Abstract
P2X7 receptor is an ATP-gated ion channel within the multiprotein inflammasome complex. Until now, little is known about regulation of P2X7 effector functions in macrophages. Here, we show that NTPDase1/CD39 is the dominant ectonucleotidase expressed by murine peritoneal macrophages and that it regulates P2X7-dependent responses in these cells. Macrophages isolated from NTPDase1-null mice (Entpd1−/−) were devoid of all ADPase and most ATPase activities when compared to wild type macrophages (Entpd1+/+). Entpd1−/− macrophages exposed to millimolar concentrations of ATP were more susceptible to cell death, released more IL-1β and IL-18 after TLR2 or TLR4 priming, and incorporated the fluorescent dye Yo-Pro-1 more efficiently (suggestive of increased pore formation) than Entpd1+/+ cells. Consistent with these observations, NTPDase1 regulated P2X7-associated IL-1β release after synthesis, and this process occurred independently of, and prior to, cytokine maturation by caspase-1. NTPDase1 also inhibited IL-1β release in vivo in the air pouch inflammatory model. Exudates of LPS-injected Entpd1−/− mice had significantly higher IL-1β levels when compared to Entpd1+/+ mice. Taken together, our studies suggest that NTPDase1/CD39 plays a key role in the control of P2X7-dependent macrophage responses.
Keywords: ATP-induced death, CD39, IL-1β, macrophage, NTPDase1, P2X7 receptor
INTRODUCTION
Monocytes/macrophages are myeloid inflammatory cells that play important roles in innate host defense and immune regulation [1]. For example, these cells represent the major source of IL-1 during inflammation, a cytokine playing a pivotal role in chronic inflammatory diseases such as rheumatoid arthritis and several neurodegenerative disorders [2, 3]. Monocytes/macrophages express functional P2 receptors specific for extracellular nucleotides, which serve as “danger” signals and initiate the immune response [4]. At the mRNA level, primary monocytes express the ion-channel P2X1,4,5,7 receptors and the G-protein-coupled P2Y1,2,4,6,11–13 receptors, and macrophages express the same receptor subtypes except P2Y13 [5]. P2Y1, P2Y2, P2X4 and P2X7 receptors have been detected in macrophages at protein level [6–8].
P2X7 receptor (formerly P2Z) plays a key role in inflammation [9, 10]. P2X7 receptor activation by extracellular ATP, released under inflammatory conditions, is a co-stimulus for a massive release of mature and bioactive cytokines of the IL-1 family, such as IL-1β and IL-18, from LPS-primed macrophages and other cell types [11]. The activation of P2X7 in leukocytes also induces cell death, intracellular pathogen clearance and membrane trafficking [12–14]. While nano to low micromolar nucleotide concentrations are sufficient for the activation of other P2 receptors, P2X7 is activated by far higher concentrations of ATP (in the order of the millimolar [mM]) and/or by mono ADP-ribosylation [15].
The concentration of P2 receptor ligands is regulated by ectonucleotidases, including members of the ectonucleoside triphosphate diphosphohydrolase (E-NTPDase) family [16, 17]. NTPDase1 (or CD39) is expressed on endothelial cells, smooth muscle cells and the majority of leukocytes [18–27]. This enzyme efficiently hydrolyzes all tri- and diphosphonucleosides, such as ATP, UTP, ADP and UDP, and thus is expected to terminate P2 receptor activation.
Hitherto, the expression of NTPDase1 in macrophages has been suggested by immunohistochemistry on human skin resident macrophages [18] and mouse testis resident macrophages [28]. Here we show that NTPDase1 is the dominant ectonucleotidase responsible for the hydrolysis of ATP and ADP at the surface of mouse primary macrophages and that this enzyme plays a key role in modulating P2X7-associated functions in these cells.
RESULTS
NTPDase1 (CD39) is the dominant ectonucleotidase on peritoneal macrophages
Previous studies indicated that peritoneal macrophages hydrolyze extracellular ATP [29, 30], suggesting the presence of ectonucleotidases at their surface. We first sought to identify the ecto-enzyme(s) responsible for this activity. RT-PCR of mouse peritoneal macrophage mRNA noted the presence of NTPDase1 and NTPDase2, but not of NTPDase3, NTPDase8 and ecto-5’-nucleotidase/CD73 (Table 1). However only NTPDase1/CD39, but not NTPDase2, was detected in peritoneal macrophages at the protein level by western blot (Fig. 1A) and flow cytometry (Fig. 1B; not shown for NTPDase2). NTPDase1 protein was also found in mouse bone marrow-derived macrophages (BMMΦ) by western blot (data not shown). The latter cells were used in a few experiments to extend the results obtained with elicited peritoneal macrophages to another type of macrophages.
Table 1.
Expression of P2 receptors and ectonucleotidases as determined by RT-PCRa)
| Gene | Entpd1+/+ | Entpd1−/− |
|---|---|---|
| P2rx1 | − | − |
| P2rx2 | − | − |
| P2rx3 | − | − |
| P2rx4 | + | + |
| P2rx5 | − | − |
| P2rx6 | +/− | +/− |
| P2rx7 | + | + |
| P2ry1 | + | + |
| P2ry2 | + | + |
| P2ry4 | − | − |
| P2ry6 | + | + |
| P2ry12 | +/− | +/− |
| P2ry13 | − | − |
| P2ry14 | + | + |
| Entpd1 | + | − |
| Entpd2 | +/− | +/− |
| Entpd3 | − | − |
| Entpd8 | − | − |
| Nt5e | − | − |
The same pattern of expression was observed in all experiment (n ≥ 3). + : strong expression; +/− : barely detectable; − : no signal detected.
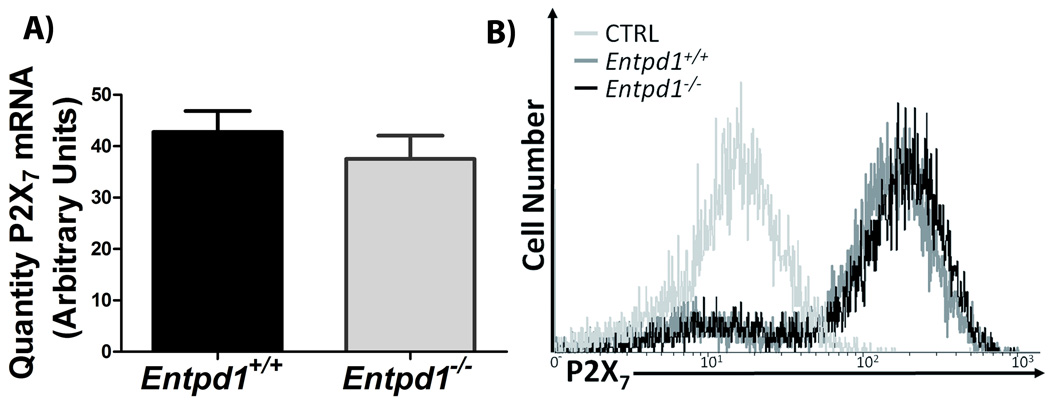
Fig. 1. NTPDase1 is the major ectonucleotidase on mouse peritoneal macrophages elicited with thioglycollate.
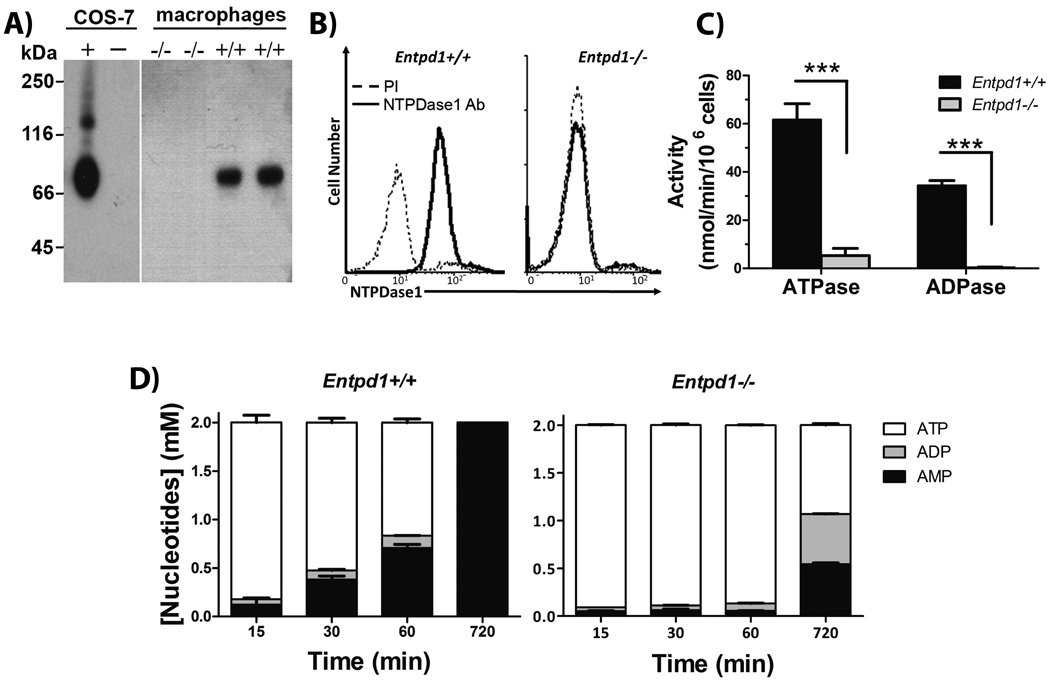
A) Western blot with rabbit polyclonal Ab against mouse NTPDase1 (mN1-2c). Left gel shows control proteins (0.5 µg) from lysates of COS-7 cells transfected with mouse NTPDase1 (+) or untransfected (−). Right gel shows a representative western blot (out of three performed with cells from individual mice) of proteins (12.5 µg) from peritoneal macrophage lysates.
