 2-3 or invasive carcinoma (Ca), 2 showed CIN 1, and one was negative for dysplasia. Further analysis of three cases with CIN1 or lower lesions suggested potential under-sampling in biopsy based on cyto-histo findings (with unequivocal cytomorphology for HSIL) and clinical details (such as with previous evidence of HSIL).
Remaining seven (25%) HSIL cases showed negative results with p16. The cervical biopsy/cone results were available in three cases, all of which showed only HPV changes without dysplasia (confirmed by p16 on biopsies). A review of initial cytology showed small atypical parakeratotic cells (SAPK)[20] which were misinterpreted initially as HSIL cells (technically should have been ASC-H).[20]
-
Fifty cases with LSIL cytology
Thirteen (26%) cases showed positive results with p16. Out of these, 12 cases had biopsies, all of which were positive for CIN1 or above lesion.
Remaining 37 (74%) LSIL cases showed negative results with p16. Cervical biopsies were available in 12 cases, all of which were negative for dysplasia but 9 (75%) of these biopsies showed the HPV cytopathic effect.
-
Twenty-one cases with ASC-H cytology
-
Fourteen (67%) cases showed positive result with p16.
Cyto-histo correlation was available during the period of study in six cases. Out of these, five cases showed CIN 3/carcinoma in situ (CIS)/Ca. The remaining one case showed CIN 1, but the cyto-histo correlation suggested that the lower grade findings in biopsy may have been related to sampling artifact with under-sampling. A review of cytology of all these ASC-H cases retrospectively showed cytomorphology favoring HSIL and could have been interpreted definitively as HSIL.
The remaining seven (33%) ASC-H cases showed negative results with p16. Cyto-histo correlation was available during the period of study in five cases. The 60% (3/5) were negative for dysplasia and 40% (2/5) were positive with CIN 3 in one and CIN 1 in the other. A review of initial cytology in both cases showed scant ASC-H cells and suggested the possibility of sampling artifact with the absence of representative abnormal cells in cell block sections immunostained for p16.
-
-
Fourteen cases with ASCUS cytology
All 14 (100%) cases showed negative results and biopsies were not available in any. The total number of ASCUS cases could not be higher due to practical limitation of unavailability of samples in the majority of the cases, where the residual LBC was sent for reflex HPV testing. As the current specimens were not sent for HPVT during this ASCUS interpretation, they were available for cell block preparation for this study. Out of these 14 cases, results of HPVT performed in last 6 months were available in seven cases. Out of these seven cases, HPVT was positive in three (43%) cases.
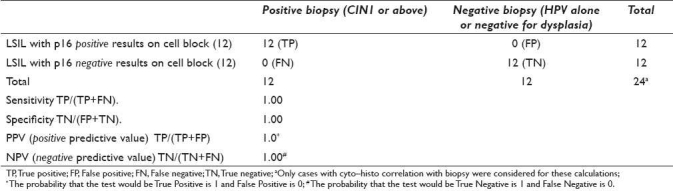
Sensitivity and specificity were calculated only for definitive categories (HSIL and LSIL) using results on cases with unequivocal biopsy results [Tables 3 and 4]. The sensitivity for p16 in HSIL cases was 100% with specificity of 75%. The sensitivity for p16 in LSIL cases was 100% with specificity of 100% [Tables 3 and 4].
Table 3.
Sensitivity, specificity, positive predictive value, and negative predictive value for HSIL category with p16 immunostaining on cell block sections of 15 LBC specimensa
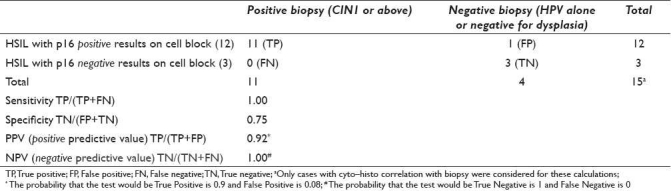
Table 4.
