Abstract
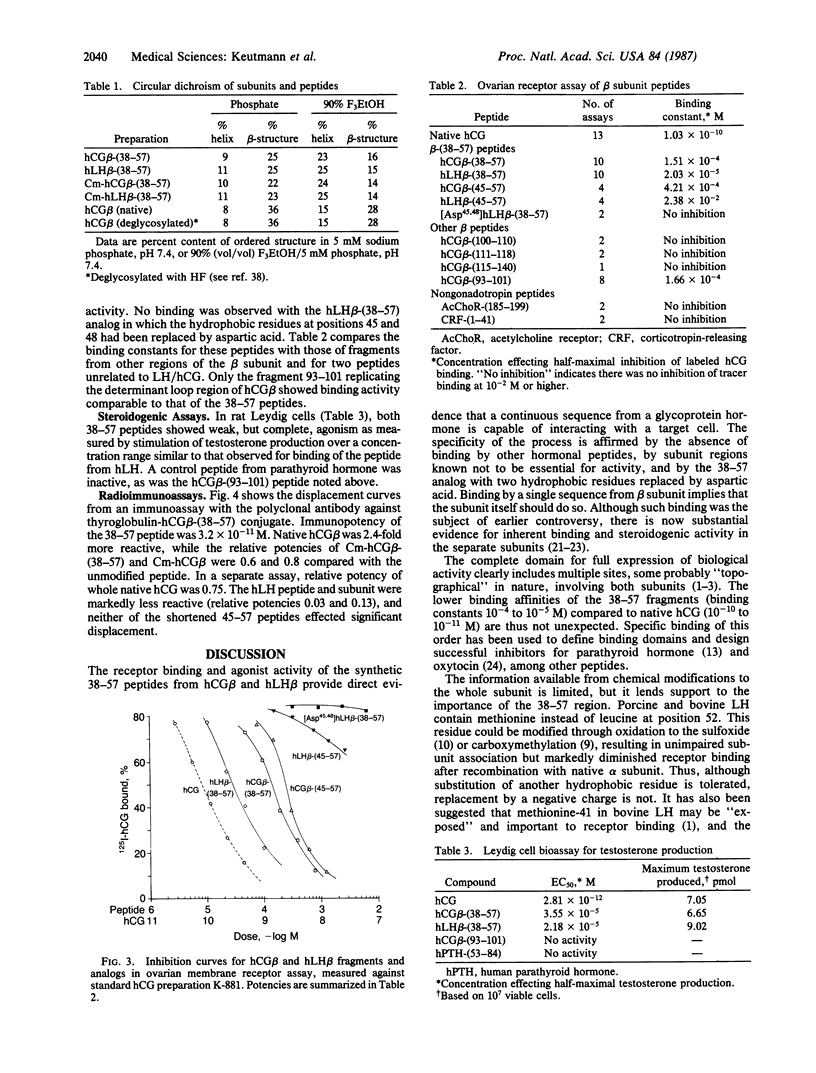
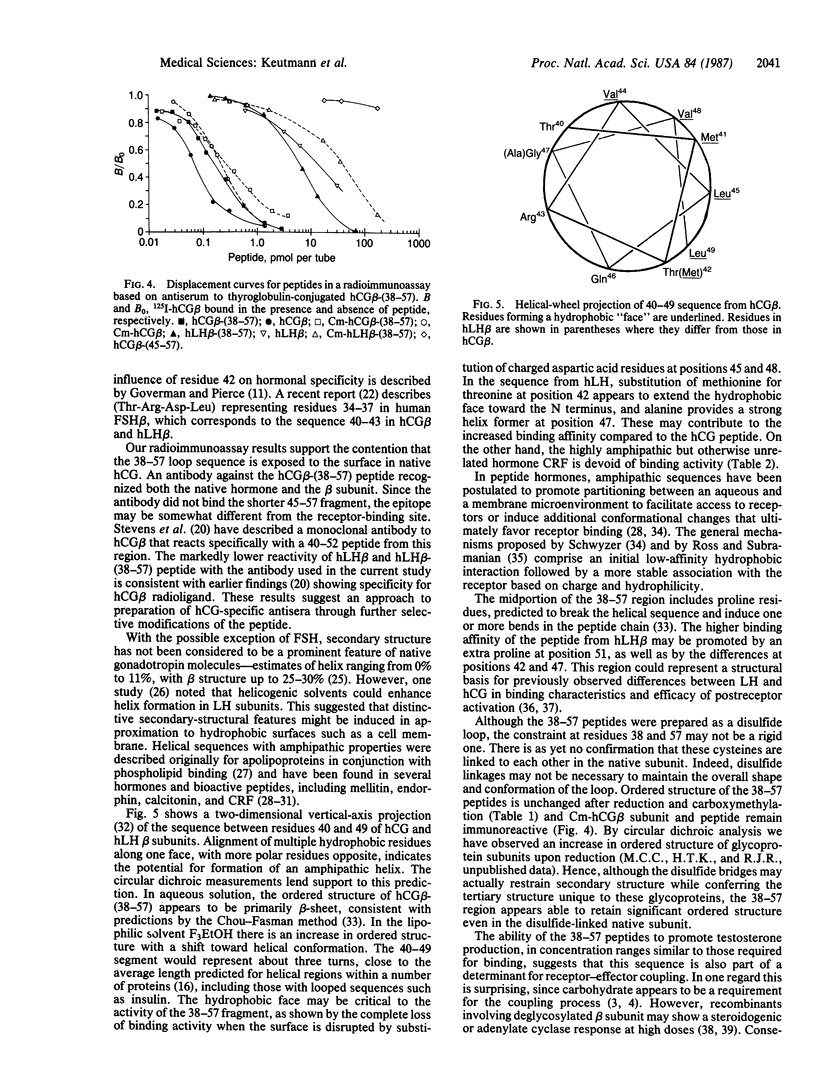
Synthetic fragments have not been widely used thus far to evaluate structure-activity relations in the glycoprotein hormones. We prepared a series of peptides representing the intercysteine "loop" sequence (residues 38-57) in human choriogonadotropin (hCG) and lutropin (hLH) beta subunits, anticipating that it might be oriented toward the surface and accessible to receptors. The peptides were characterized chemically and tested for bioactivity by binding to rat ovarian membrane receptor and stimulation of Leydig cell testosterone production. The hCG beta-(38-57) and hLH beta-(38-57) peptides inhibited binding of 125I-labeled hCG half-maximally at 1.51 X 10(-4) and 2.03 X 10(-5) M, respectively, while other peptide hormones and fragments from elsewhere in the beta subunit were inactive. Both peptides stimulated testosterone production, with half-maximal responses at 3.55 X 10(-5) M (hCG) and 2.18 X 10(-5) M (hLH). By radioimmunoassay with an antibody to thyroglobulin-conjugated hCG beta-(38-57) peptide, native hCG and beta subunit were highly reactive, as were the reduced and carboxymethylated subunit and peptide. Helical-wheel projection predicted an amphipathic region in the N-terminal portion of the 38-57 sequence, and circular dichroic measurements showed an increase in ordered structure, especially alpha-helix, when the 38-57 peptides were transferred from an aqueous to a more lipophilic (90% trifluoroethanol) environment. These results indicate that the 38-57 region of beta subunit is exposed on the surface and constitutes a component in the receptor-binding domain for hCG and hLH. A region of amphipathic-helical structure in the 38-57 sequence may promote hormone-receptor interactions in a manner proposed for several other peptide hormones.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buettner K., Ascoli M. Na+ modulates the affinity of the lutropin/choriogonadotropin receptor. J Biol Chem. 1984 Dec 25;259(24):15078–15084. [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Cheng K. W. Carboxymethylation of methionine residues in bovine pituitary luteinizing hormone and its subunits. Location of specifically modified methionine residues. Biochem J. 1976 Oct 1;159(1):79–87. doi: 10.1042/bj1590079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- De la Llosa P., El Abed A., