Abstract
Problem
Emerging evidence suggests that metabolism influences immune cell signaling and immunoregulation. To examine the immunoregulatory role of glycolysis in pregnancy, we evaluated the properties of pyruvate kinase in leukocytes from non-pregnant women and those with normal pregnancy and pre-eclampsia.
Method of study
We evaluated pyruvate kinase expression in lymphocytes and neutrophils from non-pregnant, pregnant, and pre-eclampsia patients using fluorescence microscopy and flow cytometry. Leukocyte pyruvate kinase activity and pyruvate concentrations were also evaluated. To study pyruvate’s effect on signaling, we labeled Jurkat T cells with Ca2+ dyes and measured cell responses in the presence of agents influencing intracellular pyruvate.
Results
The expression of pyruvate kinase is reduced in lymphocytes and neutrophils from normal pregnant women in comparison to those of non-pregnant women and pre-eclampsia patients. Similarly, the activity of pyruvate kinase and the intracellular pyruvate concentration are reduced in leukocytes of normal pregnant women in comparison to non-pregnant women and women with pre-eclampsia. Using Jurkat cells as a model of leukocyte signaling, we have shown that perturbations of intracellular pyruvate influence Ca2+ signals.
Conclusion
Normal pregnancy is characterized by reduced pyruvate kinase expression within lymphocytes and neutrophils. We speculate that reduced pyruvate kinase expression modifies immune cell responses due to reduced pyruvate concentrations.
Keywords: lymphocytes, neutrophils, signaling
Introduction
The immunological changes associated with pregnancy involve both local properties of the placenta and broader systemic effects. For example, there is an increase in severity of certain infections during pregnancy.1–5 Moreover, it has been reported that the spectrum of cytokines produced during pregnancy is skewed toward a Th2-type response.6–8 Interestingly, certain auto-immune diseases characterized by Th1 responses, especially multiple sclerosis and rheumatoid arthritis, have been reported to remit during pregnancy then relapse post-partum.9–11 In contrast, lupus, which is a Th2-mediated disorder, has been reported to worsen during pregnancy.12, 13 To better understand these in vivo observations, in vitro experiments have been performed. Importantly, reductions in microbial killing, chemotaxis, respiratory burst activity, phagocytosis, and adhesion have been reported for maternal leukocytes in comparison to similarly treated cells from non-pregnant women.14–20 However, the underlying biochemical mechanisms responsible for these immunomodulatory changes have not been thoroughly described.
Recent studies have suggested that metabolic pathways, including metabolite concentrations, may contribute to immunoregulation. Cross-talk between metabolic processes and immune functions have been described.21 We have previously proposed that metabolism is itself a signal affecting cell behavior.22 Suttles and co-workers have demonstrated that fatty acid-binding proteins affect leukocyte activation.23 More recently, a role for metabolism in the differentiation of memory T-cells has been reported.24, 25 We have recently shown that trophoblast contact, but not tumor cell contact, reduces glucose transport in neutrophils and, consequently, reduces cell activation.26 The regulation of glycolysis by signaling molecules, or signaling by glycolytic intermediates, is most likely to occur at irreversible, rate-limiting metabolic steps, which are characterized by large free energy changes. One of these steps is the conversion of phosphoenolpyruvate by pyruvate kinase (EC 2.7.1.40) into pyruvate. Electrophysiological studies have shown that pyruvate influences the activity of store-operated Ca2+ release-activated Ca2+ (CRAC) channels.27 The CRAC channel is a central regulator of Ca2+ signaling in immune cells including lymphocytes, monocytes, mast cells, neutrophils and others.28 CRAC channels are activated by the release of Ca2+ stored in the endoplasmic reticulum, but are rapidly inactivated by the accumulation of cytoplasmic Ca2+. Pyruvate blocks this negative feedback effect of Ca2+ on CRAC channels, resulting in a prolonged Ca2+ influx.27 Thus, the glycolytic product pyruvate is a prime candidate to regulate immune cell Ca2+ signaling. This brings up the question of how leukocytes regulate pyruvate levels without seriously compromising energy production? Five key pathways of regulating the glycolytic production of pyruvate via pyruvate kinase are: 1) the availability of substrate, phosphoenolpyruvate, 2) covalent modification of pyruvate kinase (e.g., phosphorylation), 3) feedback and feed-forward mechanisms, 4) isotype switching, and 5) the concentration of pyruvate kinase.
In the present study, we show that pyruvate kinase expression, pyruvate kinase activity, and pyruvate concentrations are substantially reduced in leukocytes from pregnant women in comparison to those of non-pregnant individuals. As leukocytes from normal pregnant women exhibit some anti-inflammatory characteristics8–20 whereas leukocytes from pregnancies complicated by pre-eclampsia demonstrate certain characteristics associated with inflammation,29–33 we further tested the properties of pyruvate kinase in leukocytes of pre-eclampsia patients. These experiments demonstrated increased concentrations of pyruvate kinase in leukocytes from pre-eclampsia patients in comparison to normal pregnant women. We suggest that pyruvate kinase expression contributes to immunoregulation during pregnancy. We further speculate that pyruvate kinase may act by influencing pyruvate concentrations and calcium signaling, thus affecting the signaling strength of transmembrane messengers and modifying immune cell behavior.
MATERIALS AND METHODS
Study design and population
This cross-sectional study included women in the following groups: 1) non-pregnant controls, 2) normal pregnancy and 3) preeclampsia. Normal pregnant women were in their 3rd trimester or term not in labor, at ≥37 weeks of gestation (n=21). Patients with preeclampsia had hypertension (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mmHg on at least two occasions, 4 hours to 1 week apart) associated with proteinuria (≥300 mg in a 24 hour urine collection, or two dipstick measurements of ≥1+, or one dipstick measurement of ≥2+) (n=25). Exclusion criteria of medical history included diabetes, chronic hypertension, asthma, heart disease, thyroid disease and autoimmune diseases; exclusion criteria for current pregnancy history included smoking, alcohol use, drugs, active sexually transmitted diseases, group B streptococcus colonization and any medications. Patients with multiple gestations, prelabor rupture of membranes, histologic chorioamnionitis, stillbirth, or fetal congenital or chromosomal abnormalities were also excluded. All patients were enrolled by the Perinatology Research Branch at the Hutzel Women’s Hospital, Detroit, MI, and provided written informed consent prior to sample collection. The utilization of samples for research purposes was approved by the Institutional Review Boards of both Wayne State University and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS).
These samples were subjected to cell isolation followed by assessment of pyruvate kinase expression, pyruvate kinase activity, and pyruvate concentration using the instrumentation and procedures described below.
Materials
The following antibodies were used in this study: rabbit polyclonal anti-pyruvate kinase M1/M2 (Alpha Diagnostic International, San Antonio, TX; Chemicon/Millipore, Temecula, CA), goat polyclonal anti-lactate dehydrogenase (Chemicon/Millipore), Alexa Fluor® 488-conjugated donkey anti-rabbit IgG (Invitrogen Corp. (Carlsbad, CA), and TRITC-conjugated donkey anti-goat IgG (Sigma-Aldrich, St. Louis, MO). Fura Red-AM, Fluo-4-AM, Indo-1-AM, BAPTA-AM, Pluronic-127, cell culture media and phosphate buffered saline (PBS) were obtained from Invitrogen Corp. Lipopolysaccharide (LPS; cat. no. L2762) and phytohemaggultinin (PHA) were obtained from Sigma-Aldrich). All other chemicals were purchased from Sigma-Aldrich.
Cell isolation
Cells were isolated by discontinuous density gradient centrifugation. Briefly, 6 ml of blood anticoagulated with EDTA were layered over 3.5 ml Histopaque 1077 and 3.5 ml Histopaque 1119 (Sigma-Aldrich) and centrifuged at 300×g for 45 min. at room temperature. After centrifugation, peripheral blood mononuclear cells (PBMCs) were collected from the interface between the plasma and Histopaque 1077 layers. The cells were then washed with PBS. CD3+ cells were isolated with human T cell enrichment kit (StemCell Technologies, Vancouver, BC, Canada). Briefly, 5×107/ml PBMCs were mixed with 50 µl T cell enrichment cocktail and then incubated at room temperature for 10 min. Magnetic nanoparticles (50 µl) were added to the cell mixture and incubated at room temperature for 10 min. Magnetically labeled cells were then separated from the unlabeled CD3+ cells by using a magnet supplied with the kit.