B) Flow cytometric anaylsis of NTPDase 1 analyis using non permeabilizing conditions with a polyclonal Ab against CD39 (C9F, ——), compared to its pre-immune serum (PI, ----). Data are representative of three independent experiments with pooled macrophages from 2 to 4 mice per experiment. C) NTPDase1 activity with either ATP or ADP as substrate, Entpd1+/+ (filled bars) and Entpd1−/− (grey bars) macrophages. Data show mean + SEM (n=3). ***p<0.001, two-way ANOVA with Bonferroni post hoc test. D) Time course of the hydrolysis of 2 mM ATP by Entpd1+/+ or Entpd1−/− peritoneal macrophages was followed for 12 hours by HPLC: ATP (□), ADP (■), AMP (■). No adenosine production was detected. Data show mean + SEM of three independent experiments.
Next, we compared the differential capacity of Entpd1+/+ and Entpd1−/− macrophages to hydrolyse extracellular ATP and ADP. Entpd1+/+ cells hydrolysed these nucleotides with specific activities of 61 ± 9 and 35 ± 2 nmol Pi × min−1 per 106 cells, respectively. In comparison Entpd1−/− macrophages had about one tenth of the ATPase activity of Entpd1+/+ cells and undetectable ADPase activity (Fig. 1C). The activity at the surface of 106 Entpd1+/+ macrophages converted about 50% of 2 mM ATP to AMP with a minimal generation of ADP in 60 min, while 106 Entpd1−/− macrophages needed around 12 hours to hydrolyse the same amount of ATP (Fig. 1D). Noteworthy, Entpd1−/− cells hydrolyzed ATP with accumulations of ADP which could be due to a modest NTPDase2 expression. This weak activity was masked by the highly expressed NTPDase1 in wild type cells. Neither Entpd1+/+ nor Entpd1−/− thioglycollate-elicited peritoneal macrophages hydrolysed AMP (Fig. 1D and data not shown) that concurs with the lack of ecto-5’-nucleotidase expression (Table 1). In comparison with elicited macrophages, resident peritoneal macrophages had similar ATPase and ADPase activities, but had ability to hydrolyze AMP (data not shown). Altogether, these results show that NTPDase1 is the major ectonucleotidase in mouse peritoneal macrophages.
Regulation of P2X7 activity by NTPDase1 (CD39)
In order to assess whether NTPDase1 can regulate P2X7-associated functions, we first verified whether Entpd1+/+ and Entpd1−/− macrophages have similar expression level of this receptor. As shown in Fig. 2, Entpd1+/+ and Entpd1−/− macrophages did not significantly differ in the expression of P2X7 receptor at both mRNA and protein levels. Moreover, these cells have similar expression of other P2 receptors (Table 1).
Fig. 2. NTPDase1 deficiency does not impact P2X7 receptor expression by peritoneal macrophages.
A) P2X7 mRNA expression was quantified by qPCR. Data show mean + SEM for qPCR experiments performed in duplicate with RNA purified from macrophages obtained from 8 to 10 individual mice done separately. There were no significant differences in the expression of P2X7 mRNA between Entpd1+/+ and Entpd1−/− peritoneal macrophages (Student’s t-test).
B) Flow cytometric analysis of surface P2X7 expression in Entpd1+/+ and Entpd1−/− peritoneal macrophages. Data are representative of three independent experiments with pooled macrophages from 2 to 4 mice per experiment.
Entpd1−/− macrophages are more susceptible to ATP-induced death
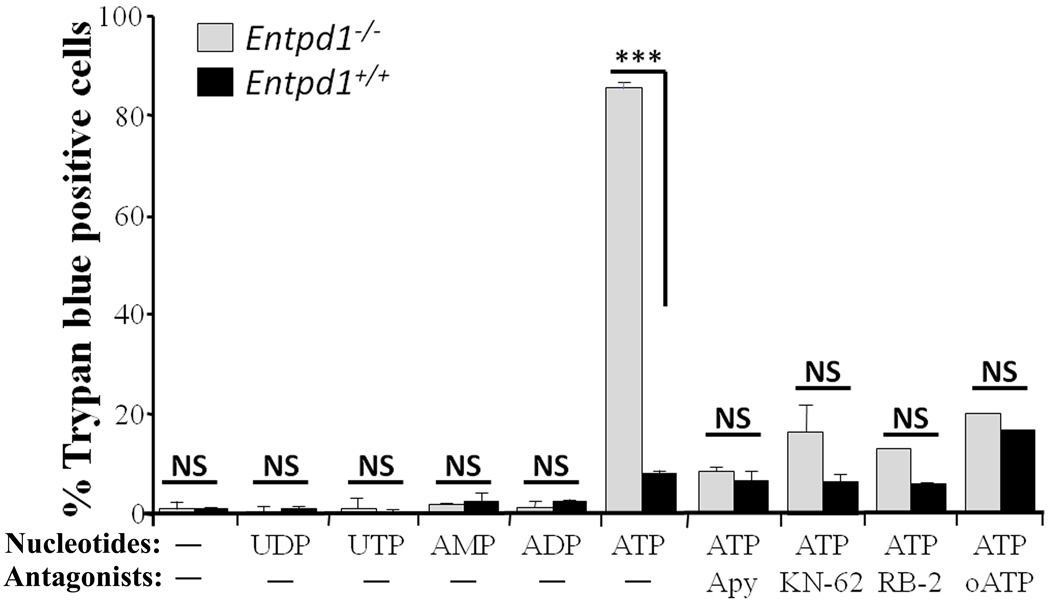
One function attributed to P2X7 receptor activation is cellular death [13, 31]. To induce this response, we incubated peritoneal macrophages with 2 mM ATP for 12 hours and measured either trypan blue incorporation or LDH release, as death indicators. This treatment killed 83 ± 4% of Entpd1−/− macrophages as compared to 13 ± 4% of Entpd1+/+ macrophages (Fig. 3) as measured by trypan blue incorporation. Similar data were obtained for LDH release (measured 3 hours after ATP addition; data not shown). Noteworthy, 4 mM ATP killed Entpd1+/+ (either from C57BL/6 or CD-1 strains) and Entpd1−/− macrophages at a similar rate (data not shown). Adenosine (0.1–2.0 mM) had no effect on macrophage viability (data not shown). In keeping with the role of NTPDase1 in ATP-induced death, exogenous NTPDase1 activity (potato apyrase, 2U/well; Fig. 3) prevented death in both null and wild type macrophages. In agreement with a role of P2X7 in ATP-induced macrophage death, P2X7 antagonists (KN-62, RB-2 and oATP) prevented this effect, and cell viability was unaffected by nucleotides other than ATP (Fig. 3). Taken together, these results suggest that the increased macrophage susceptibility to ATP-induced death in absence of NTPDase1 is due to the loss of P2X7 receptor regulation.
Fig. 3. Entpd1−/− macrophages are highly susceptible to ATP-induced death.
Peritoneal macrophages were treated 12 hours with 2 mM of the indicated nucleotides in the presence or absence of potato apyrase (Apy; 2 U) or with and without P2X7 receptor antagonists (3 µM KN-62, 100 µM RB-2, 600 µM oATP). Cells positive for trypan blue incorporation were counted in a minimum of two random fields each containing over 150 cells. Data show mean + SEM of three or more experiments for each condition tested. ***p<0.001, two-way ANOVA with Bonferroni post hoc test; NS (no significant differences), p>0.05.
NTPDase1 regulates P2X7-induced IL-1β and IL-18 secretion
We next tested whether NTPDase1 could also regulate earlier P2X7-activated macrophage responses such as IL-1β and IL-18 release, and pore formation.
NTPDase1 controls IL-1β and IL-18 release by macrophages in vitro
Macrophages accumulate pro-IL-1β in their cytosol following activation of MAPK and NF-κB [2, 3, 32] due to, for example, Toll-like receptor (TLR)-4-activation by LPS. ATP alone is a poor trigger of the synthesis of these cytokines (Fig. 4A; [3, 11]), but it is necessary for their maturation and release from macrophages and other cell types [3, 11, 33].
