Abstract
In this work, we biochemically characterized inositol phosphosphingolipid-phospholipase C (Isc1) from the pathogenic fungus Cryptococcus neoformans. Unlike Isc1 from other fungi and parasites which hydrolyze both fungal complex sphingolipids (IPC-PLC) and mammalian sphingomyelin (SM-PLC), C.neoformans Isc1 only exerts IPC-PLC activity. Genetic mutations thought to regulate substrate recognition in other Isc1 proteins do not restore SM-PLC activity of the cryptococcal enzyme. C.neoformans Isc1 regulates the level of complex sphingolipids and certain species of phytoceramide, especially when fungal cells are exposed to acidic stress. Since growth in acidic environments is required for C.neoformans to cause disease, this study has important implications for understanding of C.neoformans pathogenicity.
Keywords: Cryptococcus neoformans, phospholipase C, inositol sphingolipids, sphingomyelin, phytoceramide, plasma membrane ATPase
Introduction
In the past several years, it has become increasingly clear that sphingolipid metabolism is intimately linked with the virulence of the fungal pathogen Cryptococcus neoformans (Cn), a medically important microorganism [1]. Cn is a facultative intracellular pathogen and it can grow and replicate within the phagolysosome of phagocytic cells, such as alveolar macrophages (AMs), as well as in the extracellular spaces, such as in the alveoli or in the bloodstream [2-6].
Our laboratory has previously showed that cryptococcal sphingolipids regulates signaling events leading to the production of virulence factors [7,8] and to the regulation of phagocytosis [9,10]. Interestingly, sphingolipid not only regulate the internalization of Cn by macrophages but also promote fungal growth in these environments (intracellular and extracellular). Intracellular fungal growth is controlled by Ipc1 [11] and inositol phosphosphingolipid phospholipase C 1 (Isc1) [12] whereas extracellular growth is controlled by glucosylceramide synthase (Gcs1) [13,14]. The regulation of these processes significantly affects the interaction of Cn with macrophages, particularly in the lung environment with an important effect on the outcome of the disease.
In Saccharomyces cerevisiae (Sc) the enzyme which hydrolyzes complex sphingolipids, such as inositol phosphorylceramide (IPC), generating phytoceramide and inositol phosphate was identified as Isc1 by Sawai et al. [15]. In vitro, Sc Isc1 metabolizes not only IPC and its mannosylated derivatives mannosylinositol phosphorylceramide (MIPC) and mannosyldiinositol phosphorylceramide (M(IP)2C), thus exerting IPC-PLC activity, but it also metabolizes the mammalian complex sphingolipid sphingomyelin (SM), thus exerting SM-PLC activity, in spite of the fact that fungal cells do not contain SM [15].
In the parasite Leishmania major, the Isc1 homolog (ISCL) does possess both IPC-PLC and SM-PLC activity, even though the parasite does not produce SM and, the breakdown of host SM is an essential process for L. major to cause disease [16].
In Cn, deletion of Isc1 generated a Cn mutant (Δisc1) that is hypersensitive in vitro to nitrogen oxide, hydrogen peroxide and acidic stresses and, thus, it cannot survive within the phagolysosome of macrophages. In this study, we examined the biochemical characteristics of the Cn Isc1 enzyme and analyzed the biochemical features of the Cn strain lacking Isc1 compared to the wild type (WT) and a reconstituted strain (Δisc1REC) in acidic and neutral conditions.
Materials and Methods
Strains and growth media
Cn var. grubii serotype A strain H99 wild type (WT); Cn var. neoformans serotype D strain JEC21; Cn var. gattii serotype B (strain MMRL 1336) and serotype C (strain MMRL 1343) [the latter two strains were a kind gift from Wiley Schell, Duke University Medical Center, Durham, NC U.S.A.]; Candida albicans (Ca) wild type strain A39; Schizosaccharomyces pombe strains 972 (h-) and 975 (h+); Saccharomyces cerevisiae wild type strain (Jk9-3Dα); Sc Δisc1 strain [15]; Cn Isc1 deletion strain (Cn Δisc1) and the reconstituted strain (Δisc1REC) were used in this study. For in vitro growing condition, please see supplementary materials.