Flow cytometry
To measure pyruvate kinase expression in leukocytes, whole blood (100 µl) anti-coagulated with EDTA was incubated for 30 min. at 4°C with r-phycoerythrin conjugated anti-CD3 antibody (clone SK7; BD Biosciences, San Jose, CA). Red blood cells were lysed by incubation with FACS Lysing Solution (BD Biosciences), pelleted by centrifugation at 300×g for 5 min. at 4°C, and washed two times with Dulbecco’s PBS (D-PBS) prior to fixation with 1% paraformaldehyde in PBS for 30 min. at room temperature. Following fixation, leukocytes were washed two times with 3% FBS in D-PBS. Cells were suspended in permeabilization medium (Medium B; Invitrogen) and incubated with polyclonal goat anti-pyruvate kinase (Chemicon/Millipore), polyclonal goat anti-lactate dehydrogenase (Chemicon/Millipore), or purified goat IgG as an isotype control (Invitrogen) all at a final concentration of 100 µg/ml for 30 min. at 4°C. Incubation was terminated by the addition of 3% FBS in D-PBS. Cells were pelleted by centrifugation at 300×g for 5 min. at 4°C, and washed once with 3% FBS in D-PBS. This was followed by suspension in permeabilization medium and the addition of Alexa Fluor 488 conjugated donkey anti-goat IgG (final concentration 4 µg/ml; Invitrogen). Cells were incubated at 4°C for 15 minutes. Incubation was again terminated by adding 3% FBS in D-PBS followed by washes with 3% FBS in D-PBS and D-PBS, respectively. Stained cells were finally suspended in 0.1% paraformaldehyde in PBS for flow cytometric acquisition of CD3+ lymphocytes (gated using anti-CD3-PE versus side scatter dot plot) or neutrophils (gated on forward versus side-scatter properties). All acquisitions and analyses were performed with a FACSCalibur flow cytometer and CellQuest Pro software, version 5.2.1 (BD Biosciences). Pyruvate kinase, lactate dehydrogenase and isotype control fluorescence intensities were recorded as the median fluorescence intensity (MFI). The fluorescence intensities of isotype control antibodies were subtracted from pyruvate kinase and lactate dehydrogenase fluorescence intensities prior to statistical analyses.
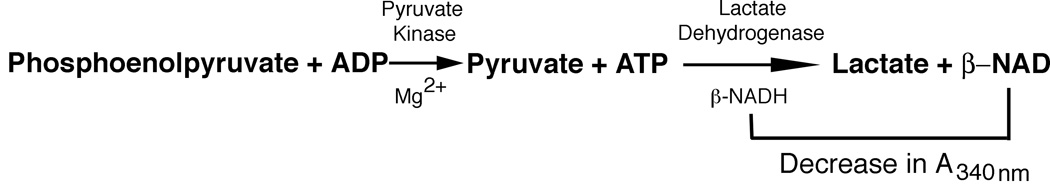
Pyruvate kinase activity assay
Frozen lymphocyte pellets were sonicated for 30 sec. and centrifuged at 15,000×g for 10 min. Aliquots of the supernatant were assayed for pyruvate kinase activity using a fluorescence-coupled assay. One ml reaction buffer (50 mM Tris-HCl pH 7.4, 225 mM KCl, 12 mM MgCl2, 0.6 mM ADP, 4.3 mM phosphoenolpyruvate (PEP), 0.6 mM NADH and 4 IU L-lactate dehydrogenase) was incubated in a spectrophotometer for 5 min. to achieve temperature equilibrium and to determine the blank reading. The reaction was initiated by adding 33 µl of a sample aliquot into the cuvette. The decrease in A340nm was recorded for 5 min. Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA). Pyruvate kinase activity was calculated using ΔA340/min. obtained from the initial linear portion of the curve and normalized to sample’s protein concentration according to the equation:
Pyruvate assay
Pyruvate was measured in PBMCs as described.34 As cell washing procedures may influence intracellular pyruvate levels, the extracellular volume in cell pellets was measured by adding fluorescein-dextran (Mr=40,000) to the cell suspension as a marker for the extracellular compartment (0.5 mg/ml final concentration). Samples were centrifuged at 600×g followed by aspiration of the supernantant. The pellet was extracted with 0.25 ml of ice-cold 0.5 M HClO4 then incubated on ice for 10 min. The mixture was neutralized with 24 µl of 2.5 M K2CO3. As Amplex Red is pH-sensitive, the pH was carefully checked at this step. After centrifugation at 10000×g for 5 min., the supernatant was collected for pyruvate measurement. Briefly, 20 µl of standards or extraction samples were pipetted into a 96-well fluorescence black plate (Corning Inc., Corning NY), 180 µl of the assay solution was added into each well, and then incubated for 30 min. at room temperature. The final reaction solution contained 200 mM potassium phosphate with 1.0 mM EDTA, pH 6.7, 1.0 mM MgCl2, 10 µM FAD, 0.2 mM thiamine pyrophosphate, 0.2 U/ml pyruvate oxidase, 50 µM amplex red and 0.2 U/ml HRP. Fluorescence at 590 nm was measured with excitation at 535 nm. Background was corrected by subtracting the value of the zero pyruvate control from sample readings. Using excitation and emission wavelengths of 485 nm and 530 nm, the intensity of fluorescein was measured then compared to a fluorescein dextran concentration calibration curve to calculate the extracellular volume of the pellet, which permitted the accurate determination of the intracellular pyruvate concentration.
Cell Labeling for Ca2+ Experiments
Human Jurkat T cells were incubated with 5.5 µM Fura Red-AM, 2.6 µM Fluo-4-AM and 2% Pluronic-127 in PBS for one hour at 37°C.35 After incubation, cells were washed with PBS, resuspended in cell buffer (150 mM NaCl, 4 mM KCl, 25 mM HEPES, 3 mM CaCl2, 10 mM glucose, and 1 mg/ml BSA, pH7.6) and incubated for a further 30 min. at 37°C, to purge excess dye. Cells were washed with cell buffer before use. For experimental studies, 10 mM phenylalanine or 5 mM pyruvate were added for 15 min. at 37°C before readings. Cells were stimulated with 10 µg/ml PHA with or without test compounds for 15 min. at 37°C then used in experiments. Fluorescence measurements were performed with a Flexstation device (Molecular Devices, Inc., Sunnyvale, CA) with excitation at 465nm and detection at 657 nm for Fura Red and 516 nm for Fluo-4.
Fluorescence microscopy
Fluorescence microscopy was performed using a 20x objective in a Nikon microscope (model E800; Melville, NY). Images were collected using an Andor (Belfast, Northern Ireland) iXon EMCCD (electron multiplying charge coupled device) camera with the following settings: digitizer, 16 bit (1 MHz); vertical speed shift, 1.7 (µsec/pixel); exposure time, 0.5 sec.; EM gain, 150; pre-amplifier gain, 2.4×; temperature of EMCCD chip, −90°C. Micrographs were recorded using MetaMorph® software (version 7.1.2.0; Molecular Devices).
Statistical analyses
Statistical analyses were performed using unpaired t-tests to evaluate the null hypothesis..
Results
Qualitative Analysis of Pyruvate Kinase Expression
As cell metabolism has been linked with signal transduction and immunoregulation,21–26 we have evaluated the potential role of pyruvate kinase, which catalyzes a key rate-limiting step of glycolysis, in leukocyte properties during pregnancy. To provide a preliminary evaluation of pyruvate kinase, we employed immunofluorescence microscopy to examine its expression in CD3+ lymphocytes of non-pregnant women, pregnant women, and pre-eclampsia patients. As illustrated in Fig. 2, pyruvate kinase can be found throughout cells, although it is most highly expressed at the cell periphery. No differences in the cytoplasmic distribution of this enzyme was detected when examining leukocytes from non-pregnant women, normal pregnant women and those with preeclampsia. These findings are consistent with our previous observations.36 However, upon closer inspection of the cell population, it appeared that the leukocyte samples from pregnant women were substantially dimmer than those of non-pregnant individuals or pre-eclampsia patients. To test this finding using a different technique, extracts of CD3+ lymphocytes were prepared then subjected to SDS-PAGE/Western blotting. These experiments showed that pyruvate kinase expression is significantly reduced in samples from pregnant women (Fig. S1). To control for sample loading, the blots were stripped and re-blotted using actin as a control (data not shown). Thus, pyruvate kinase expression is reduced during normal pregnancy, but its intracellular distribution is unaffected.
Fig. 2.
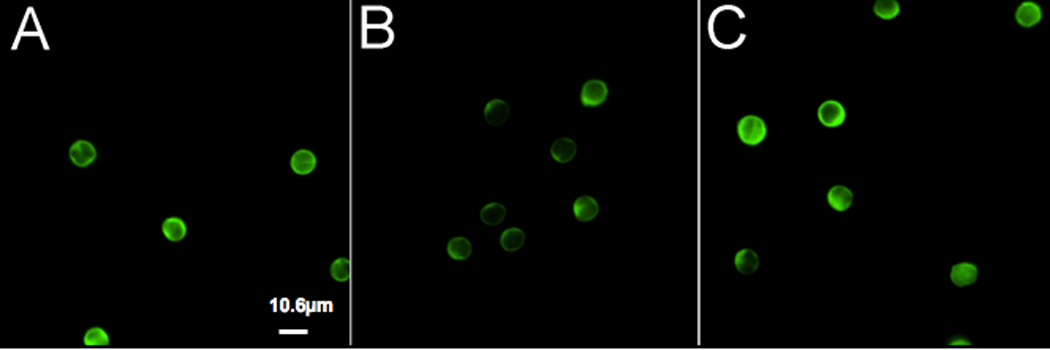
Fluorescence microscopy of pyruvate kinase. CD3+ lymphocytes were prepared from peripheral blood samples obtained from non-pregnant women (A), pregnant women (B), and pre-eclampsia patients (C). Cells were labeled with an anti-pyruvate kinase antibody. Images were collected under identical conditions, as described in the Materials and Methods. (Bar = 10.6 mm)
Quantitative Flow Cytometric Analysis of Pyruvate Kinase Expression
Flow cytometry was used to provide compelling quantitative data concerning pyruvate kinase expression by leukocyte populations. Fig. 3 shows representative flow cytometry histograms of pyruvate kinase labeling experiments and isotype controls for CD3+ lymphocyte samples from non-pregnant women and normal pregnant women. To exclude other cell types, anti-CD3-phycoerthrin was used in gated detection (see Figure 3 insert). As shown in Figure 3A, pyruvate kinase expression is substantially reduced in samples from pregnant women in comparison to non-pregnant women. However, the expression of lactate dehydrogenase was not significantly altered by pregnancy (Fig. 3B).