Sensitivity, specificity, positive predictive value, and negative predictive value for the LSIL category with p16 immunostaining on cell block sections of 24 LBC specimensa
DISCUSSION
HPV is a proven carcinogen for cervical cancers and p16 is an excellent surrogate marker for HPV-related dysplasia.[21] p16 is a cell-cycle inhibitor that binds to cyclin-dependent kinase 4 (CDK4) and prevents the phosphorylation and subsequent inactivation of the retinoblastoma protein (pRb).[21] A reciprocal relation between p16 and pRb expression has been observed.[22] Integration of high-risk HPV DNA into the host genome results in the overexpression of viral proteins E6 and E7. E7 binds to and inactivates pRB ultimately leading to overexpression of p16 through a negative feedback loop.[23,24] The overexpression of p16 indicates already advanced interference of the viral oncoproteins with cellular proteins involved in cell cycle regulation. This phenomenon translates into nuclear immunoexpression of p16 in squamous epithelial cells and correlates with HPV-related dysplasia.[25,26]
A recent meta-analysis of 61 studies published until 2007 on p16 immunoexpression which included 27 studies on cytologic specimens and 34 studies on cervical biopsies.[10] The analysis concluded that p16 immunostaining correlated with severity of cytological/histological abnormalities. However, the reproducibility was limited due to insufficiently standardized interpretation of the immunostaining. They recommended that a consensus needs to be reached for assessing p16 immunostaining. In addition, it needs to be assessed in various clinical settings addressing relevant management decisions.
Out of 27 studies performed on cytological specimens, only 2 utilized cell block sections from residual cytology specimens.[17,18] Remaining studies (n = 25) either utilized cytologic preparations or had not mentioned the details of preparation. Recently in 2010, one more study applied cell blocks to evaluate p16 immunoexpression in cytology specimens.[27] Data from these studies also support our finding that p16 is a useful marker for the objective confirmation of CIN on cell blocks of cervical cytology specimens. However, cell block preparation methods were not standardized for achieving sections with good cellularity along the cutting surface of such cell blocks.[11]
A review of these studies, surprisingly, highlights a critical flaw that the exact criteria for interpretation of p16 immunoreactivity as a marker of HPV-related dysplasias are significantly variable.[10] Studies mostly support nuclear immunoreactivity with or without cytoplasmic immunostaining in morphologically squamous epithelial cells is consistent with HPV-related squamous dysplasia – irrespective of the number of cells.[5–7,14,15,28]
Only 6 out of 61 studies in the meta-analysis spelled out the interpretation criteria for the p16 immunoreactivity pattern stressing nuclear immunoreactivity with or without cytoplasmic staining as diagnostic.[10] Others, however, stated positive p16 immunoreactivity as nuclear and/or cytoplasmic staining implying that cytoplasmic immunoreactivity alone is also positive or else did not mention the criteria. This pattern of interpretation would obviously introduce potentially false-positive interpretations in such studies.
HPVT has been recommended as ancillary reflex test for managing ASCUS cytopathologic interpretations.[29] Application of the HPVT in the concert with p16 will add further perspective as p16-positive and p16-negative cases supporting disruption or nondisruption of the cell cycle. If an HPVT is positive, then the p16-negative status indicates that the lesion is in a nondysplastic state, while the p16-positive pattern indicates that the lesion is in a dysplastic phase. Such stratification may be of practical value especially in low risk postmenopausal women and adolescents.
Bose et al. reported that the highest rate of positivity (80%) and the highest levels of expression (more than three to five positive cells/10× field) were seen in HSIL and ASC-H cases. On the other hand, p16 positivity was noted in only 21% of LSIL and ASC-US cases. They concluded that, given that only a minority of LSIL cases progress on to higher-grade lesions, p16 might be useful for triaging these patients for closer follow-up and/or further evaluation.[30] del Pino et al. in a recent report also reported comparative results on histopathology.[31]
In our study, the majority of ASCUS (14 out of 14, 100%) and LSIL (37 out of 50, 74%) cases were negative for p16 in cytology specimens and in biopsy (equivalent to negative for dysplasia). Although this may appear as overdiagnosis, many of these cases were positive for either HPVT performed in the past 6 months (in 3 out of 7, 43% ASCUS cases) or the HPV cytopathic effect in biopsy (9 out of 12, 75% LSIL cases), consistent with HPV infection without dysplasia. If we consider this as overdiagnosis for dysplasia (not for HPV infection without dysplasia as the biological status) then ASCUS and LSIL cytopathologic interpretations although not uncommon, can be corrected with objective ancillary contribution by reflex p16 on cell block sections (Rp16) on the residual cervical cytology specimen. Based on the design of this study, concurrent HPVT on the same specimen could not be performed because the entire residual specimen was used for cell block preparation for p16 immunostaining.