In vitro IPC and sphingomyelin SM phospholipase C (IPC-PLC and SM-PLC) activity
Phospholipase C activity against IPC (IPC-PLC) was performed as previously described [15]. Briefly, cell lysates containing 100 μg protein were incubated at 30°C for 30 min in 100 μl of buffer containing 50 mM Tris (pH 7.5), 5 mM MgCl2, 5 mM dithiothreitol, 0.1% Triton X-100, 10 nmoles of phosphatidylserine (Avanti Polar Lipids), 2 nmoles of unlabeled IPC, and 30,000 dpm of myo-[2-3H]inositol –labeled IPC. After the incubation, 0.8 ml of chloroform, 0.4 ml of methanol, and 0.2 ml of 1% perchloric acid were added [17], and the radioactivity in a portion (300 μl) of the upper (aqueous) phase was measured by liquid scintillation counting. Phospholipase C activity against SM (SM-PLC) was tested in a similar manner, except that 2 nmoles SM and 30,000 dpm of [choline-methyl-14C]-labeled SM were used in place of unlabeled and labeled IPC, respectively. After incubation, 0.8 ml of chloroform, 0.4 ml of methanol, and 0.2 ml of distilled water were added and the radioactivity in a portion (300 μl) of the upper phase was measured by liquid scintillation counting. As a negative control, the Sc Δisc1+pYES empty strain was assayed for Isc1 activity and its value subtracted from values obtained with Isc1 wild-type or mutated forms. Experiments were repeated at least three times. Radiolabeled IPC was prepared by incubating WT cultures with [2-3H] inositol for 4 hours and extracting sphingolipids as previously described. Non-radioactive labeled C26-IPC was custom-made by Avanti Polar Lipids, Alabaster, AL, U.S.A.
Mutagenesis Studies
The Cn Isc1 cDNA from wild-type of mutated forms were subcloned from pCR-TOPO-Cn-Isc1 [12] into the pYES vector (Invitrogen) and the Xpress tag was inserted at the 5′ of the Cn Isc1 gene to monitor protein expression in Sc Δisc1 cells. Mutagenesis studies were performed as described in the supplementary materials and the primers used are illustrated in the Supplementary Table 1. For molecular docking studies, coordinates for phosphocholine were generated and docked manually into the Bc Isc1 structure using the program O version 11 {Jones, 1991 #2404}.
In vivo labeling with 3H-inositol or 3H dihydrosphingosine
For quantitative measurements of IPC-B, IPC-C/D, MIPC and M(IP)2C in Cn wild-type, Δisc1 and Δisc1REC strain, cells were incubated for 30 minutes with 25 μCi of myo-[2-3H] inositol (20 Ci/mmol), and the lipid were extracted and loaded onto a TLC on silica gel 60 plates (EM Science) using the solvent system chloroform/methanol/water (65:25:4). The separated radioactive lipids were scraped from the TLC and quantified using a liquid scintillation counter [15]. The mass of each species was normalized to phosphorus levels of each sample. Further details are illustrated in the Supplementay Materials.
Extraction and mass spectrometry analysis of yeast sphingolipids
Lipids for mass spectrometry analysis were extracted by standard methods (please see Supplementary Materials). An aliquot of the extraction (300 μl) was used for phosphorous determination. Internal standards were added to the remaining aliquots, and sphingolipids were extracted in a one-phase neutral organic solvent (propan-2-ol/water/ethyl acetate, 30:10:60, by vol.) Samples were then analyzed by a Surveyor/TSQ 7000 liquid chromatography–MS system.
Statistical analysis
The two-way analysis of variance (ANOVA) was used. For all statistical tests, P-values less than 0.05 were considered significant
Results and Discussion
Cn lacks sphingomyelinase activity
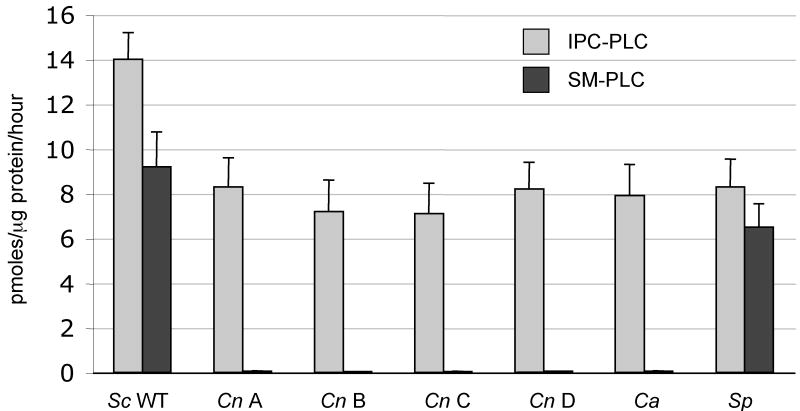
Our first interest was to investigate whether Cn cell protein lysates would hydrolyze the mammalian complex sphingolipid sphingomyelin (SM), as was observed for the Sc protein lysates. Unexpectedly, we found that Cn cannot metabolize SM (Figure 2), suggesting that the cryptococcal Isc1 does not have SM-PLC activity. In addition, we did not detect any SM-PLC activity in other serotypes (B, C and D) of Cn, nor in another pathogenic yeast, such as Candida albicans (Ca). As expected, we did detect SM-PLC activity in Sc and in Saccharomyces pombe protein lysates. We confirmed these results by in vivo labeling with NBD-labeled C6-SM, and found that whereas Sc could generate NBD-labeled ceramide, Cn could not (data not shown). These results suggest that the cryptococcal Isc1 enzyme may possess different biochemical properties than those exerted by Sc.