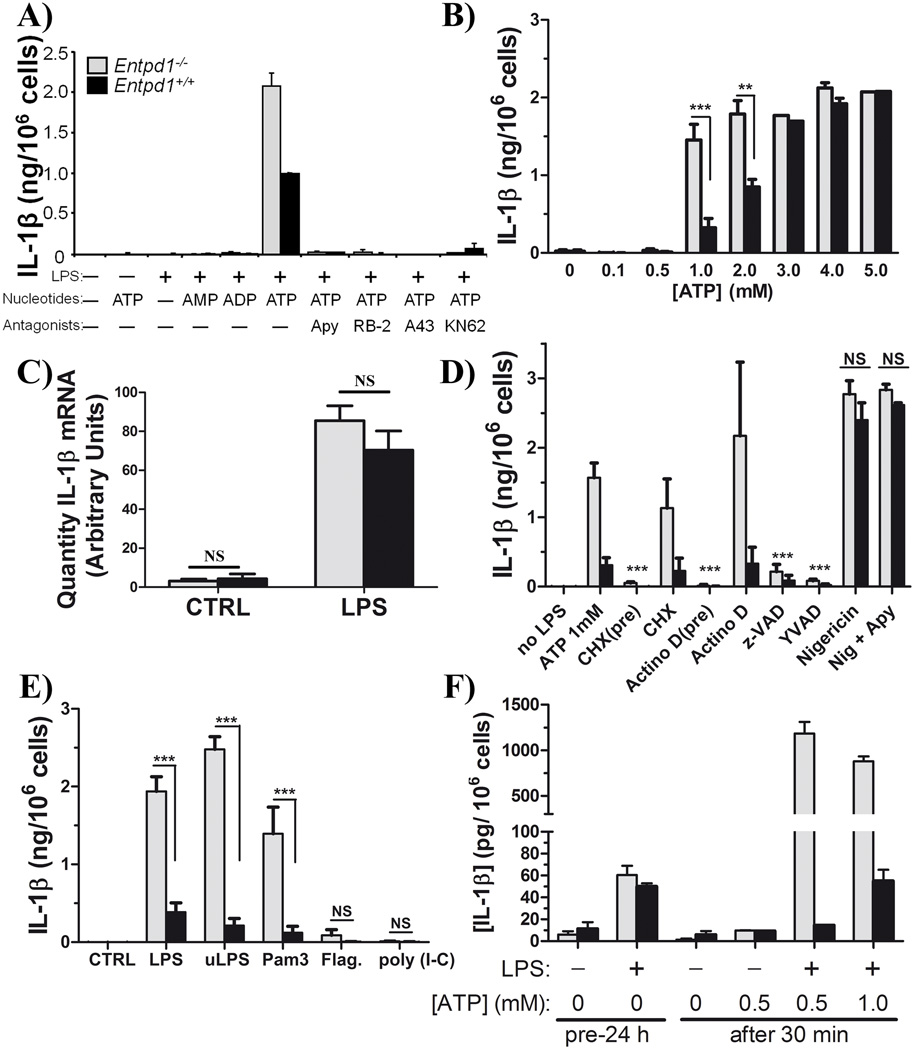
Fig. 4. NTPDase1 modulates P2X7-dependent IL-1β release from macrophages.
Macrophages (peritoneal or BMMΦ) were primed with LPS (or other TLR ligands as indicated), washed, and the medium was replaced with fresh medium containing ATP (0.1 to 5 mM) or nigericin (5 µM). Supernatants were analyzed for IL-1β concentration by ELISA.
A) IL-1β released by LPS-primed peritoneal macrophages 60 min after treatment with 2 mM ATP in the presence or absence of apyrase (Apy; 2 U) or P2X7 antagonists (100 µM RB-2, 25 µM A438079 or 3 µM KN-62). Data show mean + SD of one representative experiment performed in duplicate and are representative of least three independent experiments. Data show mean + SD of one representative experiment performed out of at least three independent experiments. In the presence of 2 mM ATP IL-1β levels ranged from 1.7 to 2.3 ng/106 cells and 0.8 to 1.1 ng/106 cells for Entpd1−/− (gray bars) and Entpd1+/+ (filled bars) macrophages, respectively.
B) IL-1β released by LPS-primed peritoneal macrophages 30 min after ATP treatment (0.1 to 4.0 mM). Data show mean + SEM of n ≥ 3 independent experiments. **p<0.01, ***p<0.001, two-way ANOVA with Bonferroni post hoc test.
C) IL-1β mRNA in Entpd1−/− and Entpd1+/+ peritoneal macrophages primed for 3 hours with LPS (10 ng/mL) or not (CTRL) was measured by qPCR. Data show mean + SEM of RNA purified from macrophages obtained from 9 (Entpd1−/−) and 8 (Entpd1+/+) mice, respectively. NS (no significant differences), p>0.05.
D) IL-1β released by LPS-primed peritoneal macrophages 30 min after 1 mM ATP or nigericin treatment was measured. The inhibitors of transcription (8 µM Actinomycin D), protein synthesis (10 µg/mL cycloheximide [CHX]), caspases (20 µM z-VAD), or caspase-1 (20 µM YVAD), were added either 15 min before LPS pre-stimulation/priming (pre) and removed before ATP stimulation, or 15 min prior to the addition of ATP when LPS was not yet removed from the media. Data show mean + SEM of n ≥ 3 experiments. After the indicated treatment, significantly less IL-1β was measured when compared to ATP 1mM (***p<0.001) for both Entpd1−/− (gray bars) and Entpd1+/+ (filled bars). There were no significant differences between Entpd1−/− and Entpd1+/+ macrophages for both nigericin (Nig) and Nig + Apy (NS, p>0.05), two-way ANOVA with Bonferroni post hoc test.
E) ATP-induced IL-1β release by peritoneal macrophages primed with various TLR ligands. Cells were pre-stimulated with an agonist to either TLR4 (10 ng/mL LPS, or ultra pure LPS (uLPS); positive controls), TLR2 (100 ng/mL Pam3CSK4 [Pam3]), TLR5 (1 µg/mL flagellin, “Flag”) or TLR3 (1 µg/mL poly I:C) and then further stimulated for 30 min with 1 mM ATP. IL-1β released by these cells was quantified by ELISA. Data show mean + SEM of 3 to 8 independent experiments. **p<0.001, two-way ANOVA with Bonferroni post hoc test.
F) IL-1β released by BMMΦ primed for 24 hours with LPS (+) or DMEM (−) and then stimulated 30 min with or without ATP (0, 0.5 or 1.0 mM), was measured. Data show mean + SEM of one experiment performed in triplicate.
Entpd1−/− peritoneal macrophages primed for 3 hours with LPS and then stimulated for 60-min with 2 mM ATP released significantly more IL-1β than their wild type counterparts (Fig. 4A). Congruent with the role of P2X7, nucleotides that are not P2X7 ligands, e.g. ADP, AMP, UTP and UDP, did not induce IL-1β release from LPS-primed macrophages (Fig. 4A and data not shown). In addition, apyrase and various P2X7 antagonists (RB-2, A438079, and KN-62) abrogated IL-1β secretion by cells of both genotypes (Fig. 4A). Thus, P2X7 activation induced IL-1β release by peritoneal macrophages, which is controlled by the ectonucleotidase NTPDase1. Noteworthy, as for cell death, at concentration of ATP > 3 mM Entpd1+/+ and Entpd1−/− macrophages acted similarly and secreted comparable amounts of IL-1β (p > 0.05; Fig. 4B).
Next, we investigated whether NTPDase1 controls IL-1β at the level of biosynthesis, maturation, or release. To limit the effect of ATP-induced death in these assays, IL-1β was measured after 30 min (instead of 60 min in Fig. 4B, D–F) already sufficient for a maximal release of this cytokine (data not shown). Firstly, the differences in IL-1β release between Entpd1+/+ and Entpd1−/− cells were not due to increased IL-1β transcription in Entpd1−/− macrophages. Low basal levels of IL-1β mRNA were detected in both naive Entpd1+/+ and Entpd1−/− macrophages, and were increased to a similar extent by LPS (∼20 fold; Fig. 4C). Comparable results were obtained for the pro-IL-1β protein expression assessed by western blot (n=1, data not shown). Furthermore, when inhibitors of transcription (actinomycin D) and of translation (cycloheximide) were added to peritoneal macrophages before LPS pre-stimulation, these near abolished IL-1β release (Fig. 4D). In contrast, these inhibitors had no significant effect when added after LPS priming, at 15 min before the addition of ATP (Fig. 4D). Taken together, these data suggest that NTPDase1 control of ATP-induced IL-1β release occurs at a level prior to biosynthesis of pro-IL-1β by peritoneal macrophages. Noteworthy, IL-1β release was also measured 60 min after the addition of ATP in at least one experiment for all conditions presented in figure 4, and data similar to 30 min incubation were obtained (data not shown). The difference in IL-1β release between Entpd1−/− and Entpd1+/+ peritoneal macrophages was more substantive at 1 mM than at 2 mM ATP (Fig. 4B), further supporting the fact that higher IL-1β release in Entpd1−/− macrophages was not due to cell death. Indeed, although some trypan blue positive cells could be observed in some experiments as early as 1 hour after the addition of 2 mM ATP, this observation was never seen with 1 mM ATP.
Finally, a general caspase inhibitor z-VAD, or the caspase-1 specific inhibitor YVAD, decreased the release of IL-1β by LPS-primed peritoneal macrophages when stimulated with ATP (Fig. 4D). In addition, nigericin, a drug that stimulates IL-1β release independently of P2X7 activation, induced the secretion of comparable amounts of IL-1β in cells from both genotypes (Fig. 4D). These data suggest that NTPDase1 controls IL-1β release by specifically modulating P2X7-induced maturation/release of IL-1β by caspase-1.
As for IL-1β, IL-18 release was also heightened in Entpd1−/− macrophages compared to Entpd1+/+ cells after ATP treatment, and equivalent after nigericin treatment (Fig. 5). Also similarly to IL-1β, the release of IL-18 was efficiently blocked by apyrase, P2X7 antagonists (KN-62, A438079) and caspases inhibitors (zVAD, YVAD; Fig. 5 and data not shown). Note however that the level of IL-18 production by elicited peritoneal macrophages was low compared to IL-1β (25–150 pg (Fig. 5) vs 2 ng (Fig. 4)).
Fig. 5. NTPDase1 modulates P2X7-dependent IL-18 release from peritoneal macrophages.