Fig. 3.
Flow cytometry histograms of pyruvate kinase abundance in human CD3+ lymphocytes are shown. Panel A: Representative histograms showing pyruvate kinase or isotype control labeling are shown. Pyruvate kinase expression is reduced in maternal lymphocytes (black dashed line) relative to lymphocytes from non-pregnant adults (black solid line). Fluorescence histograms of cells stained with an isotype control antibody are shown in gray (dashed line = maternal; solid = normal adult). Only CD3+ cells are shown in the histograms (see inset showing CD3 PE vs. side scatter dot plot). Panel B: Representative histogram overlay of cells stained with an anti-lactate dehydrogenase or isotype control antibodies. Samples from pregnant women (black dashed line) and non-pregnant women (black solid line) are shown. Isotype controls are shown in gray on the left hand side levels between maternal blood and normal adult peripheral blood CD3+ lymphocytes (dashed line = maternal; solid = normal adult). Lymphocyte pyruvate kinase expression, but not LDH expression, is substantially reduced during pregnancy.
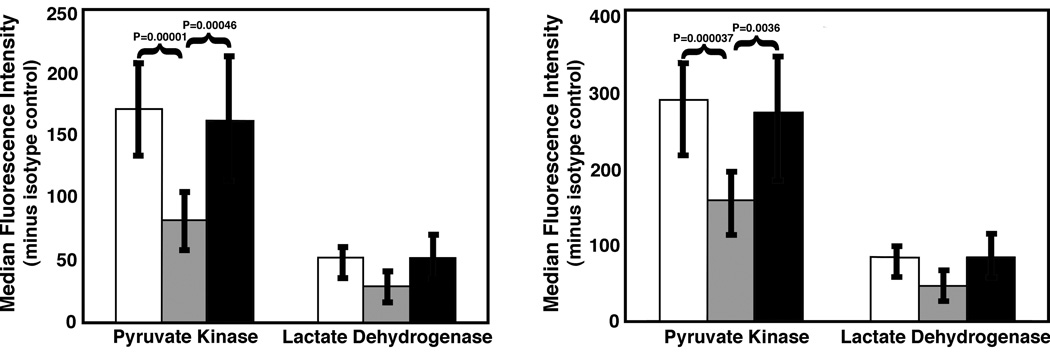
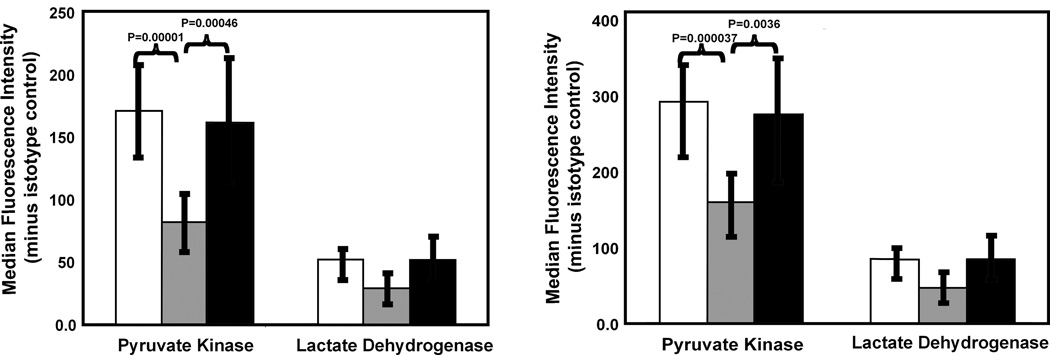
To evaluate the broad significance of reduced pyruvate kinase expression, we used flow cytometry to measure pyruvate kinase and lactate dehydrogenase levels in CD3+ lymphocytes from populations of non-pregnant and normal pregnant women as well as in those with preeclampsia (Fig. 4A). Lymphocyte pyruvate kinase levels were reduced in normal pregnancies in comparison to non-pregnant women (P=0.00001; n=10 for both groups). However, pyruvate kinase levels of lymphocytes from pre-eclampsia patients were greater than those of normal pregnant women (P=0.00046; n=10), but indistinguishable from those of non-pregnant women (see also Fig. S2). In contrast, no significant changes in lactate dehydrogenase were detected. To ascertain if these changes in CD3+ lymphocytes were found among other leukocyte populations, neutrophils were also evaluated (Fig. 4B). Neutrophils were separately catalogued based upon their 90° light scattering properties. As neutrophils are larger than lymphocytes, they express more pyruvate kinase. Nonetheless, a similar pattern of highly significant changes in pyruvate kinase expression was noted, with a reduction in expression during normal, but not pre-eclamptic, pregnancy. The expression of neutrophil lactate dehydrogenase was not influenced by pregnancy. Thus, pyruvate kinase expression is reduced in maternal leukocytes during normal pregnancy.
Fig. 4.
Summary of flow cytometry studies of CD3+ lymphocytes (A) and neutrophils (B) from non-pregnant (open bars), pregnant (gray bars) and pre-eclamptic (black bars) women. Panel A: Pyruvate kinase expression is reduced in lymphocytes from pregnant women in comparison to non-pregnant women (P=0.00001). Pyruvate kinase expression in cells from pre-eclampsia patients was significantly elevated in comparison to normal pregnant patients (P=0.00046), but not non-pregnant women. Ten individuals in each category were studied. No significant differences are detected in lactate dehydrogenase levels. Panel B: Maternal peripheral blood neutrophils have significantly less pyruvate kinase than neutrophils from both non-pregnant women (0.000037) as well as women with preeclampsia (0.0036). Eight non-pregnant women were studied. Nine normal pregnant women and nine pre-eclampsia patients were studied. As noted above for lymphocytes, no significant effect was noted for lactate dehydrogenase. Error bars represent the SD.
We have noted that pyruvate kinase expression is substantially higher in leukocytes from pre-eclampsia patients than those of normal pregnant mothers. As pre-eclampsia exhibits some characteristics of inflammation,29, 30 we considered the possibility that inflammatory signaling increases the expression of this enzyme. Fig. 5 shows flow cytometry histograms of leukocytes stained with an anti-pyruvate kinase antibody or an isotype-matched control reagent. As these data show, LPS (100 ng/ml, 30 min.) had no effect on the level of pyruvate kinase expression by neutrophils.
Fig. 5.
Flow cytometry histograms of pyruvate kinase labeling of human CD3+ lymphocytes are shown. Cells were untreated (control) or treated with LPS (100 ng/ml, 30 min.). Isotype controls are shown on the left hand side. No differences in pyruvate kinase levels could be noted in the presence of LPS.
Evaluation of Enzymatic Activity and Product Levels
To verify that enzyme expression levels correlate with enzyme activity, we measured pyruvate kinase activity in lymphocyte extracts. Pyruvate kinase activity was measured as described in the Materials and Methods and illustrated in Fig. 1. Pyruvate kinase activity in leukocyte samples from normal pregnant women was 23±4 ΔA/min./mg protein (mean±SD; n=8) in comparison to 40±9 ΔA/min./mg protein (n=7) for non-pregnant women (P<0.001). Although the pyruvate kinase activity of leukocytes from pre-eclampsia patients (45±22 ΔA/min./mg protein; n=6) was similar to that of samples from non-pregnant women, it was significantly greater than that found for normal pregnancy samples (P<0.029). As observed in these studies and the flow cytometry experiments (Fig. 4), leukocytes from women with pre-eclampsia demonstrated greater variability in these measures than did cells from non-pregnant or normal pregnant women. As expected based upon the flow cytometry studies, these enzyme activity measurements showed that total pyruvate kinase activity was depressed in leukocytes from normal pregnant women, but not those of pre-eclampsia patients, with respect to those of non-pregnant women (data not shown). Hence, pyruvate kinase is reduced in expression and enzymatic function in normal pregnancy.
Fig. 1.
Pyruvate kinase activity assay. Pyruvate kinase activity was measured using phosphoenolpyruvate as substrate and coupling pyruvate production to the disappearance of NADH, which can be quantified using fluorescence detection.