The application of Rp16 on cell blocks of positive cytology specimens has very high potential of targeting selective intervention and preventing nonindicated invasive procedures by identifying p16 positive subset with increased probability of higher-grade lesions. This p16 assisted approach with reduced intervention should decrease the morbidity and cost associated with potential overtreatment.[4] The p16 immunoexpression pattern in cytology specimens with positive cytologic interpretation for squamous epithelial lesions such as ASCUS, LSIL, ASC-H (including LSIL cannot rule out high grade (LSIL-H)[32] ), and HSIL could steer definitive management with significant savings and prevention of avoidable interventions. A comprehensive prospective ASCUS-LSIL Traige Study (ALTS) type multiinstitutional study evaluating Rp16 on cell block sections of residual cervical cytology specimens with positive result (ASCUS and above) in comparison to other alternatives including the HPV status would benefit patients and should be initiated with cost analysis. In addition to other benefits, such a prospective multiinstitutional study will achieve higher number of cases by including almost all potential specimens by preventing unavoidable exclusion of any of these relatively rare cases in routine setting similar to this study.
Currently, tests and procedures such as HPVT, colposcopy, and biopsy are applied to manage various cytologic interpretations as recommended by American Society for Colposcopy and Cervical Pathology (ASCCP).[33] However, all these techniques are resource and manpower intensive, with related patient discomfort (colposcopy) and morbidity (cervical biopsy/conization) such as hemorrhage, cervical stenosis, cervical incomptence, and preterm delivery.[4,34] HPVT is noninvasive, but it lacks specificity and only identifies the subset of cases with higher risk without any information-related to the absence or presence of dysplasia, which is a better decisive feature deciding the definitive management to minimize the invasive encounters. The specificity without decreasing the sensitivity of cervical cytology interpretations is highly desirable. This may be optimized by introducing the Rp16 as ancillary test[35] on appropriately prepared cell block in all cases with positive cytologic interpretations. This allows an excellent noninvasive opportunity for appropriate decision making in the management algorithm. Rp16 on cell blocks of the residual LBC specimen in all positive cytology interpretations would achieve these features [Figure 5] and lead to significant savings with opportunity to decrease morbidity. The cell block protocol used in this study is an economical and easily available method.[11]
Figure 5.
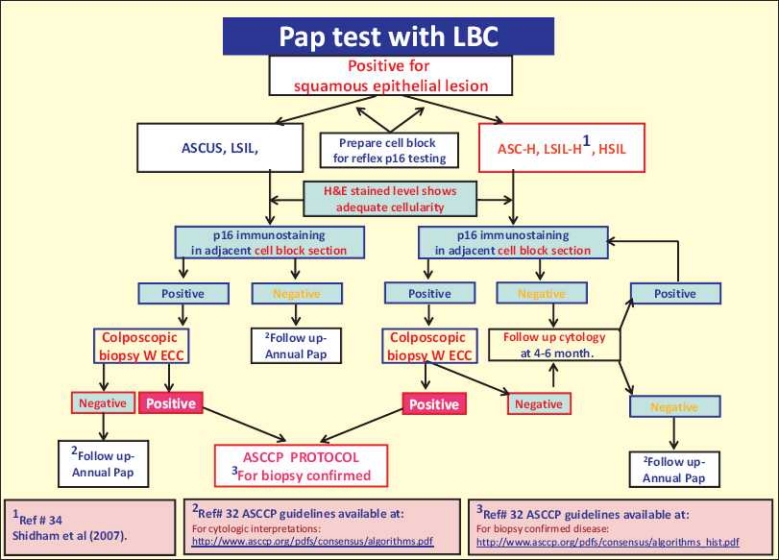
The recommended management algorithm using reflex p16 immunostaining on cell block sections of LBC specimens.