Macrophages were primed with LPS (10 ng/mL) for 3 hours and then treated for 30 min with either 1 or 2 mM ATP in the presence or absence of P2X7 antagonists (25 µM A438079 or 3 µM KN-62). The supernatants were analyzed for IL-18 by ELISA. Data show mean + SEM of n = 3–4 experiments. IL-18 release was normalized with nigericin as a stimulus; 100% release varied from 15 to 150 pg/106 cells depending on the experiment. *p<0.05, ***p<0.001, two-way ANOVA with Bonferroni post hoc test.
NTPDase1 modulates IL-1β secretion also in TLR-2 primed macrophages
To determine whether TLR agonists other than LPS, the TLR4 agonist, stimulate the production of pro-IL-1β in peritoneal cells, and to examine whether NTPDase1 also plays a role in this process, we pre-stimulated these cells for 3 hours with the agonists of TLR expressed by macrophages, namely TLR2 (Pam3CSK4), TLR5 (flagellin) and TLR3 (poly I:C) [34], and then added 1 mM ATP for 30 min (Fig. 4E). Only Entpd1−/− macrophages pre-stimulated with TLR2 and TLR4 agonists, but not with TLR3 and TLR5 agonists, released significantly more IL-1β than Entpd1+/+ cells (p < 0.001; Fig. 4E).
NTPDase1 modulates IL-1β secretion by BMMΦ
We verified whether the increased IL-1β release from Entpd1−/− peritoneal macrophages could be extrapolated to another source of macrophages. Figure 4F shows that Entpd1−/− BMMΦ released more IL-1β than Entpd1+/+ cells due to either 0.5 mM or 1.0 mM ATP.
Enhanced levels of IL-1β in Entpd1−/− mice in vivo
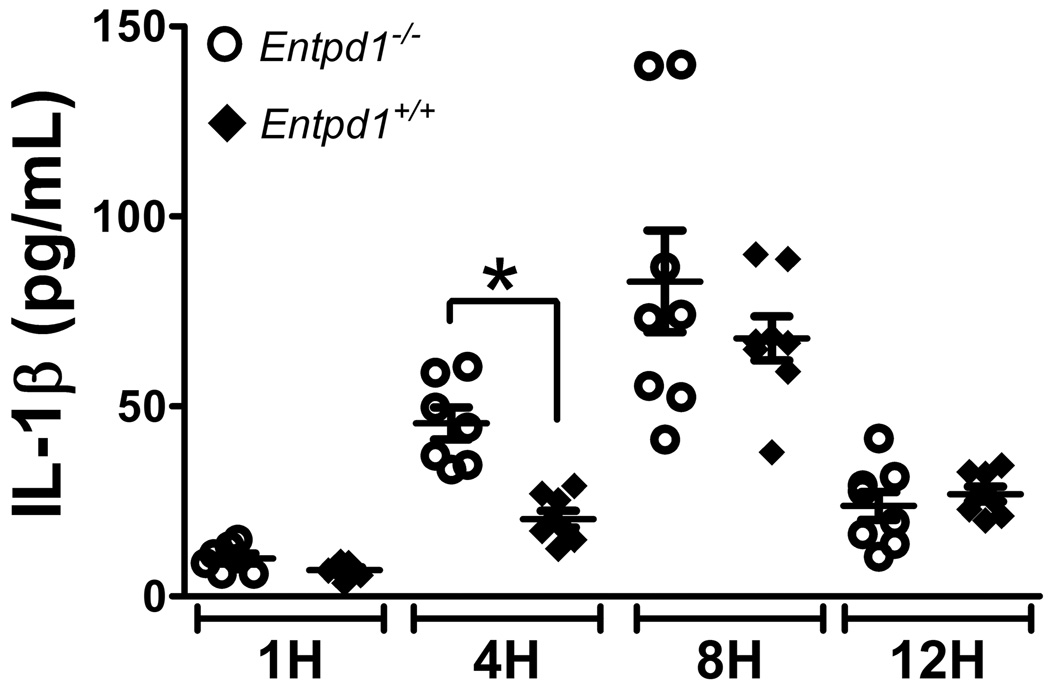
Next, we investigated whether NTPDase1 regulates IL-1β production in vivo using LPS-treated mouse air-pouches. LPS was chosen as an inflammatory inducer as it stimulates ATP release from monocytes, peritoneal macrophages as well as from other cells [35–38], and because LPS-primed cells can release IL-1β upon ATP stimulation (Fig. 4). LPS injection in the air pouches resulted in a robust migration of leukocytes peaking at 4 hours post injection that was comparable in Entpd1+/+ and Entpd1−/− mice (data not shown). However, Entpd1−/− mice produced significantly more IL-1β at this time point when compared to wild type mice (Fig. 6). Thus, NTPDase1 also controls IL-1β production in vivo.
Fig. 6. NTPDase1 deficiency increases IL-1β production in inflammatory air pouches.
Air pouches were raised on the back of female Entpd1+/+ and Entpd1−/− C57BL/6 mice. At the indicated time in hours (H) post s.c. injection of LPS in the pouches, inflammatory exudates were collected and analyzed for IL-1β level by ELISA. Data show mean ± SEM of n = 7–8 mice per group. *p<0.05, one-way ANOVA with Bonferroni multiple comparison test.
Entpd1−/− peritoneal macrophages exhibit increased pore formation
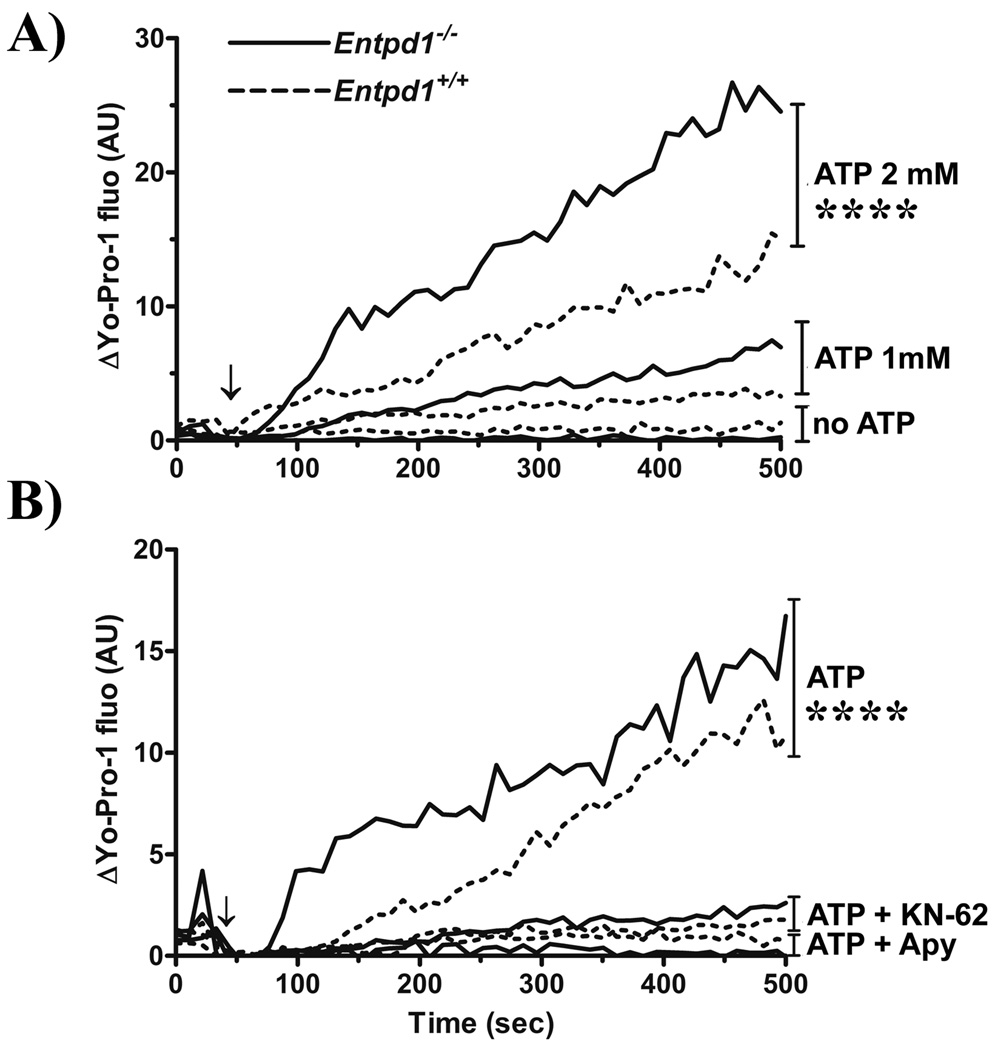
ATP, via P2X7 activation, induces a pannexin-1 (panx-1)-dependent pore formation that can be detected by measuring uptake of fluorescent dyes, such as Yo-Pro-1 [39, 40]. This effect takes place a few minutes after ATP addition to the cells, well before IL-1β release. Here, we investigated whether NTPDase1 regulates Yo-Pro-1 uptake in macrophages stimulated with or without ATP (0.1 to 4.0 mM). A linear model with a common slope and 12 additional individual slopes (deviations from the common slope) was adjusted for each ATP concentration. Pairwise comparisons of individual slope coefficients showed significantly higher intensity increase for Entpd1−/− compared to Entpd1+/+ macrophages for all ATP concentrations tested (p ≤ 0.002). When we evaluated Yo-Pro-1 uptake compared to the absence of ATP, slope coefficients were significantly different only at concentration of 2 and 4 mM (p < 0.0001; Fig. 7A and data not shown). Yo-Pro-1 intensities were not significantly different from baseline at either 0.1, 0.5 or 1.0 mM ATP (p > 0.05; not shown).