Figure 2. neoformans (Cn) and C. albicans (Ca) lack SM-PLC Activity.
Crude cell lysates from fungi were assessed for neutral phospholipase C (PLC) activity against either inositol phosphorylceramide (IPC) or sphingomyelin (SM). The fungi tested included Sc (Sc), Cn var. grubii serotype A strain H99 (Cn A), Cn var. gattii serotype B (Cn B), Cn var. gattii serotype C (Cn C), Cn var. neoformans serotype D (Cn D), Ca, and S. pombe (Sp).
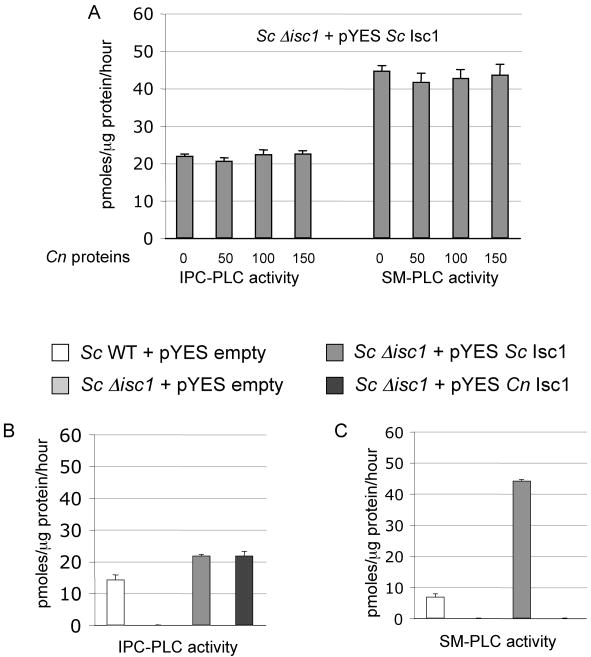
Because we observed that Cn protein extracts do not possess SM-PLC activity (Figure 2), we wondered whether a cryptococcal factor(s) may inhibit such activity. To address this hypothesis, different concentrations of protein lysates isolated from Cn Δisc1 mutant were mixed with protein lysates isolated from Sc Δisc1 + pYES Sc Isc1, and IPC-PLC and SM-PLC activities were measured. We found that presence of Cn proteins did not inhibit SM-PLC or IPC-PLC activities of Sc Isc1 (Figure 3A). Next, we wondered whether a factor(s) from Sc would be necessary for SM-PLC activity. Thus, the Cn Isc1 cDNA was expressed into the Sc Δisc1 strain and both IPC- and SM-PLC activity were measured. Sc Isc1 cloned into pYES was used as a positive control, whereas the pYES empty plasmid was used as a negative control. As additional controls for Isc1 protein expression, we also included the Sc wild-type JK-3Dα transformed with pYES empty vector. We found that IPC-PLC activity is promptly restored in the Sc Δisc1 strain when over-expressing either Sc Isc1 or Cn Isc1 (Figure 3B). However, only the expression of Sc Isc1 and not Cn Isc1 restores SM-PLC activity in the Sc Δisc1 strain (Figure 3C). These results suggest that Cn Isc1 is biochemically different from Sc Isc1 in terms of substrate specificity.
Figure 3. Expression of C. neoformans (Cn) Isc1 in S. cerevisiae (Sc) Δisc1 does not restore SM-PLC activity.
A) IPC-PLC and SM-PLC activity using protein lysates obtained from ScΔisc1 + pYES Sc Isc1 mixed with 50, 100 or 150 μg of Cn protein lysates. B) IPC-PLC and C) SM-PLC activity of Sc wild-type (WT) + pYES empty, ScΔisc1 + pYES empty, ScΔisc1 + pYES Sc Isc1, and ScΔisc1 + pYES Cn (Cn) Isc1. Data are average ± standard deviation of three separate experiments.