The cell block sections have additional benefits of sequential sectioning and immunostaining with other additional immunomarkers and future techniques including two color immunostaining.[13,36] The location of AV marker in the section allows the application of the subtractive coordinate immunoreactivity pattern (SCIP) approach for proper evaluation of multiple immunomarkers in serial levels of cell block sections with singly scattered cells.[37,38]
It is concluded that p16 on cell block sections of cervical cytology specimens with positive squamous interpretation including ASCUS, LSIL, ASC-H, LSIL-H, and HSIL showed distinct patterns with excellent correlation with biopsy results. Rp16 immunostaining of cell blocks prepared by a properly standardized protocol is recommended. An algorithmic approach with reference to p16 results to manage cytologically positive squamous lesions is proposed [Figure 5]. A multiinstitutional comprehensive prospective study to evaluate this approach is recommended as the next step to evaluate this algorithm with cost analysis.
List of abbreviations
ALTS, ASCUS-LSIL Triage Study; ASCCP, American Society for Colposcopy and Cervical Pathology; ASC-H, Atypical squamous cells Cannot exclude high-grade intraepithelial lesion; ASCUS, Atypical squamous cells of undetermined significance; Ca, invasive carcinoma; CDK4, cyclin-dependent kinase 4; CIN, cervical intraepithelial neoplasia; ECC, endocervical curettage; H & E, Hematoxylin and Eosin; HPV, human papilloma virus; HPVT, HPV DNA testing; HSIL, high-grade squamous intraepithelial lesion; p16, p16INK4a; IHC, immunohistochemistry; IRB, institutional review board; LBC, liquid based cytology; LSIL, low-grade squamous intraepithelial lesion; LSIL-H, low-grade squamous intraepithelial lesion, cannot exclude HSIL; NILM, negative for intraepithelial lesion or malignancy; Pap test, cervical cytology; pRb, retinoblastoma protein; Rp16, Reflex p16; SAPK cells, small atypical parakeratotic cells; SCIP, subtractive coordinate immunoreactivity pattern; SP, SurePathTM specimen (TriPath Imaging, Burlington, NC).
COMPETING INTERESTS
The author(s) declare that they do not have competing commercial interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author
Each author has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article.
Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted at Medical College of Wisconsin, Milwaukee, WI, USA with approval from Institutional Review Board (IRB) at Medical College of Wisconsin.
EDITORIAL / PEER-REVIEW STATEMENT
CytoJournal editorial team thanks the academic editor : Hormoz Ehya, M.D. Professor & Director of Cytopathology, Department of Pathology, Fox Chase Cancer Center, Philadelphia, PA, USA, for organizing and completing the double-blind peer-review process for this manuscript as per CytoJournal's peer-review policy.
Acknowledgments
This study was conducted at the Medical College of Wisconsin, (MCW) Milwaukee, WI, USA (during VS affiliation at MCW) and was presented in parts at different Annual Meetings of United States and Canadian Academy of Pathology, from 2004 through 2009. This article is a consolidation of these parts.
RM contributed to authorship during his participation in manuscript writing including a review of literature as an Indian Council of Medical Research (ICMR) Senior Visiting Faculty under mentorship of VB at the Department of Pathology, Medical College of Wisconsin, Milwaukee, WI, United States during March, 2010.
The authors appreciate and thank Glen Dawson, BS, HT, IHC(ASCP), Jerome Jacobson, HT, QIHC(ASCP), and Katherine Wertz, BS,HTL,QIHC(ASCP) for the technical immunocytochemistry assistance. We also thank Anushree Shidham for her secretarial and copy-editing support.
Footnotes
Available FREE in open access from: http://www.cytojournal.com/text.asp?2011/8/1/1/76379
Contributor Information
Vinod B. Shidham, Email: vshidham@med.wayne.edu.
Ravi Mehrotra, Email: rm8509@gmail.com.
George Varsegi, Email: varsegi@yahoo.com.
Krista L. D'Amore, Email: D'Amore@phci.org.
Bryan Hunt, Email: hunbry@yahoo.com.
Raj Narayan, Email: rnarayan@mcw.edu.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Previous Version: SEER Cancer Statistics Review, 1975-2005. [Last accessed on 2010 Mar 14]. Available from: http://www.seer.cancer.gov/csr/1975_2006/results_single/sect_05_table.04.pdf .
- 3.Centers for Disease Control and Prevention. Cervical Cancer Trends. [Last accessed On 2010 Mar 14]. Available from: http://www.cdc.gov/cancer/cervical/statistics/trends.htm .
- 4.Park SY, Bae DS, Nam JH, Park CT, Cho CH, Lee JM, et al. Quality of life and sexual problems in disease-free survivors of cervical cancer compared with the general population. Cancer. 2007;110:2716–25. doi: 10.1002/cncr.23094. [DOI] [PubMed] [Google Scholar]
- 5.Chivukula M, Komorowski R, Shidham V. Ancillary application of p16INK4a immunoexpression for objective grading of dysplasia in cervical biopsies. Mod Pathol. 2004;17:1a–388. [Google Scholar]
- 6.Chivukula M, Komorowski R, Shidham V. Application of p16INK4A to evaluate the distribution pattern of mitotic figures and apoptotic bodies for grading cervical dysplasia. Mod Pathol. 2004;17:1a–388. [Google Scholar]
- 7.Behmaram B, Kotov P, Basir Z, Cafaro A, Novoa-Takara L, Shidham VB. Comparison of inter and intra-observer reproducibility of evaluating cervical dysplasia in HandE stained and p16 immunostained sections. [Last accessed on 2010 Jun 12];Mod Pathol. 2006 19:1a–359. Available from: http://www.nature.com/modpathol/journal/v19/n1s/pdf/3800847a.pdf . [Google Scholar]
- 8.Wentzensen N, Bergeron C, Cas F, Vinokurova S, von Knebel Doeberitz M. Evaluation of a nuclear score for p16INK4a-stained cervical squamous cells in liquid-based cytology samples. Cancer. 2007;111:58–66. doi: 10.1002/cncr.21378. [DOI] [PubMed] [Google Scholar]
- 9.Wentzensen N, Bergeron C, Cas F, Eschenbach D, Vinokurova S, von Knebel Doeberitz M. Evaluation of a nuclear score for p16 INK4a -stained cervical squamous cells in liquid-based cytology samples. Cancer. 2005;105:461–7. doi: 10.1002/cncr.21378. [DOI] [PubMed] [Google Scholar]
- 10.Tsoumpou I, Arbyn M, Kyrgiou M, Wentzensen N, Koliopoulos G, Martin-Hirsch P, et al. 2009 p16 INK4a immunostaining in cytological and histological specimens from the uterine cervix: A systematic review and meta-analysis. Cancer Treat Rev. 2009;35:210–20. doi: 10.1016/j.ctrv.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varsegi GM, Shidham V. Cell block preparation from cytology specimen with predominance of individually scattered cells. [Last accessed on 2010 Jun 12];J Vis Exp. 2009 29 doi: 10.3791/1316. 29.doi: 10.3791/1316. PMID: 19623160 Video article is available FREE on web as open access from http://www.jove.com/index/Details.stp?ID=1316 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ungureanu C, Socolov D, Anton G, Mihailovici MS, Teleman S. Immunocytochemical expression of p16INK4a and HPV L1 capsid proteins as predictive markers of the cervical lesions progression risk. Rom J Morphol Embryol. 2010;51:497–503. [PubMed] [Google Scholar]
- 13.Chivukula M, Austin RM, Duwe A, Friedman T, Masko J, Mauser N, et al. Dual-Stain for P16 and Ki67 in the Interpretation of Abnormal PAP Cytology Results: A Prospective Study. [Last accessed on 2010 Jun 12];Mod Pathol. 2010 23:85a–109. doi:10.1038/modpathol.2010.10 Abstract #395. Available from: http://www.nature.com/modpathol/journal/v23/n1s/pdf/modpathol201010a.pdf . [Google Scholar]