Fig. 7. NTPDase1 deficiency increases P2X7-associated Yo-Pro-1 uptake by macrophages.
A) ATP dose-dependent Yo-Pro-1 uptake by peritoneal macrophages (Entpd1−/− [——], Entpd1+/+ [----]) was evaluated by measuring differences in Yo-Pro-1 mean fluorescence intensity (ΔYo-Pro-1 MFI) using flow cytometry. Cells were incubated with or without ATP (0, 1.0 and 2.0 mM) for the indicated time period. Data show ΔYo-Pro-1 expressed in arbitrary units (AU) for 275–550 cells per 11 seconds. A representative experiment out of 3 is shown. The time point corresponding to ATP addition to the cells (60 sec) is indicated on the graphs by an arrow (↓). Note that statistical analyses were carried out on complete data (n = 15–30 cells per 0.6 sec). Pairwise comparison of individual slope indicate significantly higher intensity growth for Entpd1−/− when compared to Entpd1+/+ macrophages (p≤0.002), but slope were significantly different from the baseline slope only when indicated (****, p<0.0001).
B) Effect of KN-62 and apyrase (Apy) on ATP-induced Yo-Pro-1 uptake by peritoneal macrophages (Entpd1−/− [——], Entpd1+/+ [----]). The cells were stimulated with 2 mM ATP alone or in combination with KN-62 (3 µM) or Apy (2U) for the indicated period of time. Yo-Pro-1 incorporation measurement and statistical analysis were done as above. Pairwise comparison of individual slope indicate significantly higher intensity growth compared to KN-62 and Apy treatment (****p<0.0001) for both genotype.
Moreover, the addition of KN-62 and apyrase decreased Yo-Pro-1 incorporation in cells of both genotypes (Fig. 7B). A linear model with a common slope and six additional individual slopes (deviations from the common slope) was adjusted for each treatment (ATP 2 mM; ATP+KN-62 and ATP+Apy) for Entpd1−/− and Entpd1+/+ macrophages. Pairwise comparisons of individual slope coefficients showed significantly lower fluorescence intensity increase for both ATP+KN-62 and ATP+Apy when compared to ATP 2 mM for Entpd1−/− and Entpd1+/+ macrophages (p < 0.0001). These data demonstrate that NTPDase1 modulates P2X7-dependent pore formation in murine macrophages.
DISCUSSION
NTPDase1 is the dominant ectonucleotidase on macrophages
We have demonstrated that resident and thioglycollate-elicited peritoneal macrophages, as well as BMMΦ, express the ectonucleotidase NTPDase1. Moreover, Entpd1−/− macrophages were almost completely devoid of ATPase and ADPase activities. These results are in agreement with previous works reporting the presence of an ecto-ATPase activity on macrophages [29, 30, 41] and immunoreactivity with NTPDase1/CD39 antibodies in resident macrophages of skin and testis [18, 28]. Macrophages may also express low levels of NTPDase2 as a faint mRNA signal was detected by RT-PCR (Table 1). This would also explain the hydrolysis, albeit minor, of ATP to ADP seen in figure 1D. Finally, thioglycollate-elicited peritoneal macrophages do not express ecto-5’-nucleotidase/CD73 (Table 1) and lack AMPase activity (data not shown). These data are in agreement with the important decrease in ecto-5’-nucleotidase activity reported in elicited or activated peritoneal macrophages when compared to resident macrophages [42].
NTPDase1 regulates P2X7 receptor functions
NTPDase1 influences ATP-induced cell death
In this study, we show that NTPDase1 protects peritoneal macrophages from ATP-induced death via the suppression of P2X7 activation by 2 mM ATP (Fig. 3). These results are in agreement with a previous work that reported a high Ca2+- and Mg2+-dependent ATPase activity in cytotoxic T lymphocytes (CTL), which protected these cells from P2X7-mediated LDH release [43]. In contrast to peritoneal macrophages, CTL ATPase activity was attributed to more than one ectonucleotidase, although their identity has not yet been established [43]. Another study has shown that the over expression of NTPDase1 in HUVEC decreased ATP-induced DNA degradation [44]. This cell death was ascribed to P2X7 that is upregulated in activated HUVEC [45, 46]. At ATP concentrations higher than 4 mM, NTPDase1 could not prevent ATP-induced death in wild type peritoneal macrophages which is in agreement with previous works [13, 47].
In contrast, no differences in LDH release were observed between Entpd1−/− and Entpd1+/+ DC exposed for 4 hours to 2.5 mM ATP, and apyrase added to DC together with ATP enhanced LDH release in both, Entpd1−/− and Entpd1+/+ DC, after 1 hour incubation [23]. This LDH release might have been induced by ATP and/or adenosine. Indeed, adenosine was reported to induce death in several cell lines [48–50], but had no effect on the viability of peritoneal macrophages (Entpd1+/+ or Entpd1−/−) even at 2 mM (not shown).
NTPDase1 modulates release of IL-1β and IL-18
Extracellular ATP via P2X7 activation causes K+ efflux essential in the process of caspase-1 activation [9, 51] and the release of bioactive IL-1β and IL-18 [11, 52, 53] from activated macrophages which represent the major source of these cytokines during inflammation [2, 3]. Here, we have demonstrated that NTPDase1 controls the release of these cytokines by mouse macrophages as Entpd1−/− macrophages stimulated with ATP released more IL-1β and IL-18 than Entpd1+/+ cells (Fig. 4 and 5). NTPDase1 might have a general role in the control of IL-1β release from macrophages as BMMΦ devoid of NTPDase1 also released more IL-1β than wild type BMMΦ (Fig. 4F). Furthermore, our results indicate that NTPDase1 controls IL-1β release upstream of caspase-1/inflammasome complex activation, and downstream of pro-IL-1β synthesis, which is in agreement with the control of P2X7 activation. In keeping with the idea that the control of IL-1 cytokine release is regulated by NTPDase1, we have previously shown that IL-1α is decreased by NTPDase1 overexpression in HUVEC [44].
Murine macrophages express several TLR [34] and the primary stimulation of some of these receptors results in IL-1β and IL-18 release after a subsequent addition of 5 mM ATP [9, 54]. Entpd1−/− peritoneal macrophages primed for 3 hours with LPS (an agonist of TLR4) and Pam3CSK4 (TLR1/2), (but not when primed with poly I:C (TLR3) and flagellin (TLR5)), released high level of IL-1β after stimulation with 2 mM ATP (Fig. 4E). The failure of IL-1β release by macrophages when primed with flagellin was unexpected as Mariathason et al. reported the opposite result [52]. It is possible that TLR5 was absent in peritoneal macrophages which is consistent with the fact that it is not expressed by all mouse macrophage lines, being present in J774 and SV-40 MES-13 macrophages but absent in RAW264.7 cells [34]. Another possibility is that flagellin and poly I:C require longer incubation times. Indeed, poly I:C needs to be internalized to activate TLR3 which is present in intracellular vesicles and flagellin might have its effect via the activation of IPAF-inflammasome in the cytosol of macrophages as it was recently suggested [55].
NTPDase1 has also a role in IL-1β production in vivo as IL-1β levels in exudates from air pouch were increased in Entpd1−/− compared to Entpd1+/+ mice when LPS was injected in the air pouch of these mice. Although macrophages are present in air pouches, it is unclear whether these cells were responsible for all IL-1β production in this mode as the majority of cells migrating into the air pouch are neutrophils. However, no functional P2X7 has been shown on neutrophils and their ability to release IL-1β upon LPS stimulation remains to be established, even though neutrophils can produce pro-IL-1β mRNA and protein [56].
NTPDase1 controls pore formation
ATP-induced cell death and IL-1β release are often linked to an earlier feature of P2X7 activation, namely pore formation which happen after short exposure to ATP (2–3 min). Entpd1−/− macrophages incorporated more Yo-Pro-1 and in a faster way when compared to wild type cells (Fig. 7) indicating a regulation of NTPDase1 on the P2X7-associated pore formation. In agreement with early control of P2X7 activation by NTPDase1, high ATPase activity and expression of NTPDase1 correlated with a lack of Ca2+ mobilization in response to 5 mM ATP in the leukemia B cell line LCL-H [57]. In the latter cells, Ca2+ was mobilized when this ATPase activity was blocked with βγMeATP, and this mobilization could be inhibited by the P2X7 antagonist KN-62 [57]. Interestingly, figure 1D reveals that NTPDase1 present on Entpd1+/+ macrophages converted less than 2% of 2 mM ATP after 15 min. This small change in the bulk ATP concentration cannot directly explain the dramatic effect observed on P2X7 activation in absence of NTPDase1. This suggests that NTPDase1 may be impressed adjacent to P2X7 in macrophage plasma membrane and could impact local concentration more importantly than what could be seen in the bulk phase. In agreement, both P2X7 and NTPDase1 can be palmitoylated and localized in detergent-resistant membrane domains, where these proteins might partially colocalize with caveolin [58–60].
NTPDase1 may impact inflammation by controlling P2X7 receptor activation
Regulation of P2X7 activation by NTPDase1 can help maintain low inflammation in resting conditions and favour homeostasis. Inflammatory conditions can modulate NTPDase1 expression and/or activity which is decreased in ischemia reperfusion injury [61, 62] and brain ischemia [63], and increased in certain chronic inflammatory conditions such as colitis and pancreatitis [64–67]. This modulation of NTPDase1 expression and activity may in turn affect P2X7 mediated signaling and alter inflammation. Indeed, the lack of P2X7 receptor in knockout mice or the addition of antagonists to wild type animals have been shown to attenuate inflammation in rat brain [68] and in mice unilateral ureteral obstruction [69], to reduce inflammatory and neuropathic pain [70], and to diminish IL-1β and IL-18 release [11].