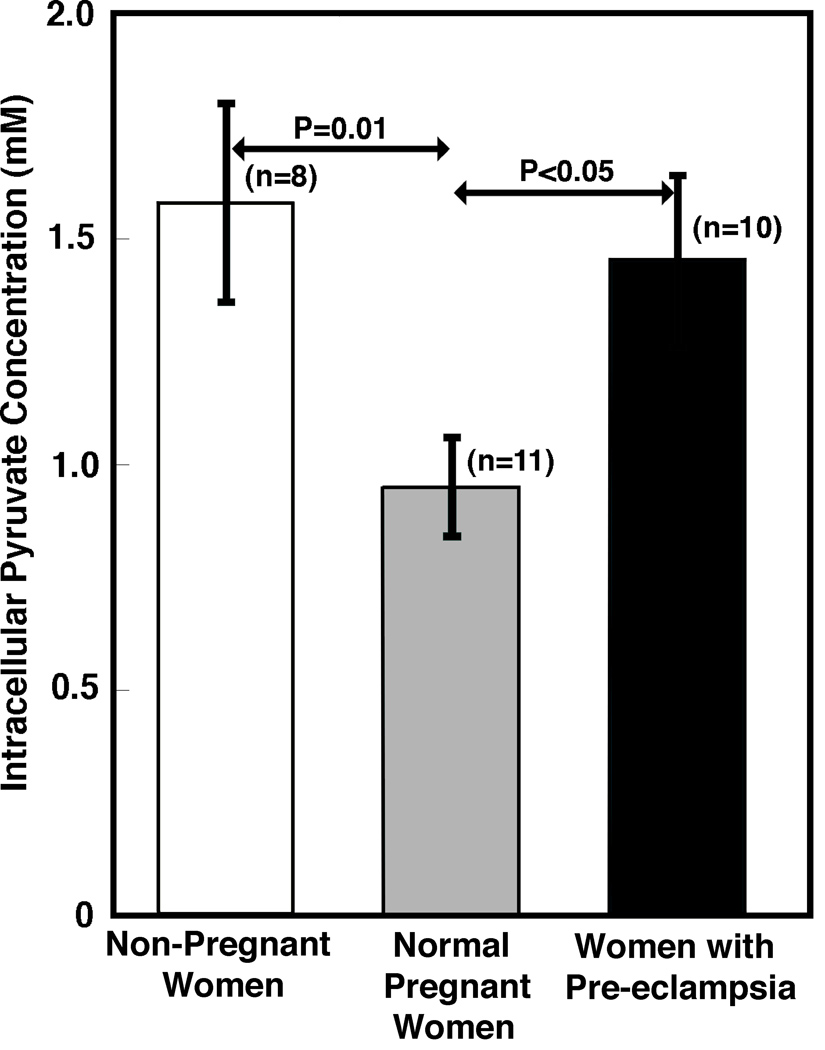
Given the highly significant differences in pyruvate kinase expression and activity in the leukocytes of non-pregnant women, normal pregnant women and those with preeclampsia, we assessed intracellular pyruvate concentrations. We developed a novel fluorescence assay for pyruvate of high sensitivity and specificity for use with small volumes of natural human biological specimens.34 Using this assay, we measured the number of picomoles of pyruvate per 106 cells in cell extracts. To calculate the intracellular pyruvate concentration, an estimate of cell volume is required. We used PBMCs because there was a limited amount of blood available for studies and purified CD3+ cells yielded the similar values in preliminary experiments. The volume of lymphocytes has been determined to be 210 µm3.37 The volume of small monocytes is 332 mm3 whereas that of large monocytes is 380 µm3.38 As large monocytes make up 2/3 of the total monocyte population,38 the average monocyte volume is approximately 364 µm3. As the ratio of lymphocytes to monocytes is 3:1 for non-pregnant and normal pregnant women,39, 40 the average peripheral blood mononuclear cell volume is roughly 249 µm3 for leukocytes from non-pregnant women. As cell counts were performed for all samples before extraction, variations in the leukocyte numbers during pregnancy40 do not affect our results. In addition, previous studies have shown that the mean cell volume of leukocytes is not affected by pregnancy.41, 42 Based upon this information, we calculated the intracellular pyruvate concentrations in cells from non-pregnant, pregnant, and pre-eclamptic women. As shown in Fig. 6, the intracellular pyruvate concentration in leukocytes from non-pregnant women is 1.58 mM, which is substantially greater than that of samples from pregnant women (0.95 mM). Pyruvate levels in leukocytes of pre-eclamptic women, again, track with pyruvate kinase expression and activity and are higher. As these means represent good measures of the underlying population means, these differences in pyruvate concentration may be sufficient to affect Ca2+ signaling in cells.27
Fig. 6.
Evaluation of intracellular pyruvate concentrations. Intracellular pyruvate concentrations were determined by measuring pyruvate levels in cell extracts then correcting for cell volume. The pyruvate concentration in PBMCs from non-pregnant women (1.58±0.22 mM; mean±SEM; n=8) was significantly higher than that of pregnant women (0.95±0.11 mM; n=11) (P=0.01). The pyruvate concentration in cells from pre-eclamptic patients (1.45±0.19; n=10) was significantly higher than that of normal pregnant women (P<0.05). As indicated by the standard error of the means, these values are a good measure of the underlying population means, thus supporting the proposed mechanistic contribution to signaling.
Calcium Signaling
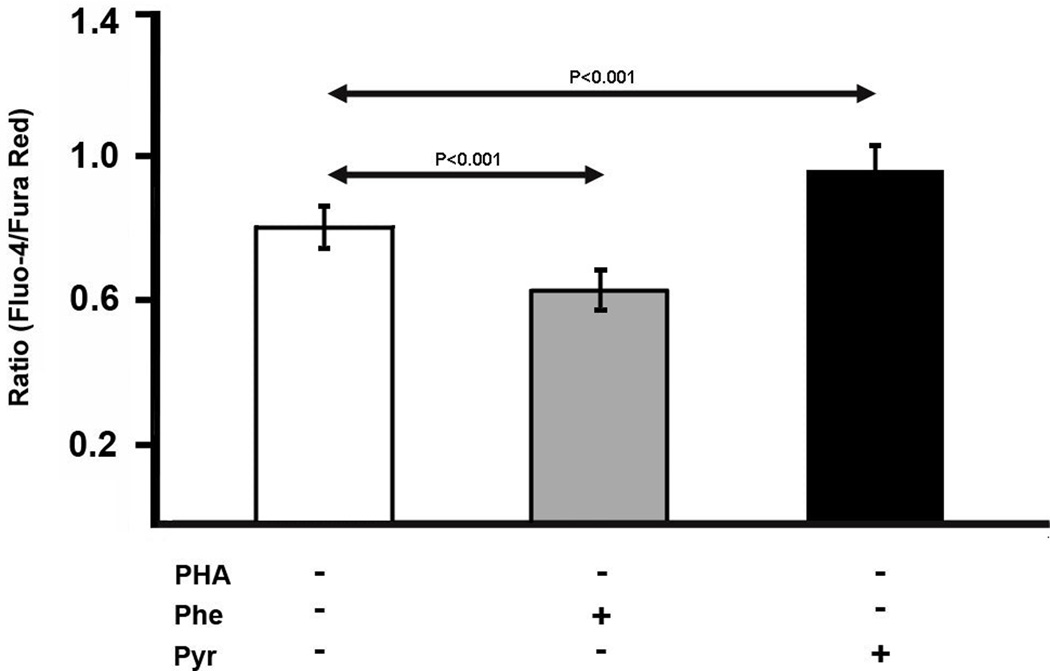
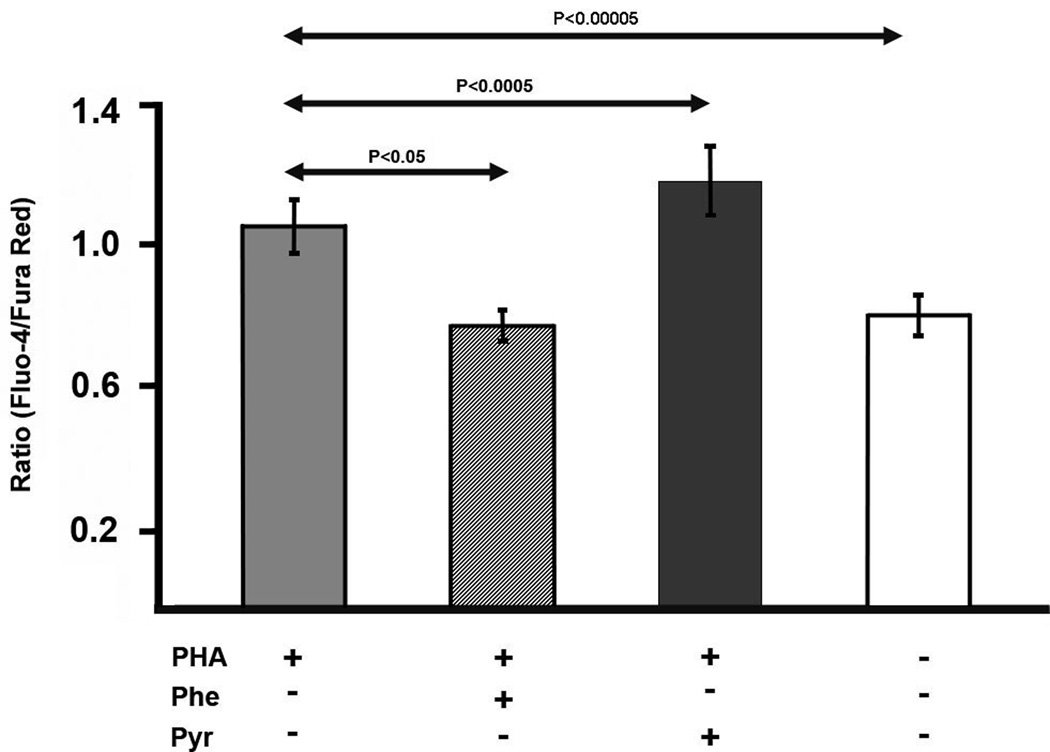
If leukocyte function is affected by the pyruvate kinase expression level, it may be possible to manipulate cell signaling by changing pyruvate levels. As Ca2+ experiments are performed over a short period of time, we required a model system in which pyruvate levels could be easily altered. As preliminary experiments showed that exogenous pyruvate could quickly alter the intracellular level of pyruvate in human Jurkat T cells, we used this as a model for leukocyte signaling. (Similarly, washing Jurkat cells in a pyruvate-free medium rapidly washes out intracellular pyruvate.) To reduce pyruvate levels, phenylalanine, a pyruvate kinase inhibitor was employed.43 To study Ca2+, we labeled Jurkat cells with the dyes Fluo-4 and Fura Red, as described in the Materials and Methods section. The Fluo-4/Fura Red ratio was measured as an indicator of intracellular Ca2+ concentration. In unstimulated lymphocytes, pyruvate addition enhanced Ca2+ levels whereas addition of phenylalanine, reduced Ca2+ levels (Fig. 7); although these changes were relatively small, they were statistically significant. To test leukocyte activation in this model, PHA stimulation was employed, which leads to an increase in intracellular Ca2+ as judged by the Fluo-4/Fura Red ratio (P<0.00005; Fig. 8). PHA-stimulated cells exhibited a decrease in Ca2+ in the presence of phenylalanine and an increase in the presence of pyruvate (Fig. 8). Thus, exogenous factors affecting pyruvate influence the Ca2+ signaling apparatus of Jurkat cells.