- 14.Kotov P, Parameswaran L, Parisi JA, Chivkula M, Cafaro A, Fuentes M, et al. p16INK4a Immunostaing of Liquid Based Cervical Cytology Smears with SurePath® - Comparison of Two Antibodies. [Last accessed on 2010 Jun 12];Mod Pathol. 2005 18:1a–359. Available from: http://www.nature.com/modpathol/journal/v18/n1s/pdf/3800908a.pdf . [Google Scholar]
- 15.Kotov P, Parameswaran L, Parisi JA, Chivkula M, Cafaro A, Fuentes M, et al. Application of p16INK4A Immunostaing for Definitive Interpretation of ASC-H in Liquid Based Cervical Cytology Smears with SurePath ®. [Last accessed on 2010 Jun 12];Mod Pathol. 2005 18:1a–359. Available from: http://www.nature.com/modpathol/journal/v18/n1s/pdf/3800908a.pdf . [Google Scholar]
- 16.Shidham V, D'Amore K, Varsegi G. Objective and definitive subcategorization of LSIL with p16INK Immunocytochemistry on Cell block Sections of Cervical Cytology Specimens. Cancer Cytopathol. 2009;117:349–450. [Google Scholar]
- 17.Liu H, Shi J, Wilkerson M, Huang Y, Meschter S, Dupree W, et al. Immunohistochemical detection of p16INK4a in liquid-based cytology specimens on cell block sections. Cancer. 2007;111:74–82. doi: 10.1002/cncr.22577. [DOI] [PubMed] [Google Scholar]
- 18.Akpolat I, Smith DA, Ramzy I, Chirala M, Mody DR. The utility of p16INK4a and Ki-67 staining on cell blocks prepared from residual thin-layer cervicovaginal material. Cancer. 2004;102:142–9. doi: 10.1002/cncr.20258. [DOI] [PubMed] [Google Scholar]
- 19.Meyer JL, Hanlon DW, Andersen BT, Rasmussen OF, Bisgaard K. Evaluation of p16INK4a expression in ThinPrep cervical specimens with the CINtec p16INK4a assay. Cancer. 2007;111:83–92. doi: 10.1002/cncr.22580. [DOI] [PubMed] [Google Scholar]
- 20.Chivukula M, Shidham V. ASC-H in Pap test- definitive categorization of cytomorphological spectrum. [Last accessed on 2010 Jun 12];Cytojournal. 2006 3:14. doi: 10.1186/1742-6413-3-14. Free full text is Available from: http://www.cytojournal.com/content/3/1/14.PDF. http://www.cytojournal.com/content/pdf/1742-6413-3-14.pdf . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalof AN, Cooper K. p16INK4a immunoexpression: Surrogate marker of high-risk HPV and high-grade cervica intraepithelial neoplasia. Adv Anat Pathol. 2006;13:190–4. doi: 10.1097/00125480-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Tringler B, Gup CJ, Singh M, Groshong S, Shroyer AL, Heinz DE, et al. Evaluation of p16INK4a and pRb expression in cervical squamous and glandular neoplasia. Hum Pathol. 2004;35:689–96. doi: 10.1016/j.humpath.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Klaes R, Woerner SM, Ridder R, Wentzensen N, Duerst M, Schneider A, et al. Detection of high risk cervical intraepithelial neoplasia and cervical cancer byamplification of transcripts derived from integrated papillomavirus oncogenes. Cancer Res. 1999;59:6132–6. [PubMed] [Google Scholar]
- 24.Klaes R, Benner A, Friedrich T, Ridder R, Herrington S, Jenkins D, et al. p16INK4a immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol. 2002;26:1389–99. doi: 10.1097/00000478-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Leversha MA, Fielding P, Watson S, Gosney JR, Field JK. Expression of p53, pRB, and p16 in lung tumours: A validation study on tissue microarrays. J Pathol. 2003;200:610–9. doi: 10.1002/path.1374. [DOI] [PubMed] [Google Scholar]
- 26.Mäkitie AA, MacMillan C, Ho J, Shi W, Lee A, O'Sullivan B, et al. Loss of p16 expression has prognostic significance in human nasopharyngeal carcinoma. Clin Cancer Res. 2003;9:2177–84. [PubMed] [Google Scholar]