Functional analysis of Cn Isc1 by site-directed mutagenesis of the P-loop-like domain
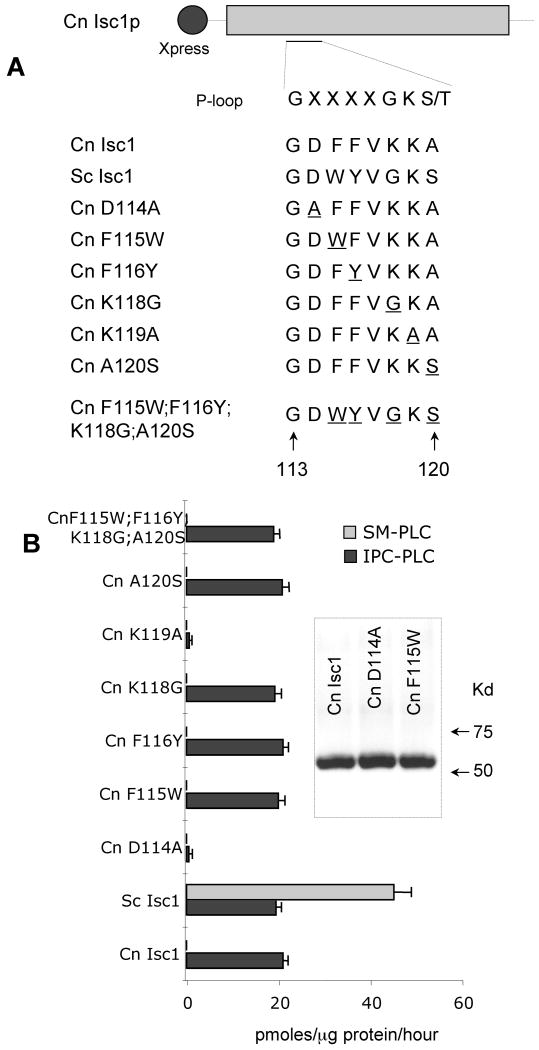
Studies on the Sc Isc1 [18,19] and Bacillus cereus SMase [20] have identified distinct domains within the enzyme which are essential for substrate binding and hydrolysis. One such domain, the P-loop-like domain, shows homology among the Isc1 superfamily, including bacterial and human SMases. The Cn Isc1 P-loop domain is contained between G113 and A120 and is illustrated in Figure 4A. Okamoto et al. have shown that substituting the second glycine of the P-loop with an alanine decreased Sc Isc1 activity against SM nearly 100-fold, though activity against IPC was not assayed [19]. Of interest, Cn has a lysine instead of a glycine at this position and additional differences between the two Isc1 homolog exist (Figure 4A). Thus, we wondered whether the differences in amino acid residues found in the Cn Isc1 are responsible for the lack of SM-PLC activity. We expressed Cn Isc1 in S.cerevisiae Δisc1 and assessed the role of the P-loop residues on both SM-PLC and IPC-PLC activities. The inset in Figure 4B is representative Western blot of the expressed proteins showing that the level of expression was found to be comparable. We found that D114A and K119A mutations lost IPC-PLC activity completely Figure 4B, suggesting that these two amino acids are essential for catalysis of Cn Isc1. In contrast to Sc, neither of the remaining mutated forms lost IPC-PLC activity nor they did restore SM-PLC activity, even when the Cn Isc1 P-loop was identical to the Sc Isc1 P-loop (Figure 4B) [19]. These results suggest that either the P-loop is not involved in the substrate recognition or that it is not sufficient to discriminate between IPC and SM.
Figure 4. Effect of mutations of the C. neoformans Isc1 P-loop domain on IPC-PLC and SM-PLC activity.
(A) Alignment of amino acid sequences of P-loop-like wild-type domain and mutant constructs. Amino acids sequences are given for residues 113-120 of Cn Isc1. The residue(s) mutated from the wild-type is underlined. (B) Effect of mutations in the P-loop on Isc1 activity. Isc1 activity was measured using SM (SM-PLC) or IPC (IPC-PLC) as a substrate, as described. Inset in B shows a representative Western blot of P-loop-like domain mutants of Cn Isc1 with anti-Xpress antibody. Shown are two different clones (Cn D114A) and Cn F115W) of each over expressed cells compared to Cn Isc1 wild-type.
We therefore performed docking studies of Cn Isc1 protein in the attempt to identify key residues that would interact with IPC and not with SM based on the available structure of B. cereus SMase [21] and found none. Amino acids predicted to interact with the choline phoshate of SM, such as D195, N197, W232, D295, W284 and F285, are either conserved in Cn Isc1 or absent in other eukaryotic SMase. Thus, which amino acid(s) are most likely to be responsible for substrate (IPC or SM) recognition or exclusion by Cn Isc1 is still unknown.
The observation that Cn Isc1 is not able to breakdown host SM as other microbes do is intriguing. It is possible that, since SM is an essential sphingolipid for mammalian cell viability [22,23], opportunistic pathogenic yeasts, such as Cn, may have lost SM-PLC activity to prevent mammalian cell death due to their close interaction with their host. this hypothesis is supported by the observation that Ca, an opportunistic and commensal fungal pathogen, also does not diplay SM-PLC activity. In non-opportunistic pathogens, such as L. major, the ability to breakdown host SM is maintained by their Isc1 homolog ISCL and, importantly, the authors elegantly showed that this activity is required for virulence [16].