Fig. 7.
Effect of pyruvate and phenylalanine, a pyruvate kinase inhibitor, on the Fluo-4/Fura Red ratio (a measure of intracellular Ca2+) in unstimulated Jurkat cells. The Fluo-4/Fura Red ratio is listed at the ordinate (mean±SD). Experiments were performed as described in the Materials and Methods. Phenylalanine, a pyruvate kinase inhibitor,43 is known to perturb the concentration of metabolites in Jurkat cells.76 At 6 mM, phenylalanine significantly reduced the Fluo-4/Fura Red ratio. On the other hand, addition of pyruvate to cells promoted an increase in the Fluo-4/Fura Red ratio. Although significant, these changes were not dramatic and required multiple repetitions to reach these high levels of significance. (n=10)
Fig. 8.
Effect of pyruvate and phenylalanine on the Fluo-4/Fura Red ratio (mean±SD) in stimulated Jurkat cells. In the first three columns, cells were activated using PHA. Addition of phenylalanine promoted a decrease in the Fluo-4/Fura Red ratio whereas the addition of pyruvate promoted an increase in the Fluo-4/Fura Red ratio. Untreated cells are shown on the right hand side for comparison. (n=10)
Discussion
In the present study we have demonstrated that pyruvate kinase expression is reduced in leukocytes from normal pregnant women in comparison to non-pregnant women. In addition, leukocyte pyruvate kinase expression is higher in women with preeclampsia than in normal pregnant women. The biochemical significance of a change in enzyme expression depends in complex fashion upon the chemical properties of its reaction mechanism and reaction network. For example, using in vitro systems, a four-to-five-fold increase in phosphofructokinase expression has no effect on glycolytic flux or cell growth.44, 45 However, similar changes in pyruvate kinase expression dramatically alter carbon flux and cell growth.46, 47 Pyruvate kinase is a highly regulated enzyme catalyzing an irreversible step of glycolysis that is associated with a large decrease in free energy.48 Pyruvate kinase is also at a unique biochemical “choke-point” in metabolism, which is a key factor regarding its importance in cancer cell grow.49 To illustrate the functional importance of our observations, we have explored changes in pyruvate kinase activity and intracellular pyruvate concentrations in leukocytes from non-pregnant women, normal pregnant women, and women with preeclampsia. The findings suggest that a roughly two-fold change in pyruvate kinase expression is associated with a similar change in intracellular pyruvate concentration.
Our measurements of intracellular pyruvate coupled with estimates of leukocyte cell volume suggest an intracellular pyruvate concentration of ~ 1 mM. As neutrophils and lymphocytes contain relatively few mitochondria and rely primarily upon aerobic glycolysis for energy production,50, 51 mitochondrial pyruvate catabolism is reduced in these cells, which may contribute to higher intracellular pyruvate concentrations. Furthermore, our experimental assessments of intracellular pyruvate concentration are similar to estimates of peri-membrane pyruvate levels provided by others.27, 52 However, as washing protocols and the requirement of correcting for extracellular pyruvate in extracts complicate published estimates of intracellular pyruvate concentrations, we sought to more accurately determine this value. Using newly developed methods of greater sensitivity, this study provides the first direct measurements of intracellular pyruvate in immune cells, although the values are considered to be estimates. Nonetheless, the intracellular concentration of pyruvate in leukocytes from normal pregnant women is considerably lower than that of cells from non-pregnant women.
Signaling strength
Recently, Bakowski and Parekh27 have shown that pyruvate concentrations of roughly 1 mM increase store-operated Ca2+ entry by inhibiting Ca2+-mediated inactivation of these channels (Fig. 9). These findings are important because: 1) they were observed at physiologically-relevant voltages and 2) substantial differences in fast CRAC channel inactivation are observed in the range of pyruvate concentrations associated with leukocytes from pregnant and non-pregnant women. Thus, the differences in leukocyte pyruvate kinase expression of normal pregnant and non-pregnant women would be anticipated to influence CRAC channel signaling thereby blunting signal strength during pregnancy. However, it remains possible that pyruvate may also act through other Ca2+ channels53 to influence signal transduction during pregnancy.
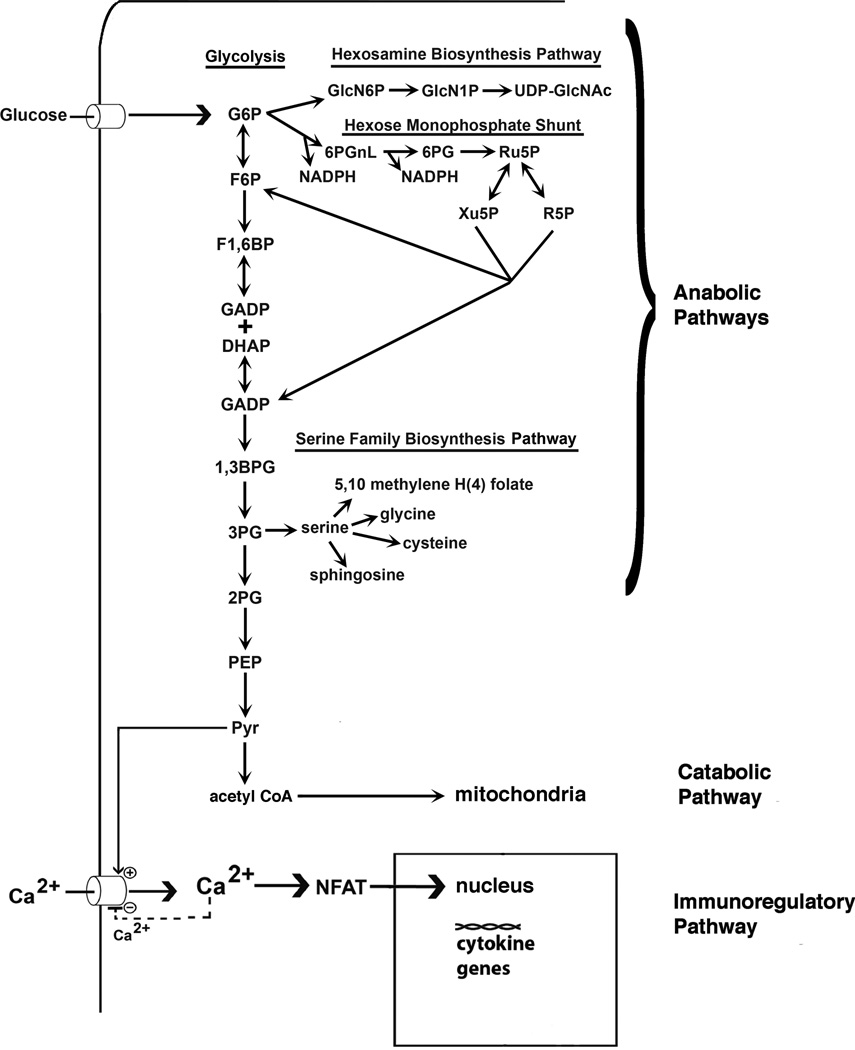
Fig. 9.
Proposed mechanism of pyruvate-mediated influence on cell growth and immunologic modifications. Pyruvate is produced by pyruvate kinase, the final enzyme of glycolysis. When pyruvate kinase levels are depressed at this rate-limiting step of glycolysis, upstream glycolytic phosphometabolite intermediates accumulate to higher levels. Higher levels of glycolytic intermediates support the synthesis of carbohydrates, nucleic acids, amino acids, and lipids, as indicated in the diagram.
Pyruvate promotes Ca2+ signaling by inhibiting the negative Ca2+ feedback at the CRAC channel.27 Ca2+ directly influences NFAT. However, when pyruvate levels are reduced, signaling is reduced.