In conclusion, NTPDase1 is the major ectonucleotidase on murine macrophages, where this ecto-enzyme governs P2X7-mediated responses such as ATP-induced death, IL-1β and IL-18 secretion, and Yo-Pro-1 permeable pore formation. As NTPDase1 participates in the regulation of the extracellular concentrations of both nucleotides and adenosine, it might solely impact P2X7 activation, but also other P2 and P1 receptors. NTPDase1 and could therefore influence inflammation in a more general manner.
MATERIAL AND METHODS
Reagents
The general inhibitor of caspases z-VAD(OMe)-fmk, the caspase-1 inhibitor Ac-YVAD-AOM, and cycloheximide (CHX) were purchased from Calbiochem (Gibbstown, NJ, USA). Flagellin from S. typhimurium, Pam3CSK4, and poly I:C were obtained from Invivogen (San Diego, CA, USA). Actinomycin D, LPS from Escherichia coli O111:B4, nucleotides (ATP, ADP, AMP, UTP, UDP), oxidized ATP (oATP), potato apyrase grade VII were purchased from Sigma-Aldrich (Oakville, ON, Canada). The P2X7 antagonists A-438079 and KN-62 were provided by Tocris Bioscience (Ellisville, MO, USA), BBL thioglycollate medium by Fischer Scientific (Ottawa, ON, Canada), Reactive blue 2 (RB-2) by ICN Biochemicals (Aurora, OH, USA) and ultra pure LPS from Escherichia coli O55:B5 by List Biological Laboratories (Campbell, CA, USA). Yo-Pro-1 was acquired from Invitrogen (Burlington, ON, Canada).
Animals
CD1 mice were purchased from Charles River (Pointe-Claire, QC, Canada). NTPDase1-deficient mice backcrossed 7 times to the C57BL/6 genetic background were previously reported [71]. Experimental procedures were done according to the Canadian Council on Animal Care policy and were approved by the Université Laval Animal Welfare Committee.
Isolation of Peritoneal Macrophages
Peritoneal macrophages were obtained either from CD1 or C57BL/6 wild type (Entpd1+/+) or NTPDase1 knockout (Entpd1−/−) mice. Unless otherwise indicated, the peritoneal macrophages used in this study were elicited with thioglycollate and prepared by a method similar to the one described by Zhang et al. [72]. Macrophages were collected 4 days after i.p. injection of 1 mL of 3% sterile thioglycollate medium by a lavage of the peritoneal cavity with 8 mL of sterile PBS. The cells consisted of 70–85% macrophages, as confirmed with CD11b and F4/80 Ab, were washed twice with sterile PBS and resuspended in DMEM/F12 medium containing 1% FBS. To enrich this preparation in macrophages, the cells were transferred into 6 (2×106 cells/well), 24 (5×105−106 cells/well) or 48 (2×105 cells/well) well-plates and allowed to attach for 2 hours. Unattached cells were wash out with DMEM/F12 containing 1% FBS. The attached cells, mainly peritoneal macrophages, were used for the experiments thereafter.
Isolation of BMMΦ
Mice were anesthetized with Ketamine/Xylazine and sacrificed by cervical dislocation. Femur and tibia were isolated aseptically. Bone marrows were collected in 10% FBS-DMEM/F12 medium, disrupted, and cultured for 7–10 days in 10% FBS-DMES/F12 supplemented with 1 ng/ml of Mouse CSF (Peprotech) in bacterial petri-dish. Cells were harvested and replated in 12-well plates at 0.5 × 106/well in 10% FBS-DMEM/F12 medium [73].
Nucleotidase Activity Assays
Enzymatic activity was evaluated by two methods, a Malachite green colorimetric assay to quantify the release of inorganic phosphate (Pi) [74], and by analysis of the nucleotide products by HPLC. Activity was determined for 106 cells at 37°C in a 24 well-plate using 0.5 mL of incubation medium (5 mM CaCl2, 145 mM NaCl, 80 mM Tris, pH 7.4). The reaction was initiated by the addition of 500 µM ATP or ADP and stopped after 15 min by sampling an aliquot of 0.2 mL promptly mixed with 50 µL of malachite reagent and Pi released was calculated as before [16]. HPLC analysis was used to determine and quantify the nucleotide products of ATP hydrolysis. Two milliliter of DMEM medium without phenol red containing 2 mM ATP was added to macrophages at 37°C, aliquots of 200 µL were withdrawn from the wells at different time points, and transferred to 200 µL ice cold 1 M HClO4. Further sample preparation and nucleotide content quantification by HPLC were done as before [16].
RT and Quantitative PCR
Total RNA was isolated using Trizol method according to the manufacturer’s recommendation (Invitrogen). RNA quantity and quality was assessed using an Agilent Technologies 2100 bioanalyzer and RNA 6000 Nano LabChip kit (Agilent, Mountain View, CA, USA). Primers were designed using Primer Express 2.0 (Applied Biosystems) and their respective sequences are included in Supp. Table 1. For RT-PCR, cDNA was synthesized with Supercript III (Invitogen) from 500 ng of total RNA with oligo (dT)18 as the primer, according to the instructions of the manufacturer (Invitrogen). For amplification, 1/20 of the reverse transcription (RT) reaction volume was used as a template in a final volume of 25 µL, containing 0.4 µM primer, 200 µM dNTP, and 1.5 unit of Taq DNA polymerase (New England Biolab, Ipswich, MA, USA). Amplification was started with 10 min at 94°C followed by 35 cycles of denaturation for 45 sec at 94°C, annealing for 45 sec at 55–61°C, as indicated in Table 2), and elongation for 45 sec at 74°C, and ended by 7 min incubation at 74°C. Glyceraldehyde dehydrogenase (GAPDH) amplification was used as a control of amplification.
For quantitative PCR (qPCR), cDNA was generated from 75 ng of total RNA using random nanomers as primers (Sigma-Aldrich) following the protocol for Superscript III (Invitrogen). Equal amounts of cDNA were run in triplicate and amplified in a final volume of 15 µl containing 7.5 µL of 2X Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA), 10 nM of Z-tailed forward primer, 100 nM of reverse primer, 250 nM of Amplifluor Uniprimer probe (Chemicon, Temecula, CA, USA), and 80 ng of cDNA target. The mixture was incubated at 50°C for 2 min, at 95°C for 4 min, and then cycled 55 times at 95°C for 15 sec and at 55°C for 30 sec using the Applied Biosystems Prism 7900 Sequence Detector. For all genes quantified, no template controls where run, amplification efficiencies were validated and normalized to 18S ribosomal gene and quantity of target gene was calculated according to a standard curve. Primers were designed using Primer Express 2.0 (Applied Biosystems) and their respective sequences are included in Suppl. Table 1. Amplicons were detected using the Amplifuor UniPrimer system where forward primers contained a 5’ Z sequence: ACTGAACCTGACCGTACA.
Western Blot
Protein extracts of cells were prepared as previously described [16]. Mouse NTPDase1 was revealed with mN1–2c (2 hours; 1:1000; [28]) and C9F (2 hours, 1:1000) and IL-1 with 3ZD (1 hour, 1:1000), followed by 1 hour incubation with appropriate horseradish peroxidase-conjugated secondary antibody (anti-rabbit, Amersham Biosciences, Boston, MA; anti-guinea pig, GE Healthcare, QC, Canada; anti-mouse, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). Bands were visualized using lightning western blot Chemiluminescence Reagent Plus (Perkin Elmer, Boston, MA, USA).
Flow Cytometry
The expression of selected surface proteins by peritoneal macrophages was evaluated by cytometry using the following Ab: a rat PE-conjugated anti-mouse CD11b (clone M1/70; BD Biosciences, Mississauga, ON, Canada), a rat anti-mouse F4/80 (clone A3-1; AbD Serotec, Raleigh, NC, USA), a rabbit polyclonal anti-mouse CD39 (C9F; [71]), a rabbit polyclonal anti-mouse NTPDase2 (mN2–36L; [75]) and a rabbit polyclonal anti-P2X7 (APR-004; Alomone Labs, Jerusalem, Israel). Briefly, the cells were incubated 30 min with the above primary antibodies diluted in PBS 1X, 1% FBS, 0.1% sodium azide (PFA), and, when necessary, with secondary FITC-conjugated goat anti-rabbit IgG antibodies for 30 min (Santa Cruz Biotech, Santa Cruz, CA, USA), with a minimum of 2 washes with PFA after each incubation. Cell surface fluorescence was measured with EPICS XL flow cytofluorometer (Beckman-Coulter, Mississauga, ON, Canada) and analysed with WinMDI 2.9 software (Scripps Research Institute, La Jolla CA, USA).
ATP-induced Cell Death Assays
The cell death of peritoneal cells treated with nucleotides was determined based on two techniques: trypan blue (0.2%) incorporation and LDH release. In both assays, the cells were incubated with 2 mM nucleotides (ATP, ADP, AMP, UTP and UTP) with or without apyrase, or P2X7 antagonists, for various times up to 12 hours at 37°C in a CO2 incubator. For the trypan blue incorporation assay, medium was removed after stimulation and cells were incubated with trypan blue for 5 min, then washed twice with PBS and analyzed with a microscope. The lactate dehydrogenase (LDH) activity released in the supernatants of the treated peritoneal cells was compared with the total activity in cells lysed with 2% Triton X-100 using the “Cytotoxicity Detection Kit (LDH)” following the manufacturer’s recommendations (Roche Diagnosis, Indianapolis, IN, USA).