- 27.Yu L, Wang L, Zhong J, Chen S. Diagnostic value of p16INK4A, Ki-67, and human papillomavirus l1 capsid protein immunochemical staining on cell blocks from residual liquid-based gynecologic cytology specimens. Cancer Cytopathol. 2010;118:47–55. doi: 10.1002/cncy.20061. [DOI] [PubMed] [Google Scholar]
- 28.Varsegi G, D'Amore K, Shidham V. p16ink4a immunocytochemistry as an adjunct to cervical cytology - potential reflex testing on specially prepared cellblocks from residual liquid based cytology (lbc) specimens. [Last accessed on 2010 Jun 12];Mod Pathol. 2009 22:97a. doi: 10.4103/1742-6413.76379. Available from: http://www.nature.com/modpathol/journal/v22/n1s/pdf/modpathol2008212a.pdf . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoler MH, Schiffman M. Interobserver Reproducibility of Cervical Cytologic and Histologic Interpretations: Realistic Estimates From the ASCUS-LSIL Triage Study. JAMA. 2001;285:500–5. doi: 10.1001/jama.285.11.1500. [DOI] [PubMed] [Google Scholar]
- 30.Bose S, Evans H, Lantzy L, Scharre K, Youssef E. p16(INK4A) is a surrogate biomarker for a subset of human papilloma virus-associated dysplasias of the uterine cervix as determined on the Pap smear. Diagn Cytopathol. 2005;32:21–4. doi: 10.1002/dc.20175. [DOI] [PubMed] [Google Scholar]
- 31.del Pino M, Garcia S, Fusté V, Alonso I, Fusté P, Torné A, et al. Value of p16(INK4a) as a marker of progression/regression in cervical intraepithelial neoplasia grade 1. Am J Obstet Gynecol. 2009;201(488):e1–7. doi: 10.1016/j.ajog.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 32.Shidham VB, Kumar N, Narayan R, Brotzman GL. Should LSIL with ASC-H (LSIL-H) in cervical smears be an independent category? A study on SurePath™ specimens with review of literature. [Last accessed on 2010 Jun 12];Cytojournal. 2007 4:7. doi: 10.1186/1742-6413-4-7. Free full text is Available from: http://www.cytojournal.com/content/4/1/7 . PDF Available from: http://www.cytojournal.com/content/pdf/1742-6413-4-7.pdf . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Society for Colposcopy and Cervical Pathology guidelines. [Last accessed on 2010 Mar 02]. Available from: http://www.asccp.org/pdfs/consensus/algorithms_cyto_07.pdf. (ASCCP- Available from: http://www.asccp.org/hpv.shtml .
- 34.Luesley DM, McCrum A, Terry PB, Wade-Evans T, Nicholson HO, Mylotte MJ, et al. Complications of cone biopsy related to the dimensions of the cone and the influence of prior colposcopic assessment. Br J Obstet Gynaecol. 1985;92:158–64. doi: 10.1111/j.1471-0528.1985.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 35.Wentzensen N, Bergeron C, Cas F, Vinokurova S, von Knebel Doeberitz M. Triage of women with ASCUS and LSIL cytology. Cancer. 2006;111:58–66. doi: 10.1002/cncr.22420. [DOI] [PubMed] [Google Scholar]
- 36.Shidham VB, Varsegi G, D'Amore K. Two-color immunocytochemistry for evaluation of effusion fluids for metastatic adenocarcinoma. [Last accessed on 2010 Jun 12];Cytojournal. 2010 7:1. doi: 10.4103/1742-6413.59887. Available from: http://alturl.com/x4rg . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halloush RA, Akpolat I, Jim Zhai Q, Schwartz MR, Mody DR. Comparison of ProEx C with p16INK4a and Ki-67 immunohistochemical staining of cell blocks prepared from residual liquid-based cervicovaginal material: A pilot study. Cancer. 2008;114:474–80. doi: 10.1002/cncr.23951. [DOI] [PubMed] [Google Scholar]
- 38.Shidham VB, Atkinson BF. Immunocytochemistry of effusion fluids: Introduction to the SCIP approach. In: Shidham VB, Atkinson BF, editors. 'Cytopathologic Diagnosis of Serous Fluids' Ch 5. 1st ed. Elsevier: W. B. Saunders Company; 2007. pp. 55–78. [Google Scholar]