Using Jurkat cells, a model human T lymphocyte, to assess the role of pyruvate kinase in signaling, we have manipulated pyruvate levels by adding high concentrations of exogenous pyruvate, which is able to enter these cells, and the pyruvate kinase inhibitor phenylalanine. We observed that experimentally-induced reductions in pyruvate concentrations diminish Ca2+ levels, whereas an elevation of pyruvate concentration also elicits an increase in intracellular Ca2+ levels. These findings are consistent with our proposed role of pyruvate kinase in Ca2+ signaling.
Signal strength, including Ca2+ signaling strength, is a key factor in determining the differentiation of CD4+ T cells into Th1 and Th2 cells.54, 55 As a result of reduced intracellular pyruvate levels in leukocytes, cells from normal pregnant women would be expected to exhibit a reduction in the Th1/Th2 ratio. This is consistent with prior studies suggesting a reduction in the Th1/Th2 ratio during pregnancy.6–8 As CRAC channels are important in many leukocyte populations,28 this mechanism may contribute to Ca2+-sensitive leukocyte activating pathways of monocytes, neutrophils and other cell types. As the NADPH oxidase is activated in granule membranes of neutrophils,56 our model suggests that at low signal levels (e.g., normal pregnancy) Ca2+ -dependent degranulation is reduced thereby enhancing intracellular oxidant levels30, 57 and decreasing extracellular release14, 15 whereas higher signal levels (e.g., cells from non-pregnant individuals) would promote greater NADPH oxidase delivery to the plasma membrane and extracellular oxidant release.
Leukocytes from patients with preeclampsia had a higher intracellular pyruvate concentration than that of the same cells of normal pregnant women. It can be anticipated that the increased intracellular concentration of pyruvate also leads to increased Ca2+ levels. Such findings have been previously reported for women with preeclampsia.31, 32 This may be a contributory factor in the modified leukocyte properties associated with pre-eclampsia including: increased levels of pro-inflammatory cytokines, enhanced reactive oxygen metabolite production, nuclear translocation of NF-κB, and enhanced surface CD11b, CD11a, CD49b expression.29, 30, 33
As described above, significant differences have been found in leukocyte pyruvate kinase levels in non-pregnant and pregnant women. It seems possible that serum levels of pyruvate or phenylalanine might contribute to the measured pyruvate kinase activity and pyruvate levels. For non-pregnant and normal pregnant women, serum pyruvate levels have been found to be 0.04–0.20 mM and 0.042–0.09 mM, respectively.58, 59 Moreover, serum phenylalanine levels of 0.06v0.24 mM and 0.03–0.10 mM have been found for non-pregnant and normal pregnant women, respectively.60–64 Thus, in surveying studies in this field, there are no significant differences in pyruvate or phenylalanine levels in non-pregnant and pregnant women. Thus, the differences we have observed cannot be accounted for by serum levels of pyruvate or phenylalanine.
Potentially Related Metabolic Pathways
In contrast to many glycolytic enzymes, pyruvate kinase action is irreversible.48 In some cell types pyruvate levels could be affected by enzymes involved in gluconeogenesis; specifically pyruvate carboxylase and phosphoenolpyruvate carboxykinase. During gluconeogenesis, pyruvate carboxylase participates in the conversion of pyruvate to phosphoenolpyruvate. It is unlikely that differences in pyruvate carboxylase levels affected the measured and calculated pyruvate concentrations. First, pyruvate kinase and pyruvate carboxylase are prevented by the signaling apparatus from functioning at the same time to prevent a futile cycle.65 Second, circulating leukocytes express extremely low levels of this enzyme.66 Thus, this enzyme is unlikely to be a significant contributor to our findings.
In another step of gluconeogenesis, phosphoenolpyruvate carboxykinase converts oxaloacetate to phosphoenolpyruvate. We used an anti-human pyruvate carboxylase (PCK-1) antibody (Novus Biologicals, Littleton, CO) to measure the expression of this molecule by leukocytes. We could not detect the expression of this enzyme in leukocytes using flow cytometry (data not shown). Therefore, this enzyme cannot interfere with the experiments reported herein. These observations further support our conclusions.
Potentially Related Disease Pathways
Our proposed mechanism also finds support in previous clinical studies. For example, conventional lactate-based peritoneal dialysis solutions (L-PDS) have been employed in continuous ambulatory peritoneal dialysis for many years. It has been demonstrated that L-PDS solutions reduce leukocyte cytokine production (TNF-α and IL-1β), chemotaxis and oxidant release as well as mesothelial cell proliferation.67, 68 Use of L-PDS is associated with complications such as peritonitis and peritoneal membrane perforations, which are minimized by using pyruvate-based peritoneal dialysis solutions (P-PDS).67, 68 As extracellular pyruvate may affect signaling, our mechanistic model may account for these descriptive clinical findings.
Alterations in pyruvate kinase levels have also been noted in neutrophils from patients following polytrauma.69 A staggering 600-fold increase in pyruvate kinase activity was noted in neutrophils, suggesting that pyruvate kinase is no longer a rate-limiting step in pyruvate production. Neutrophil activation, as judged by oxidant release, is greatly enhanced in these cells, as our hypothetical mechanism would suggest.
Additional support for our hypothesis can be found in sepsis. In healthy individuals, peripheral blood pyruvate levels are 0.04–0.08 mM. However, during sepsis these levels climb to 0.4 mM.70 As higher serum levels reduce the diffusion potential of pyruvate across the cell membrane, pyruvate will increase within cells, thus causing more robust stimulation of TNF-α production, as seen during sepsis. Thus, our model is consistent with previous clinical investigations from other laboratories.
A Model for the Glycolytic Regulation and Further Consideration of its Implications
We have used fluorescence microscopy, Western blotting, flow cytometry, activity measurements, and intracellular pyruvate determinations to confirm the reduced levels of cytosolic pyruvate kinase observed in leukocytes from normal pregnant women. Although Carboné et al.71 did not observe these changes in pyruvate kinase activity, their results were confounded by the use of detergents for enzyme extraction, which promote enzyme denaturation and the release of mitochondrial pyruvate kinase activity.72 Fig. 9 shows an integration of the glycolytic and signaling pathways that illustrates the principles described above. Glycolytic phosphometabolite intermediates enter the hexosamine biosynthesis pathway, the hexose monophosphate shunt, and the serine family biosynthesis pathways. When the final step of glycolysis mediated by pyruvate kinase is reduced, glycolytic phosphometabolite intermediates accumulate in the cytoplasm, which supports the synthesis of sugars, nucleotides, amino acids and lipids. In tumor cell biology, a reduction in pyruvate kinase activity is essential in supporting cell growth.73 The potential role of pyruvate kinase expression levels in cell growth in normal and pre-eclamptic pregnancies awaits further study. As illustrated at the bottom of this figure and discussed previously, when pyruvate levels are reduced, as in normal pregnancy, rising intracellular Ca2+ levels feedback to decrease CRAC activity thereby reducing further Ca2+ entry.
Our findings are particularly exciting because they describe glycolytic enzyme changes in leukocytes during pregnancy that may provide a mechanism contributing to immunoregulation. It will be important to determine whether the changes in intracellular pyruvate concentrations occur in early pregnancy, and whether the increase observed in preeclampsia is detectable before the diagnosis of the disease. If this is the case, then intracellular pyruvate concentrations may have predictive value. Longitudinal studies are required to test this hypothesis. Similarly, it is important to determine whether such findings are specific to preeclampsia, or if they can be observed in other complications of pregnancy. We have previously reported changes in the phenotypic and metabolic characteristics of neutrophils and monocytes in other obstetrical syndromes, such as preterm labor, preterm PROM and SGA.74–77 One possibility that cannot be excluded is that the general mechanisms described herein apply to more than one complication of pregnancy, and that it may be observed not only in the maternal compartment, but also the fetal compartment. Furthermore, it may be possible to design drugs that mildly suppress pyruvate kinase activity to diminish pro-inflammatory signaling, such as that observed during pre-eclampsia. In a subsequent paper, we explore this possibility.
Supplementary Material
Acknowledgements
This work was supported, in part, by the Division of Intramural Research of the Eunice Kennedy Schriver National Instutute of Child Health and Human Development, NIH, DHHS, contract number N01-HD-2-3342 and subcontract WSU04055. We thank L. Nikita for assistance in recruiting patients.