IL-1β and IL-18 ELISA
Peritoneal macrophages were primed 3 hours with the following TLR ligands: LPS 10 ng/mL (TLR4), Pam3CSK4 100 ng/mL (TLR1/2), or flagellin 1 µg/mL (TLR5). After washes with PBS, these macrophages were stimulated with various nucleotides, at concentrations ranging from 0.1 to 5.0 mM for 30 min, in order to induce the maturation/release of pro-IL-1 cytokines, produced upon stimulation with TLR ligands. In some of these experiments, various inhibitors and P2X7 antagonists were added to macrophages 15 min before the addition of TLR ligands or ATP. The mature form of IL-1β and IL-18 released from macrophages was quantified by sandwich ELISA following manufacturers’ protocols (eBioscience (San Diego, CA, USA) and Bender Medsystems Inc. (Burlingame, CA, USA), respectively). In figure 4a, IL-1β was measured using ELISA kit from Thermo Scientific (Nepean, ON, Canada).
BMMΦ were primed with LPS (Sigma 0111:B4) at 300 ng/ml for 24 hours in 10% FBS-DMEM/F12. After washing with serum free DMEM/F12, cells were treated for 30 min with 0.5 or 1.0 mM ATP. The supernatant was then harvested and kept at −80°C until used. IL-1β was measured using Quantikine ELISA kit as recommended by the manufacturer, R&D Systems Inc. (Minneapolis, MN, USA).
Air Pouch Model
Air pouches were formed on the dorsum of 10 to 12 week-old C57BL/6 wild type or Entpd1−/− mice by s.c. injection of 4 mL sterile air on day 0, and 3 mL on day 4, as described [37]. On day 7, 0.1 µg LPS diluted in 1 mL PBS was injected into the pouches and mice were euthanized 1, 4, 8 or 12 hours later by CO2 asphyxiation. The inflammatory exudates with the accumulated cells were collected from the air pouches with a 2 mL wash (PBS-5 mM EDTA) and centrifuged (500 g, 10 min, 4°C). The cells present in the pellet were resuspended in PBS and counted manually with a hemacytometer. Leukocyte subpopulations were distinguished by Diff Quick staining of cytospins and/or by flow cytometry. The supernatants were analyzed for the level of IL-1β by ELISA, as described above.
Statistical Analysis
Student’s t-test and two-way ANOVA analysis with Bonferroni post hoc test were performed using Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). For Yo-Pro-1 incorporation, statistical analysis was performed using SAS 9.2 statistical software (SAS Institute Inc., NC, USA). Briefly, the increase of fluorescence intensity over time for Entpd1−/− and Entpd1+/+ macrophages was compared for each treatment. A linear model with a common slope and additional individual slopes (deviations from the common slope) was adjusted and a global test F performed on the slope coefficients indicated if the common slope was significantly different from zero and if there was a significant difference between individual slopes. Since both test were positives (p < 0.0001), we proceeded to t tests and to pairwise comparisons of these individual slope coefficients.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the contribution of the Gene Quantification core laboratory of the Centre de Génomique de Québec, CRCHUL, for qPCR experiments and of Olga Gordynska, M.Sc., Statistical Consulting Service, Department of mathematics and statistics, Université Laval, for the statistical analysis of Yo-Pro-1 uptake data. This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and from The Arthritis Society of Canada to JS, and the NIH HL08 and HL094400 to SCR. SAL was a recipient of scholarships from Fond Hydro-Québec established at La Fondation de l’Université Laval, and from the Fonds de la Recherche en Santé du Québec (FRSQ), F.K. of a fellowship from the CIHR/Wyeth Pharmaceuticals, and JS of a New Investigator award from the CIHR and of a junior 2 scholarship from FRSQ.
Abbreviations
- A438079
3-[[5-(2,3-Dichlorophenyl)-1H-tetrazol-1-yl]methyl]pyridine hydrochloride
- ADP
adenosine diphosphate
- AMP
adenosine monophosphate
- ATP
adenosine triphosphate
- BMMΦ
mouse bone marrow-derived macrophages
- CHX
cycloheximide
- NTPDase
nucleoside triphosphate diphosphohydrolase
- KN-62
1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazine
- LDH
lactate deshydrogenase (EC 1.1.1.27)
- NTPDase
nucleoside triphosphate diphosphohydrolase
- oATP
oxidized ATP
- Pi
inorganic phosphate (PO4)
- RB-2
Reactive blue-2
- UDP
uridine diphosphate
- UTP
uridine triphosphate
Footnotes
CONFLICT OF INTEREST
The authors declare no financial or commercial conflict of interest.
REFERENCES
- 1.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1–S13. [PubMed] [Google Scholar]
- 3.Pelegrin P. Targeting Interleukin-1 Signaling in Chronic Inflammation: Focus on P2X7 Receptor and Pannexin-1. Drug News Perspect. 2008;21:424–433. doi: 10.1358/dnp.2008.21.8.1265800. [DOI] [PubMed] [Google Scholar]
- 4.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5'-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 6.del Rey A, Renigunta V, Dalpke AH, Leipziger J, Matos JE, Robaye B, Zuzarte M, et al. Knock-out mice reveal the contributions of P2Y and P2X receptors to nucleotide-induced Ca2+ signaling in macrophages. J Biol Chem. 2006;281:35147–35155. doi: 10.1074/jbc.M607713200. [DOI] [PubMed] [Google Scholar]
- 7.Coutinho-Silva R, Ojcius DM, Gorecki DC, Persechini PM, Bisaggio RC, Mendes AN, Marks J, et al. Multiple P2X and P2Y receptor subtypes in mouse J774, spleen and peritoneal macrophages. Biochem Pharmacol. 2005;69:641–655. doi: 10.1016/j.bcp.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Brône B, Moechars D, Marrannes R, Mercken M, Meert T. P2X currents in peritoneal macrophages of wild type and P2X4−/− mice. Immunol Lett. 2007;113:83–89. doi: 10.1016/j.imlet.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, Lee WP, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 10.Lister MF, Sharkey J, Sawatzky DA, Hodgkiss JP, Davidson DJ, Rossi AG, Finlayson K. The role of the purinergic P2X7 receptor in inflammation. J Inflamm (Lond) 2007;4:5. doi: 10.1186/1476-9255-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 12.Qu Y, Dubyak GR. P2X7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways. Purinergic Signal. 2009;5:163–173. doi: 10.1007/s11302-009-9132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari D, Los M, Bauer MK, Vandenabeele P, Wesselborg S, Schulze-Osthoff K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999;447:71–75. doi: 10.1016/s0014-5793(99)00270-7. [DOI] [PubMed] [Google Scholar]
- 14.Kusner DJ, Adams J. ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J Immunol. 2000;164:379–388. doi: 10.4049/jimmunol.164.1.379. [DOI] [PubMed] [Google Scholar]
- 15.Haag F, Adriouch S, Brass A, Jung C, Moller S, Scheuplein F, Bannas P, et al. Extracellular NAD and ATP: Partners in immune cell modulation. Purinergic Signal. 2007;3:71–81. doi: 10.1007/s11302-006-9038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kukulski F, Lévesque SA, Lavoie EG, Lecka J, Bigonnesse F, Knowles AF, Robson SC, et al. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal. 2005;1:193–204. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2006;2:409–430. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kansas GS, Wood GS, Tedder TF. Expression, distribution, and biochemistry of human CD39. Role in activation-associated homotypic adhesion of lymphocytes. J Immunol. 1991;146:2235–2244. [PubMed] [Google Scholar]
- 19.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Hopner S, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 21.Beldi G, Wu Y, Banz Y, Nowak M, Miller L, Enjyoji K, Haschemi A, et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. 2008;48:841–852. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corriden R, Chen Y, Inoue Y, Beldi G, Robson SC, Insel PA, Junger WG. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J Biol Chem. 2008;283:28480–28486. doi: 10.1074/jbc.M800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizumoto N, Kumamoto T, Robson SC, Sévigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 24.Berchtold S, Ogilvie AL, Bogdan C, Muhl-Zurbes P, Ogilvie A, Schuler G, Steinkasserer A. Human monocyte derived dendritic cells express functional P2X and P2Y receptors as well as ecto-nucleotidases. FEBS Lett. 1999;458:424–428. doi: 10.1016/s0014-5793(99)01197-7. [DOI] [PubMed] [Google Scholar]
- 25.Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D'Orleans-Juste P, Marceau F, et al. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res. 2010;85:204–213. doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulte ED, Broekman MJ, Olson KE, Drosopoulos JH, Kizer JR, Islam N, Marcus AJ. CD39/NTPDase-1 activity and expression in normal leukocytes. Thromb Res. 2007;121:309–317. doi: 10.1016/j.thromres.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart DN, McKenzie JL. Isolation and characterization of human tonsil dendritic cells. J Exp Med. 1988;168:157–170. doi: 10.1084/jem.168.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martín-Satué M, Lavoie EG, Pelletier J, Fausther M, Csizmadia E, Guckelberger O, Robson SC, et al. Localization of plasma membrane bound NTPDases in the murine reproductive tract. Histochem Cell Biol. 2009;131:615–628. doi: 10.1007/s00418-008-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon S, Ripps CS, Cohn Z. The preparation and properties of macrophage-L cell hybrids. J Exp Med. 1971;134:1187–1200. doi: 10.1084/jem.134.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beigi RD, Dubyak GR. Endotoxin activation of macrophages does not induce ATP release and autocrine stimulation of P2 nucleotide receptors. J Immunol. 2000;165:7189–7198. doi: 10.4049/jimmunol.165.12.7189. [DOI] [PubMed] [Google Scholar]