Isc1 regulates the level of complex sphingolipids and specific phytoceramide species
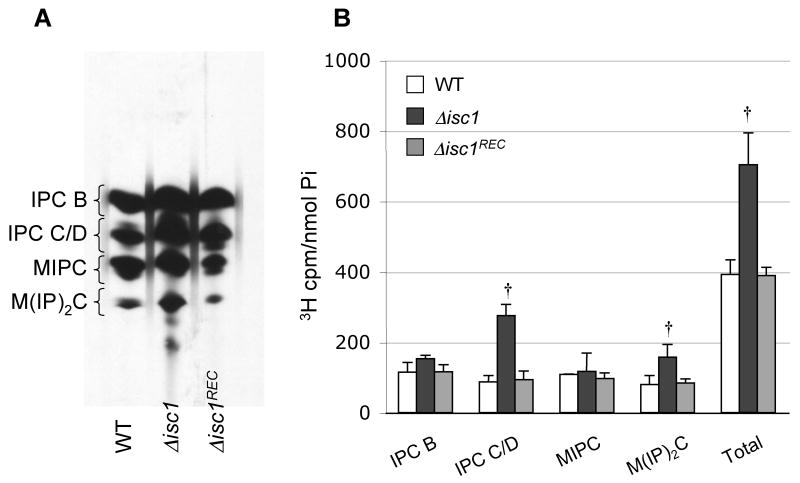
Since Isc1 generates phytoceramide during the hydrolysis of complex sphingolipids, we next investigated how the loss of Isc1 modulated the level of complex sphingolipids and phytoceramide. Complex sphingolipids were measured by pulse chase labeling using [3H]-inositol because mass spectrometry analysis could not be performed due the lack of IPC, MIPC and M(IP)2C standards. Cn Δisc1 cells showed a significantly increased of IPC C/D and M(IP)2C compared to Cn WT or Δisc1REC strains when cells were grown at either pH 4.0 (Figure 5) or 7.0 (data not shown). Similar results were obtained when cultures were labeled with tritiated dihydrosphingosine.
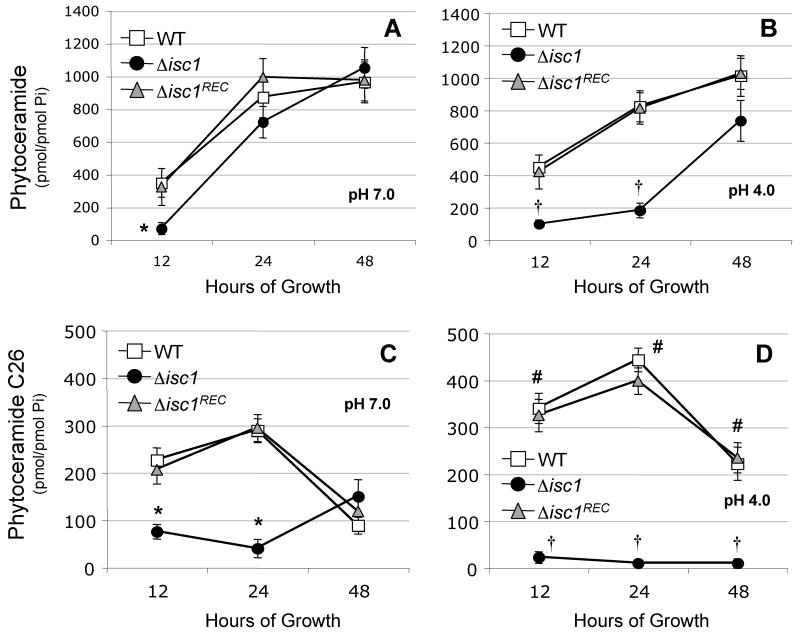
Figure 5. In vivo labeling of WT, Δisc1, and Δisc1REC strains of C. neoformans.
Cultures were labeled with 3H-inositol for thirty minutes, and lipids were extracted and resolved in a TLC (A) as described in “Materials and Methods.” Panel A is a representative of three separate experiments. B) Quantitative analysis of scraped lipids from the TLC normalized to phosphorus (Pi) content. † P< 0.05, Δisc1 versus WT or Δisc1REC. Data are average ± standard deviation of three separate experiments.
Interestingly, deletion of Isc1 causes the accumulation of two complex sphingolipids more polar than M(IP)2C. In other fungi, such as Ca, a new inositol containing sphingolipid, dimmannose inositol phosphorylceramide (M2IPC), has been identified {Trinel, 2002 #1577}. In contrast to IPC, MIPC and M(IP)2C that are anchored to the plasma membrane, M2IPC can “diffuse” from the plasma membrane to the cell wall. In the dimorphic fungus Histoplasma capsulatum, in addition to M2IPC another sphingolipid has been identified as galactosyldimmannose inositolphosphorylceramide (GalM2IPC) which exists in two forms depending on the addition of a galactofuranose residue at the 6-position of mannose (yeast form specific) or the addition of galactopyranose residue at the 4-position of mannose (hyphal form specific) {Barr, 1984 #1578; Barr, 1984 #1579}. Although not described thus far, most likely these new complex sphingolipid species are also present in C. neoformans, and, since they are more polar than M(IP)2C, they would run below M(IP)2C on a TLC plate. Presumably, M2IPC and GalM2IPC are breakdown by Isc1 and, thus, they would accumulate in condition in which Isc1 is deleted. Further studies are clearly needed to investigate such hypotheses.