References
- 1.Brabin BJ. Epidemiology of infection in pregnancy. Rev Infect Dis. 1985;7:579–603. doi: 10.1093/clinids/7.5.579. [DOI] [PubMed] [Google Scholar]
- 2.Larsen B, Galask RP. Host-parasite interactions during pregnancy. Obstet Gynecol Survey. 1978;33:297–318. doi: 10.1097/00006254-197805000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Reinhardt MC. Effects of parasitic infections in pregnant women. Ciba Found Symp. 1979;77:149–170. doi: 10.1002/9780470720608.ch10. [DOI] [PubMed] [Google Scholar]
- 4.Luft BJ, Remington JS. Effect of pregnancy on resistance to Listeria monocytogenes and Toxoplasma gondii infections in mice. Infect Immun. 1982;38:1164–1171. doi: 10.1128/iai.38.3.1164-1171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham FG, Leveno KJ, Hankins GDV, Whalley PJ. Respiratory insufficiency associated with pyelonephritis during pregnancy. Obstet Gynecol. 1984;63:121–125. [PubMed] [Google Scholar]
- 6.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 7.Miyaura H, Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol. 2002;168:1087–1094. doi: 10.4049/jimmunol.168.3.1087. [DOI] [PubMed] [Google Scholar]
- 8.Khil LY, Jun HS, Kwon H, Yoo JK, Kim S, Notkins AL, Yoon JW. Human chorionic gonadotropin is an immune modulator and can prevent autoimmune diabetes in NOD mice. Diabetologia. 2007;50:2147–2155. doi: 10.1007/s00125-007-0769-y. [DOI] [PubMed] [Google Scholar]
- 9.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 10.Spector TD, da Silva JAP. Pregnancy and rheumatoid arthritis. Clin Rheumatol. 1992;11:189–194. doi: 10.1007/BF02207955. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal RK, Chan C-C, Wiggert B, Caspi RR. Pregnancy ameliorates induction and expression of experimental autoimmune uveitis. J Immunol. 1999;162:2648–2654. [PubMed] [Google Scholar]
- 12.Kaaja RJ, Greer IA. Manifestations of chronic disease during pregnancy. JAMA. 2005;294:2751–2757. doi: 10.1001/jama.294.21.2751. [DOI] [PubMed] [Google Scholar]
- 13.Doria A, Iaccarino L, Arienti S, Ghirardello A, Zampieri S, Rampudda ME, Cutolo M, Tincani A, Todesco S. Th2 immune deviation induced by pregnancy: the two faces of autoimmune rheumatic diseases. Reprod Toxicol. 2006;22:234–241. doi: 10.1016/j.reprotox.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Cotton DJ, Seligmann B, O’Brian B, Gallin JI. Selective defect in human neutrophil superoxide anion generation elicited by the chemoattractant N-formylmethionylleucylphenylalanine in pregnancy. J Infect Dis. 1983;148:194–199. doi: 10.1093/infdis/148.2.194. [DOI] [PubMed] [Google Scholar]
- 15.Tsukimori KI, Maeda H, Ishida K, Nagata H, Koyanagi T, Nadano H. The superoxide generation of neutrophils in normal and preeclamptic pregnancies. Obstet Gynecol. 1993;81:536–540. [PubMed] [Google Scholar]
- 16.Benjamin JL, Remington JS. The adverse effect of pregnancy on macrophage activation. Cell Immunol. 1984;85:94–99. doi: 10.1016/0008-8749(84)90281-8. [DOI] [PubMed] [Google Scholar]
- 17.El-Maallem H, Fletcher J. Impaired neutrophil function and myeloperoxidase deficiency in pregnancy. Br J Hematol. 1980;44:375–381. doi: 10.1111/j.1365-2141.1980.tb05906.x. [DOI] [PubMed] [Google Scholar]
- 18.Bjoksten B, Soderstrom T, Damber MG, Von Schoultz B, Stigbrand T. Polymorphonuclear leucocyte function during pregnancy. Scand J Immunol. 1978;8:257–262. doi: 10.1111/j.1365-3083.1978.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 19.Krause PJ, Ingardia CJ, Pontius LT, Malech HL, Lobello TM, Maderazo EG. Host defense during pregnancy: neutrophil chemotaxis and adherence. Am J Obstet Gynecol. 1987;157:274–280. doi: 10.1016/s0002-9378(87)80150-3. [DOI] [PubMed] [Google Scholar]
- 20.Persellin RH, Thoi LL. Human polymorphonuclear leukocyte phagocytosis in pregnancy: development of inhibition during gestation and recovery in the post partum period. Am J Obstet Gynecol. 1979;134:250–255. doi: 10.1016/s0002-9378(16)33028-9. [DOI] [PubMed] [Google Scholar]
- 21.Matarese G, La Cava A. The intricate interface between immune system and metabolism. Trends Immunol. 2004;25:193–200. doi: 10.1016/j.it.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Kindzelskii AL, Eszes MM, Todd RF, Petty HR. Proximity oscillations of complement receptor type 4 and urokinase receptors on migrating neutrophils are linked with signal transduction/metabolic oscillations. Biophys J. 1997;73:1777–1784. doi: 10.1016/S0006-3495(97)78208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds JM, Liu Q, Brittingham KC, Liu Y, Gruenthal M, Gorgun CZ, Hotamisligil GS, Stout RD, Suttles J. Deficiency of fatty acid-binding proteins in mice confers protection from development of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:313–321. doi: 10.4049/jimmunol.179.1.313. [DOI] [PubMed] [Google Scholar]
- 24.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petty HR, Kindzelskii A, Espinoza J, Romero R. Trophoblast contact de-activates human neutrophils. J Immunol. 2006;176:3205–3214. doi: 10.4049/jimmunol.176.5.3205. [DOI] [PubMed] [Google Scholar]
- 27.Bakowski D, Parekh AB. Regulation of store-operated calcium channels by the intermediary metabolite pyruvic acid. Curr Biol. 2007;17:1076–1081. doi: 10.1016/j.cub.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 28.Feske S. Calcium signaling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 29.Luppi P, Tse H, Lain KY, Markovic N, Piganelli JD, DeLoia JA. Preeclampsia activates circulating immune cells with engagement of the NF-kappaB pathway. Am J Reprod Immunol. 2006;56:135–144. doi: 10.1111/j.1600-0897.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- 30.Gervasi MT, Chaiworapongsa T, Pacora P, Naccasha N, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–797. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 31.Hojo M, Suthanthiran M, Helseth G, August P. Lymphocyte intracellular free calcium concentration is increased in preeclampsia. Am J Obstet Gynecol. 1999;180:1209–1214. doi: 10.1016/s0002-9378(99)70618-6. [DOI] [PubMed] [Google Scholar]
- 32.von Dadelszen P, Wilkins T, Redman CW. Maternal peripheral blood leukocytes in normal and pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1999;106:576–581. doi: 10.1111/j.1471-0528.1999.tb08327.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee VM, Quinn PA, Jennings SC, Ng LL. Neutrophil activation and production of reactive oxygen species in pre-eclampsia. J Hypertens. 2003;21:395–402. doi: 10.1097/00004872-200302000-00032. [DOI] [PubMed] [Google Scholar]
- 34.Zhu A, Romero R, Petty HR. An enzymatic fluorimetric assay for pyruvate. Anal Biochem. 2009;396:146–151. doi: 10.1016/j.ab.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Floto RA, Mahaut-Smith MP, Somasundaram B, Allen JM. IgG-induced Ca2+ oscillations in differentiated U937 cells; a study using laser scanning confocal microscopy and co-loaded fluo-3 and fura-red fluorescent probes. Cell Calcium. 1995;18:377–389. doi: 10.1016/0143-4160(95)90053-5. [DOI] [PubMed] [Google Scholar]
- 36.Kindzelskii AL, Huang J-B, Chaiworapongsa T, Kim YM, Romero R, Petty HR. Pregnancy alters glucose-6-phosphate dehydrogenase trafficking, cell metabolism and oxidant release of maternal neutrophils. J Clin Invest. 2002;110:1801–1811. doi: 10.1172/JCI200215973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segel GB, Cokelet GR, Lichtman MA. The measurement of lymphocyte volume: importance of reference particle deformability and counting solution tonicity. Blood. 1981;57:894–899. [PubMed] [Google Scholar]
- 38.Yasaka T, Mantich NM, Boxer LA, Baehner RL. Functions of human monocyte and lymphocyte subsets obtained by countercurrent centrifugal elutriation: differing functional capacities of human monocyte subsets. J Immunol. 1981;127:1515–1518. [PubMed] [Google Scholar]
- 39.Matyushichev VB, Shamratova VG, Muzafarova DA. Correlation between the quantity of white blood cells and their volume distribution parameters in humans. Human Physiol. 2001;27:110–114. [PubMed] [Google Scholar]
- 40.Pitkin RM, Witte DL. Platelet and leukocyte counts in pregnancy. JAMA. 1979;242:2696–2698. [PubMed] [Google Scholar]
- 41.Balloch AJ, Cauchi MN. Reference ranges for haematology parameters in pregnancy derived from patient populations. Clin Lab Haematol. 1993;15:7–14. doi: 10.1111/j.1365-2257.1993.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 42.Harvery JW, Asquith RL, Pate MG, Kivipelto J, Chen CL, Ott EA. Haematological findings in pregnant, postparturient and nursing mares. Comp Haematol Internat. 1994;4:25–29. [Google Scholar]