- 31.Brough D, Rothwell NJ. Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci. 2007;120:772–781. doi: 10.1242/jcs.03377. [DOI] [PubMed] [Google Scholar]
- 32.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175:7611–7622. doi: 10.4049/jimmunol.175.11.7611. [DOI] [PubMed] [Google Scholar]
- 33.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 34.Applequist SE, Wallin RP, Ljunggren HG. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int Immunol. 2002;14:1065–1074. doi: 10.1093/intimm/dxf069. [DOI] [PubMed] [Google Scholar]
- 35.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imai M, Goepfert C, Kaczmarek E, Robson SC. CD39 modulates IL-1 release from activated endothelial cells. Biochem Biophys Res Commun. 2000;270:272–278. doi: 10.1006/bbrc.2000.2410. [DOI] [PubMed] [Google Scholar]
- 37.Kukulski F, Ben Yebdri F, Lefebvre J, Warny M, Tessier PA, Sévigny J. Extracellular nucleotides mediate LPS-induced neutrophil migration in vitro and in vivo. J Leukoc Biol. 2007;81:1269–1275. doi: 10.1189/jlb.1206758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sperlagh B, Hasko G, Nemeth Z, Vizi ES. ATP released by LPS increases nitric oxide production in raw 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem Int. 1998;33:209–215. doi: 10.1016/s0197-0186(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg TH, Newman AS, Swanson JA, Silverstein SC. ATP4-permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987;262:8884–8888. [PubMed] [Google Scholar]
- 40.Pelegrin P, Surprenant A. The P2X(7) receptor-pannexin connection to dye uptake and IL-1beta release. Purinergic Signal. 2009 doi: 10.1007/s11302-009-9141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goepfert C, Sundberg C, Sévigny J, Enjyoji K, Hoshi T, Csizmadia E, Robson S. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104:3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- 42.Morahan PS, Edelson PJ, Gass K. Changes in macrophage ectoenzymes associated with anti-tumor activity. J Immunol. 1980;125:1312–1317. [PubMed] [Google Scholar]
- 43.Filippini A, Taffs RE, Agui T, Sitkovsky MV. Ecto-ATPase activity in cytolytic T-lymphocytes. Protection from the cytolytic effects of extracellular ATP. J Biol Chem. 1990;265:334–340. [PubMed] [Google Scholar]
- 44.Goepfert C, Imai M, Brouard S, Csizmadia E, Kaczmarek E, Robson SC. CD39 modulates endothelial cell activation and apoptosis. Mol Med. 2000;6:591–603. [PMC free article] [PubMed] [Google Scholar]
- 45.Zandberg M, van Son WJ, Harmsen MC, Bakker WW. Infection of human endothelium in vitro by cytomegalovirus causes enhanced expression of purinergic receptors: a potential virus escape mechanism? Transplantation. 2007;84:1343–1347. doi: 10.1097/01.tp.0000287598.25493.a5. [DOI] [PubMed] [Google Scholar]
- 46.Wilson HL, Francis SE, Dower SK, Crossman DC. Secretion of intracellular IL-1 receptor antagonist (type 1) is dependent on P2X7 receptor activation. J Immunol. 2004;173:1202–1208. doi: 10.4049/jimmunol.173.2.1202. [DOI] [PubMed] [Google Scholar]
- 47.Zheng LM, Zychlinsky A, Liu CC, Ojcius DM, Young JD. Extracellular ATP as a trigger for apoptosis or programmed cell death. J Cell Biol. 1991;112:279–288. doi: 10.1083/jcb.112.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wen LT, Knowles AF. Extracellular ATP and adenosine induce cell apoptosis of human hepatoma Li-7A cells via the A3 adenosine receptor. Br J Pharmacol. 2003;140:1009–1018. doi: 10.1038/sj.bjp.0705523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu LF, Li GP, Feng JL, Pu ZJ. Molecular mechanisms of adenosine-induced apoptosis in human HepG2 cells. Acta Pharmacol Sin. 2006;27:477–484. doi: 10.1111/j.1745-7254.2006.00302.x. [DOI] [PubMed] [Google Scholar]
- 50.Jacobson KA, Hoffmann C, Cattabeni F, Abbracchio MP. Adenosine-induced cell death: evidence for receptor-mediated signalling. Apoptosis. 1999;4:197–211. doi: 10.1023/a:1009666707307. [DOI] [PubMed] [Google Scholar]
- 51.Kahlenberg JM, Dubyak GR. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am J Physiol Cell Physiol. 2004;286:C1100–C1108. doi: 10.1152/ajpcell.00494.2003. [DOI] [PubMed] [Google Scholar]
- 52.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 53.Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pelegrin P, Surprenant A. Pannexin-1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem. 2007;282:2386–2394. doi: 10.1074/jbc.M610351200. [DOI] [PubMed] [Google Scholar]
- 55.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 56.Meda L, Gasperini S, Ceska M, Cassatella MA. Modulation of proinflammatory cytokine release from human polymorphonuclear leukocytes by gamma interferon. Cell Immunol. 1994;157:448–461. doi: 10.1006/cimm.1994.1241. [DOI] [PubMed] [Google Scholar]
- 57.Nie K, Zheng GG, Zhang XJ, Lin YM, Wang L, Li G, Song YH, et al. CD39-associated high ATPase activity contribute to the loss of P2X7-mediated calcium response in LCL cells. Leuk Res. 2005;29:1325–1333. doi: 10.1016/j.leukres.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 58.Gonnord P, Delarasse C, Auger R, Benihoud K, Prigent M, Cuif MH, Lamaze C, et al. Palmitoylation of the P2X7 receptor, an ATP-gated channel, controls its expression and association with lipid rafts. FASEB J. 2008;23:795–805. doi: 10.1096/fj.08-114637. [DOI] [PubMed] [Google Scholar]
- 59.Barth K, Weinhold K, Guenther A, Young MT, Schnittler H, Kasper M. Caveolin-1 influences P2X7 receptor expression and localization in mouse lung alveolar epithelial cells. FEBS J. 2007;274:3021–3033. doi: 10.1111/j.1742-4658.2007.05830.x. [DOI] [PubMed] [Google Scholar]
- 60.Koziak K, Sévigny J, Robson SC, Siegel JB, Kaczmarek E. Analysis of CD39/ATP diphosphohydrolase (ATPDase) expression in endothelial cells, platelets and leukocytes. Thromb Haemost. 1999;82:1538–1544. [PubMed] [Google Scholar]
- 61.Robson SC, Daoud S, Begin M, Côté YP, Siegel JB, Bach FH, Beaudoin AR. Modulation of vascular ATP diphosphohydrolase by fatty acids. Blood Coagul Fibrinolysis. 1997;8:21–27. doi: 10.1097/00001721-199701000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Robson SC, Kaczmarek E, Siegel JB, Candinas D, Koziak K, Millan M, Hancock WW, et al. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J Exp Med. 1997;185:153–163. doi: 10.1084/jem.185.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hyman MC, Petrovic-Djergovic D, Visovatti SH, Liao H, Yanamadala S, Bouis D, Su EJ, et al. Self-regulation of inflammatory cell trafficking in mice by the leukocyte surface apyrase CD39. J Clin Invest. 2009;119:1136–1149. doi: 10.1172/JCI36433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kunzli BM, Nuhn P, Enjyoji K, Banz Y, Smith RN, Csizmadia E, Schuppan D, et al. Disordered pancreatic inflammatory responses and inhibition of fibrosis in CD39-null mice. Gastroenterology. 2008;134:292–305. doi: 10.1053/j.gastro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neshat S, deVries M, Barajas-Espinosa AR, Skeith L, Chisholm SP, Lomax AE. Loss of purinergic vascular regulation in the colon during colitis is associated with upregulation of CD39. Am J Physiol Gastrointest Liver Physiol. 2009;296:G399–G405. doi: 10.1152/ajpgi.90450.2008. [DOI] [PubMed] [Google Scholar]
- 67.Friedman DJ, Kunzli BM, YI AR, Sévigny J, Berberat PO, Enjyoji K, Csizmadia E, et al. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27:4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goncalves RG, Gabrich L, Rosario A, Jr, Takiya CM, Ferreira ML, Chiarini LB, Persechini PM, et al. The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int. 2006;70:1599–1606. doi: 10.1038/sj.ki.5001804. [DOI] [PubMed] [Google Scholar]
- 70.Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain. 2005;114:386–396. doi: 10.1016/j.pain.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Enjyoji K, Sévigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, Imai M, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 72.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008 doi: 10.1002/0471142735.im1401s83. Chapter 14: Unit 14 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davies JQ, Gordon S. Isolation and culture of murine macrophages. Methods Mol Biol. 2005;290:91–103. doi: 10.1385/1-59259-838-2:091. [DOI] [PubMed] [Google Scholar]
- 74.Baykov AA, Evtushenko OA, Avaeva SM. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem. 1988;171:266–270. doi: 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- 75.Bartel DL, Sullivan SL, Lavoie EG, Sévigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.