When we measured the level of phytoceramide using mass spectrometry, we found that, except at 12 hours of growth, at pH 7.0 the WT or Δisc1REC strain contained approximately the same level of total phytoceramide than Δisc1 mutant (Figure 6A) but not at pH 4.0, in which the level of phytoceramide at 12 and 24 hours of growth is significantly lower (Figure 6B). Interestingly, the Δisc1 strain grown at pH 7.0 recovered its total phytoceramide pools very quickly and by 24 hours its level is similar to the one measured in the WT or Δisc1REC strain. In contrast, the Isc1 mutant grown at pH 4.0 does recover its phytoceramide pool to a level similar to the WT strains at 48 hours of growth.
Figure 6. Isc1 regulates phytoceramide levels in C. neoformans.
Mass spectrometry quantification of total phytoceramide pools (A and B) and very long chain (C26) phytoceramide (C and D) of WT, Δisc1, and Δisc1REC grown at pH 7.0 (A and C) or pH 4.0 (B and D). * P< 0.05, Δisc1 versus WT or Δisc1REC at pH 7; † P< 0.05, Δisc1 versus WT or Δisc1REC at pH 4; # P< 0.05, WT at pH 4.0 versus pH 7.0. Data are average ± standard deviation of three separate experiments.
An advantage of mass spectrometry analysis is the ability to quantify the different species of lipids. Using this method, we found that the level of C26 phytoceramide in WT or Δisc1REC strain is significantly higher when the strains are grown at low compared to neutral pH (Figure 6C and 6D). Importantly, the Δisc1 strain displayed a significantly lower level of C26 phytoceramide compared to WT and Δisc1REC strains, especially at low pH (Figure 5D). This suggests that Isc1 may directly regulate the level of C26 phytoceramide in Cn. The lack of C26-phytoceramide in the Δisc1 mutant is intriguing, as it suggests a possible involvement of the plasma membrane ATPase (Pma1), a proton-extruding plasma membrane pump, in the regulation of Cn growth when cells are exposed to low pH [24], which has also been proposed in Sc [25-27].
Although the involvement of sphingolipids in the fungal stress response appears to be conserved, our study adds a new dimension because Cn is a facultative intracellular pathogen. Clinically treating cryptococcal infections is often hampered by the persistence of fungal cells in infected organs. Similar to Mycobacterium tuberculosis, cryptococcal persistence may be a result of its intracellular ability to survive within macrophages [3]. This hypothesis is supported by studies suggesting that Cn may lay dormant in the lungs of individuals many years before disease occurs [28,29], and by studies in M. tuberculosis that, peculiarly, accumulates C26 triacylglycerides during hypoxia, an in vitro condition that mimics the in vivo dormancy prior reactivation [30]. Thus, through the production of C26-phytoceramide, Cn may be protected against acidic stresses so that it can survive within the hostile phagolysosome of macrophages.
In conclusion, we show that Cn Isc1 is biochemically different than Sc Isc1 and it synthesizes mainly C26 phytoceramide especially in acidic environments. Since the intracellular niche of host cells in which Cn grows is characteristically acidic, these results may have important implications for the interaction of Cn with the host.
Supplementary Material
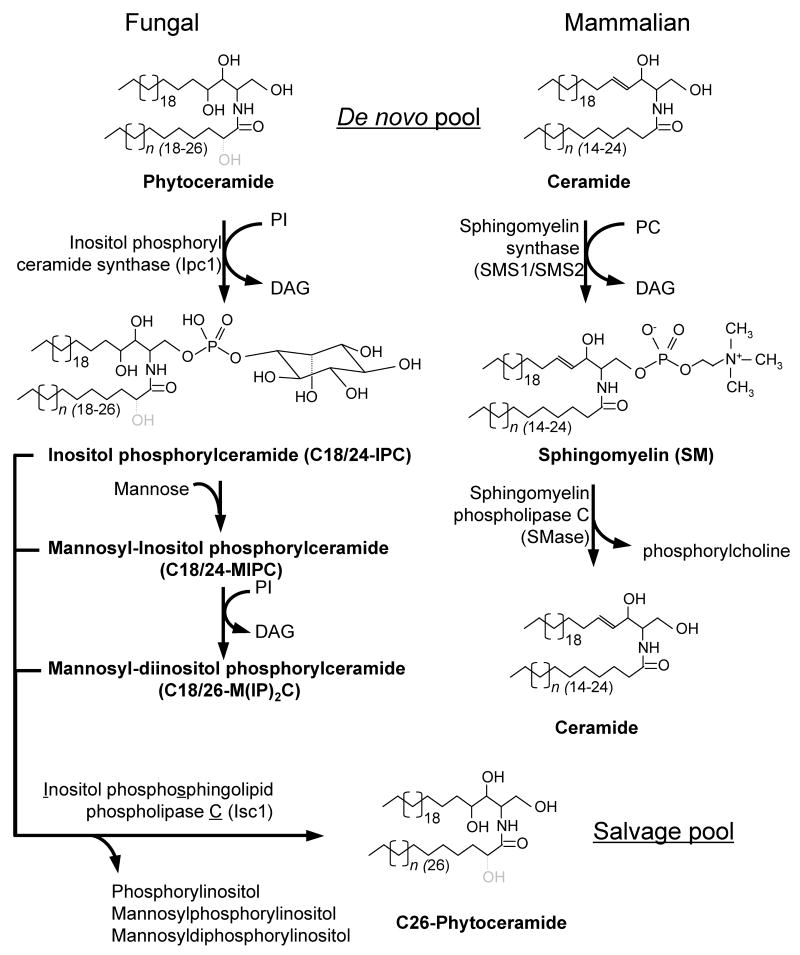
Figure 1. Ipc1 and Isc1 biosynthetic pathway.
