Abstract
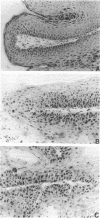
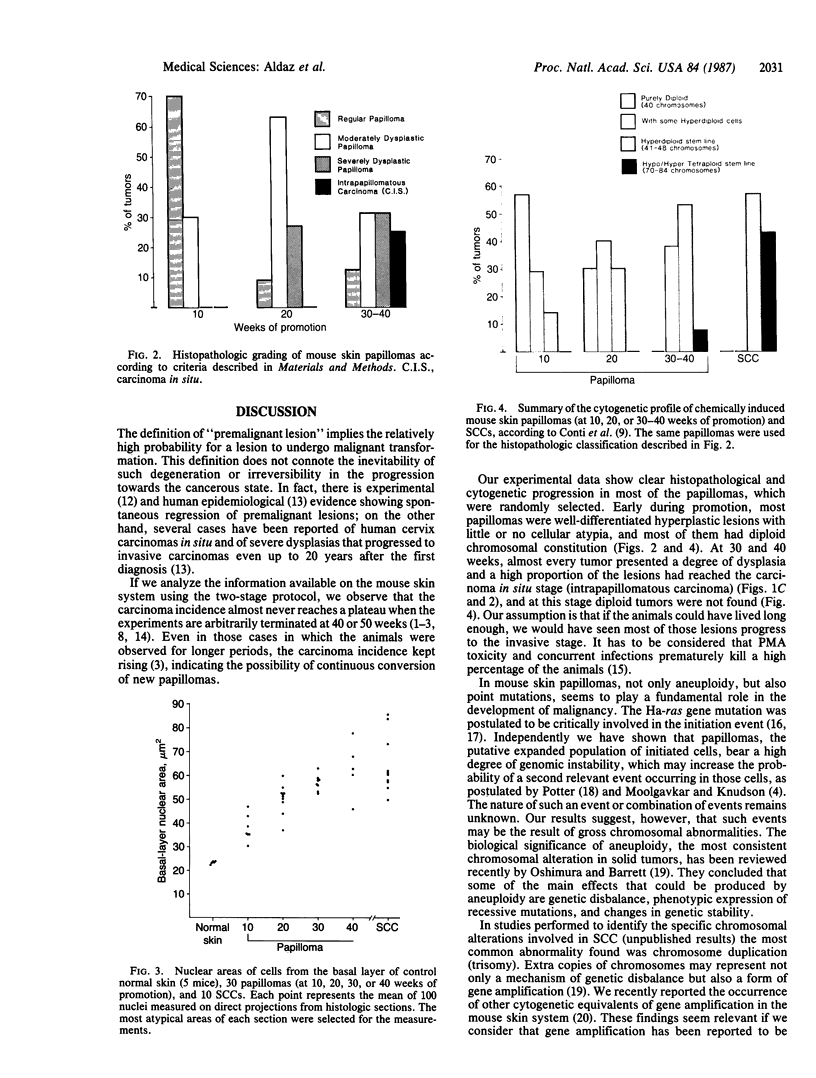
We report a systematic histopathologic study of papillomas at different times during promotion, correlating the results with those from cytogenetic analysis of the same tumors. Papillomas were induced in SENCAR mice by two-stage carcinogenesis (7,12-dimethylbenz[a]anthracene and phorbol 12-myristate 13-acetate). Individual tumors were randomly sampled at different times during promotion, and histopathologic and cytogenetic studies were carried out on every tumor. Early during promotion (10 weeks), most papillomas were well-differentiated hyperplastic lesions with mild or no cellular atypia. No tumors showed severe dysplastic changes. By 20 weeks of promotion, a dramatic drop had occurred in the number of lesions with no dysplasia. Most of the tumors presented moderate dysplasia, and some already showed severe dysplastic changes. At later stages (30-40 weeks), most of the papillomas were classified as moderately or severely dysplastic papillomas, and several were considered to be intrapapillomatous carcinomas. This histopathologic evaluation was supported by nuclear measurements performed on papillomas at different time points. Chromosomal abnormalities followed a similar trend. Papillomas seem to start as diploid lesions, but between 10 and 20 weeks of promotion, hyperdiploid cells can be observed in almost every tumor. In some cases the stem line was taken over by aneuploid clones. At 40 weeks of promotion, all papillomas were aneuploid, most of them with hyperdiploid stem lines. A positive correlation was found between the histological and cytogenetic studies, with the most aggressive and atypical tumors being the more aneuploid. These results support the idea that most, if not all, papillomas are truly premalignant lesions in different stages of the potential progression toward malignancy. Chromosomal abnormalities might play an important role in the sequence of events leading to malignancy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldaz C. M., Conti C. J., Klein-Szanto A. J., Slaga T. J. A direct cytogenetic technique for mouse skin carcinomas and papillomas. Cancer Genet Cytogenet. 1986 Feb 15;20(3-4):223–229. doi: 10.1016/0165-4608(86)90077-4. [DOI] [PubMed] [Google Scholar]
- Aldaz C. M., Conti C. J., O'Connell J., Yuspa S. H., Klein-Szanto A. J., Slaga T. J. Cytogenetic evidence for gene amplification in mouse skin carcinogenesis. Cancer Res. 1986 Jul;46(7):3565–3568. [PubMed] [Google Scholar]
- Auer G., Ono J., Nasiell M., Caspersson T., Kato H., Konaka C., Hayata Y. Reversibility of bronchial cell atypia. Cancer Res. 1982 Oct;42(10):4241–4247. [PubMed] [Google Scholar]
- Balmain A., Ramsden M., Bowden G. T., Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984 Feb 16;307(5952):658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- Brodeur G. M., Seeger R. C. Gene amplification in human neuroblastomas: basic mechanisms and clinical implications. Cancer Genet Cytogenet. 1986 Jan 1;19(1-2):101–111. doi: 10.1016/0165-4608(86)90377-8. [DOI] [PubMed] [Google Scholar]
- Brown K., Quintanilla M., Ramsden M., Kerr I. B., Young S., Balmain A. v-ras genes from Harvey and BALB murine sarcoma viruses can act as initiators of two-stage mouse skin carcinogenesis. Cell. 1986 Aug 1;46(3):447–456. doi: 10.1016/0092-8674(86)90665-3. [DOI] [PubMed] [Google Scholar]
- Conti C. J., Aldaz C. M., O'Connell J., Klein-Szanto A. J., Slaga T. J. Aneuploidy, an early event in mouse skin tumor development. Carcinogenesis. 1986 Nov;7(11):1845–1848. doi: 10.1093/carcin/7.11.1845. [DOI] [PubMed] [Google Scholar]
- Hennings H., Shores R., Mitchell P., Spangler E. F., Yuspa S. H. Induction of papillomas with a high probability of conversion to malignancy. Carcinogenesis. 1985 Nov;6(11):1607–1610. doi: 10.1093/carcin/6.11.1607. [DOI] [PubMed] [Google Scholar]
- Hennings H., Shores R., Wenk M. L., Spangler E. F., Tarone R., Yuspa S. H. Malignant conversion of mouse skin tumours is increased by tumour initiators and unaffected by tumour promoters. Nature. 1983 Jul 7;304(5921):67–69. doi: 10.1038/304067a0. [DOI] [PubMed] [Google Scholar]
- Klein-Szanto A. J., Conti C. J., Aldaz C. M., Clapp N., Nesnow S., Slaga T. J. Effects of chronic topical application of 12-O-tetradecanoylphorbol-13-acetate on the skin and internal organs of SENCAR mice. Environ Health Perspect. 1986 Sep;68:75–80. doi: 10.1289/ehp.866875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolgavkar S. H., Knudson A. G., Jr Mutation and cancer: a model for human carcinogenesis. J Natl Cancer Inst. 1981 Jun;66(6):1037–1052. doi: 10.1093/jnci/66.6.1037. [DOI] [PubMed] [Google Scholar]
- Nowell P. C. Mechanisms of tumor progression. Cancer Res. 1986 May;46(5):2203–2207. [PubMed] [Google Scholar]
- O'Connell J. F., Klein-Szanto A. J., DiGiovanni D. M., Fries J. W., Slaga T. J. Enhanced malignant progression of mouse skin tumors by the free-radical generator benzoyl peroxide. Cancer Res. 1986 Jun;46(6):2863–2865. [PubMed] [Google Scholar]
- Oshimura M., Barrett J. C. Chemically induced aneuploidy in mammalian cells: mechanisms and biological significance in cancer. Environ Mutagen. 1986;8(1):129–159. doi: 10.1002/em.2860080112. [DOI] [PubMed] [Google Scholar]
- Potter V. R. A new protocol and its rationale for the study of initiation and promotion of carcinogenesis in rat liver. Carcinogenesis. 1981;2(12):1375–1379. doi: 10.1093/carcin/2.12.1375. [DOI] [PubMed] [Google Scholar]
- Potter V. R. Initiation and promotion in cancer formation: the importance of studies on intercellular communication. Yale J Biol Med. 1980 Sep-Oct;53(5):367–384. [PMC free article] [PubMed] [Google Scholar]
- Quintanilla M., Brown K., Ramsden M., Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986 Jul 3;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Reiners J. J., Jr, Nesnow S., Slaga T. J. Murine susceptibility to two-stage skin carcinogenesis is influenced by the agent used for promotion. Carcinogenesis. 1984 Mar;5(3):301–307. doi: 10.1093/carcin/5.3.301. [DOI] [PubMed] [Google Scholar]
- Scribner J. D., Scribner N. K., McKnight B., Mottet N. K. Evidence for a new model of tumor progression from carcinogenesis and tumor promotion studies with 7-bromomethylbenz[a]anthracene. Cancer Res. 1983 May;43(5):2034–2041. [PubMed] [Google Scholar]