- 43.Weber G. Inhibition of human brain pyruvate kinase and hexokinase by phenylalanine and phenylpyruvate: possible relevance to phenylketonuric brain damage. Proc Natl Acad Sci USA. 1969;63:1365–1369. doi: 10.1073/pnas.63.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies SE, Brindle KM. Effects of overexpression of phosphofructokinase on glycolysis in the yeast Saccharomyces cerevisiae. Biochemistry. 1992;31:4729–4735. doi: 10.1021/bi00134a028. [DOI] [PubMed] [Google Scholar]
- 45.Urbano AM, Gillham H, Groner Y, Brindle KM. Effects of overexpression of the liver subunit of 6-phosphofructo-1-kinase on the metabolism of a cultured mammalian cell line. Biochem J. 2000;352(Pt 3):921–927. [PMC free article] [PubMed] [Google Scholar]
- 46.Pearce AK, Crimmins K, Groussac E, Hewlins MJ, Dickinson JR, Francois J, Booth IR, Brown AJ. Pyruvate kinase (Pyk1) levels influence both the rate and direction of carbon flux in yeast under fermentative conditions. Microbiology. 2001;147(Pt 2):391–401. doi: 10.1099/00221287-147-2-391. [DOI] [PubMed] [Google Scholar]
- 47.Moore PA, Bettany AJ, Brown AJ. Multiple copies of the pyruvate kinase gene affect yeast cell growth. J Gen Microbiol. 1990;136:2359–2366. doi: 10.1099/00221287-136-12-2359. [DOI] [PubMed] [Google Scholar]
- 48.Mathews CK, van Holde KE. Biochemistry. Redwood City, CA: Benjamin/Cummings; 1990. [Google Scholar]
- 49.Mathupala SP, Colen CB, Parajuli P, Sloan AE. Lactate and malignant tumors: a therapeutic target at the end stage of glycolysis. J Bioenerg Biomembr. 2007;39:73–77. doi: 10.1007/s10863-006-9062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roos D, Balm AJM. The oxidative metabolism of monocytes. In: Sabarra AJ, Strauss RR, editors. The Reticuloendothelial System: A Comprehensive Treatise. Vol. 2. NY: Plenum Press; 1980. [Google Scholar]
- 51.Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol. 2008;84:949–957. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berman BG, Halperin ML. Effect of insulin on pyruvate metabolism in epididymal adipose tissue of the rat. Correlation of intracellular pyruvate contents and pyruvate dehydrogenase activity. Biochem J. 1973;134:885–889. doi: 10.1042/bj1340885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zima AV, Kockskämper J, Mejia-Alvarez R, Blatter LA. Pyruvate modulates cardiac sarcoplasmic reticulum Ca2+ release in rats via mitochondria-dependent and -independent mechanisms. J Physiol. 2003;550(Pt 3):765–783. doi: 10.1113/jphysiol.2003.040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brogdon JL, Leitenberg D, Bottomly K. The potency of TCR signaling differentially regulates NFATc/p activity and early IL-4 transcription in naïve CD4+ T cells. J Immunol. 2002;168:3825–3832. doi: 10.4049/jimmunol.168.8.3825. [DOI] [PubMed] [Google Scholar]
- 55.Leitenberg D, Bottomly K. Regulation of naïve T cell differentiation by varying the potency of TCR signal transduction. Semin Immunol. 1999;11:283–292. doi: 10.1006/smim.1999.0184. [DOI] [PubMed] [Google Scholar]
- 56.Karlsson A, Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid Redox Signal. 2002;4:49–60. doi: 10.1089/152308602753625852. [DOI] [PubMed] [Google Scholar]
- 57.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 58.Ramanathan S, Masih AK, Ashok U, Arismendy J, Turndorf H. Concentrations of lactate and pyruvate in maternal and neonatal blood with different intravenous fluids used for prehydration before epidural anesthesia. Anesth Analg. 1984;63:69–74. [PubMed] [Google Scholar]
- 59.Aynsley-Green A, Soltesz G, Jenkins PA, Mackenzie IZ. The metabolic and endocrine milieu of the human fetus at 18–21 weeks of gestation. II. Blood glucose, lactate, pyruvate and ketone body concentrations. Biol Neonate. 1985;47:19–25. doi: 10.1159/000242086. [DOI] [PubMed] [Google Scholar]
- 60.Cetin I, de Santis MS, Taricco E, Radaelli T, Teng C, Ronzoni S, Spada E, Milani S, Pardi G. Maternal and fetal amino acid concentrations in normal pregnancies and in pregnancies with gestational diabetes mellitus. Am J Obstet Gynecol. 2005;192:610–617. doi: 10.1016/j.ajog.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Soltesz G, Harris D, Mackenzie IZ, Aynsley-Green A. The metabolic and endocrine milieu of the human fetus and mother at 18–21 weeks of gestation. I. Plasma amino acid concentrations. Pediatr Res. 1985;19:91–93. doi: 10.1203/00006450-198501000-00024. [DOI] [PubMed] [Google Scholar]
- 62.Glew RH, Melah G, El-Nafaty AI, Brandt Y, Morris D, VanderJagt DJ. Plasma and urinary free amino acid concentrations in preeclamptic women in northern Nigeria. Clin Chim Acta. 2004;342:179–185. doi: 10.1016/j.cccn.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 63.Evans RW, Powers RW, Ness RB, Cropcho LJ, Daftary AR, Harger GF, Vergona R, Finegold DN. Maternal and fetal amino acid concentrations and fetal outcomes during pre-eclampsia. Reproduction. 2003;125:785–790. doi: 10.1530/rep.0.1250785. [DOI] [PubMed] [Google Scholar]
- 64.White A, Handler P, Smith EL. Principles of Biochemistry. 5th Ed. NY: McGraw-Hill; 1973. [Google Scholar]
- 65.Larsen TM, Laughlin LT, Holden HM, Rayment I, Reed GH. Structure of rabbit muscle pyruvate kinase complexed with Mn2+, K+, and pyruvate. Biochemistry. 1994;33:6301–6309. doi: 10.1021/bi00186a033. [DOI] [PubMed] [Google Scholar]
- 66.Takada G, Ohtake M, Miyabayashi S, Narisawa K, Tada K. Pyruvate carboxylase activity in lymphoblasts. J Inherit Metab Dis. 1982;5:69–70. doi: 10.1007/BF01799991. [DOI] [PubMed] [Google Scholar]
- 67.Mahiout A, Matata BM, Brunkhorst R. Effect of glucose and pyruvate in acidic and non-acidic peritoneal dialysis fluids on leukocytes cell functions. Kidney Int. 1997;51:860–867. doi: 10.1038/ki.1997.121. [DOI] [PubMed] [Google Scholar]
- 68.Mahiout A, Brunkhorst R. Pyruvate anions neutralize peritoneal dialysate cytotoxicity. Nephrol Dial Transplant. 1995;10:391–394. [PubMed] [Google Scholar]
- 69.Oehler R, Weingartmann G, Manhart N, Salzer U, Meissner M, Schlegel W, Spittler A, Bergmann M, Kandioler D, Oismüller C, Struse HM, Roth E. Polytrauma induces increased expression of pyruvate kinase in neutrophils. Blood. 2000;95:1086–1092. [PubMed] [Google Scholar]
- 70.Gore DC, Jahoor F, Hibbert JM, DeMaria EJ. Lactic acidosis during sepsis is related to increased pyruvate production, not deficits in tissue oxygen. Ann Surg. 1996;224:97–102. doi: 10.1097/00000658-199607000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carboné P, Sobreviela M, Jimenez D, Martínez C, Gonzalez de Agüero R, Pocoví M. Maternal leukocyte metabolism during pregnancy and puerperium, and its relation to fetal growth. Acta Obstet Gynecol Scand. 1992;71:266–272. doi: 10.3109/00016349209021050. [DOI] [PubMed] [Google Scholar]
- 72.Pizzuto R, Paventi G, Atlante A, Passarella S. Pyruvate kinase in pig liver mitochondria. Arch Biochem Biophys. 2010;495:42–48. doi: 10.1016/j.abb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 73.Mazurek S, Grimm H, Boschek CB, Vaupel P, Eigenbrodt E. Pyruvate kinase type M2: a crossroad in the tumor metabolome. Br J Nutr. 2002;87 Suppl 1:S23–S29. [PubMed] [Google Scholar]
- 74.Oggé G, Romero R, Chaiworapongsa T, Gervasi MT, Pacora P, Erez O, Kusanovic JP, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Mittal P, Kim YM, Hassan SS. Leukocytes of pregnant women with small-for-gestational age neonates have a different phenotypic and metabolic activity from those of women with preeclampsia. J Matern Fetal Neonatal Med. 2009 Nov 17; doi: 10.3109/14767050903216033. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim SK, Romero R, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, Mittal P, Erez O, Vaisbuch E, Gotsch F, Pacora P, Yeo L, Gervasi MT, Lamont RF, Yoon BH, Hassan SS. Evidence of changes in the immunophenotype and metabolic characteristics (intracellular reactive oxygen radicals) of fetal, but not maternal, monocytes and granulocytes in the fetal inflammatory response syndrome. J Perinat Med. 2009;37:543–552. doi: 10.1515/JPM.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gervasi MT, Chaiworapongsa T, Naccasha N, Blackwell S, Yoon BH, Maymon E, Romero R. Phenotypic and metabolic characteristics of maternal monocytes and granulocytes in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1124–1129. doi: 10.1067/mob.2001.117681. [DOI] [PubMed] [Google Scholar]
- 77.Gervasi MT, Chaiworapongsa T, Naccasha N, Pacora P, Berman S, Maymon E, Kim JC, Kim YM, Yoshimatsu J, Espinoza J, Romero R. Maternal intravascular inflammation in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:171–175. doi: 10.1080/jmf.11.3.171.175. [DOI] [PubMed] [Google Scholar]
- 76.Zhu A, Romero R, Petty HR. An enzymatic fluorimetric assay for glucose-6-phosphate: Application in an in vitro Warburg-like effect. Anal Biochem. 2009;388:97–101. doi: 10.1016/j.ab.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.