Ipc1 consumes phytoceramide and generates diacylglycerol (DAG) in the synthesis of IPC, which is subsequently transformed into MIPC and M(IP)2C. Isc1 generates phytoceramide in the hydrolysis of IPC, MIPC or M(IP)2C. The mammalian homologs, sphingomyelin synthase (SMS1 and SMS2) and sphingomyelinase (SMases), which consume and generate ceramide, respectively, are shown for comparison. In fungal cells, the fatty acid in the phytoceramide species can be hydroxylated (in gray) in position 2 (dotted line). Bracket's numbers represent the length of the sphingosine backbone and fatty acids. PI, phosphatidylinositol; DAG, diacylglycerol; PC, phosphatidylcholine.
Acknowledgments
We thank John Shea and Talar Kechichian for technical help with this work. We also thank Christopher Davies for docking studies. We thank Dr. Alicja Bielawska and Dr. Jacek Bielawski for the lipid analysis. This work was supported in part by the Burroughs Wellcome Fund, the National Institutes of Health (AI56168 and AI71142 to M.D.P.), and the Centers of Biomedical Research Excellence Program of the National Center for Research Resources (grant RR17677 Project 2 to M.D.P. and Project 6 to C.L.). The lipid analysis was partially supported by the National Institutes of Health, grant number C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. M.D.P. is a Burroughs Wellcome New Investigator in Pathogenesis of Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Casadevall A, Perfect JR. Cryptococcus neoformans. ASM Press; Washington, DC: 1998. pp. 381–405. [Google Scholar]
- 2.Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000;68:4225–4237. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman DL, Lee SC, Mednick AJ, Montella L, Casadevall A. Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression, and altered antibody responsiveness to cryptococcal polysaccharide. Infect Immun. 2000;68:832–8. doi: 10.1128/iai.68.2.832-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldmesser M, Tucker S, Casadevall A. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 2001;9:273–8. doi: 10.1016/s0966-842x(01)02035-2. [DOI] [PubMed] [Google Scholar]
- 5.Levitz SM. Cryptococcus neoformans: intracellular or extracellular? Trends Microbiol. 2001;9:417–8. doi: 10.1016/s0966-842x(01)02137-0. [DOI] [PubMed] [Google Scholar]
- 6.Steenbergen JN, Shuman HA, Casadevall A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc Natl Acad Sci USA. 2001;98:15245–50. doi: 10.1073/pnas.261418798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heung LJ, Luberto C, Plowden A, Hannun YA, Del Poeta M. The sphingolipid pathway regulates protein kinase C 1 (Pkc1) through the formation of diacylglycerol (DAG) in Cryptococcus neoformans. J Biol Chem. 2004;279:21144–21153. doi: 10.1074/jbc.M312995200. [DOI] [PubMed] [Google Scholar]
- 8.Heung LJ, Kaiser AE, Luberto C, Del Poeta M. The role and mechanism of diacylglycerol-protein kinase C1 signaling in melanogenesis by Cryptococcus neoformans. J Biol Chem. 2005;280:28547–28555. doi: 10.1074/jbc.M503404200. [DOI] [PubMed] [Google Scholar]
- 9.Mare L, Iatta R, Montagna MT, Luberto C, Del Poeta M. APP1 transcription is regulated by IPC1-DAG pathway and is controlled by ATF2 transcription factor in Cryptococcus neoformans. J Biol Chem. 2005;280:36055–36064. doi: 10.1074/jbc.M507285200. [DOI] [PubMed] [Google Scholar]
- 10.Tommasino N, Villani M, Qureshi A, Henry J, Luberto C, Del Poeta M. Atf2 transcription factor binds to the APP1 promoter in Cryptococcus neoformans: stimulatory effect of diacylglycerol. Eukaryot Cell. 2008;7:294–301. doi: 10.1128/EC.00315-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luberto C, Toffaletti DL, Wills EA, Tucker SC, Casadevall A, Perfect JR, Hannun YA, Del Poeta M. Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans. Genes Dev. 2001;15:201–212. doi: 10.1101/gad.856001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shea J, Kechichian TB, Luberto C, Del Poeta M. The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C (Isc1) confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect Immun. 2006;74:5977–5988. doi: 10.1128/IAI.00768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun. 2007;75:4792–8. doi: 10.1128/IAI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rittershaus PC, Kechichian TB, Allegood J, Merrill AHJ, Hennig M, Luberto C, Del Poeta M. Glucosylceramide is an essential regulator of pathogenicity of Cryptococcus neoformans. J Clin Invest. 2006;116:1651–1659. doi: 10.1172/JCI27890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawai H, Okamoto Y, Luberto C, Mao C, Bielawska A, Domae N, Hannun YA. Identification of ISC1 (YER019w) as inositol phosphosphingolipid phospholipase C in Saccharomyces cerevisiae. J Biol Chem. 2000;275:39793–8. doi: 10.1074/jbc.M007721200. [DOI] [PubMed] [Google Scholar]
- 16.Zhang O, et al. Degradation of host sphingomyelin is essential for Leishmania virulence. PLoS Pathog. 2009;5:e1000692. doi: 10.1371/journal.ppat.1000692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 18.Okamoto Y, Vaena De Avalos S, Hannun YA. Structural requirements for selective binding of ISC1 to anionic phospholipids. J Biol Chem. 2002;277:46470–7. doi: 10.1074/jbc.M207779200. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto Y, Vaena de Avalos S, Hannun YA. Functional analysis of ISC1 by site-directed mutagenesis. Biochemistry. 2003;42:7855–62. doi: 10.1021/bi0341354. [DOI] [PubMed] [Google Scholar]
- 20.Matsuo Y, Yamada A, Tsukamoto K, Tamura H, Ikezawa H, Nakamura H, Nishikawa K. A distant evolutionary relationship between bacterial sphingomyelinase and mammalian DNase I. Protein Sci. 1996;5:2459–67. doi: 10.1002/pro.5560051208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ago H, Oda M, Takahashi M, Tsuge H, Ochi S, Katunuma N, Miyano M, Sakurai J. Structural basis of the sphingomyelin phosphodiesterase activity in neutral sphingomyelinase from Bacillus cereus. J Biol Chem. 2006;281:16157–67. doi: 10.1074/jbc.M601089200. [DOI] [PubMed] [Google Scholar]
- 22.Okazaki T, Bell RM, Hannun YA. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989;264:19076–80. [PubMed] [Google Scholar]
- 23.Tafesse FG, Huitema K, Hermansson M, van der Poel S, van den Dikkenberg J, Uphoff A, Somerharju P, Holthuis JC. Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J Biol Chem. 2007;282:17537–47. doi: 10.1074/jbc.M702423200. [DOI] [PubMed] [Google Scholar]
- 24.Garcia J, Shea J, Alvarez-Vasquez F, Qureshi A, Luberto C, Voit EO, Del Poeta M. Mathematical modeling of pathogenicity of Cryptococcus neoformans. Molecular System Biology. 2008;4:183–195. doi: 10.1038/msb.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaigg B, Timischl B, Corbino L, Schneiter R. Synthesis of sphingolipids with very long chain fatty acids but not ergosterol is required for routing of newly synthesized plasma membrane ATPase to the cell surface of yeast. J Biol Chem. 2005;280:22515–22. doi: 10.1074/jbc.M413472200. [DOI] [PubMed] [Google Scholar]
- 26.Gaigg B, Toulmay A, Schneiter R. Very long-chain fatty acid-containing lipids rather than sphingolipids per se are required for raft association and stable surface transport of newly synthesized plasma membrane ATPase in yeast. J Biol Chem. 2006;281:34135–45. doi: 10.1074/jbc.M603791200. [DOI] [PubMed] [Google Scholar]
- 27.Lee MC, Hamamoto S, Schekman R. Ceramide biosynthesis is required for the formation of the oligomeric H+-ATPase Pma1p in the yeast endoplasmic reticulum. J Biol Chem. 2002;277:22395–401. doi: 10.1074/jbc.M200450200. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol. 1999;37:3204–9. doi: 10.1128/jcm.37.10.3204-3209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dromer F, Ronin O, Dupont B. Isolation of Cryptococcus neoformans var. gattii from an Asian patient in France: evidence for dormant infection in healthy subjects. J Med Vet Mycol. 1992;30:395–7. [PubMed] [Google Scholar]
- 30.Daniel J, Deb C, Dubey VS, Sirakova TD, Abomoelak B, Morbidoni HR, Kolattukudy PE. Induction of a novel class of diacylglycerol acyltransferases and triacylglycerol accumulation in Mycobacterium tuberculosis as it goes into a dormancy-like state in culture. J Bacteriol. 2004;186:5017–30. doi: 10.1128/JB.186.15.5017-5030.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.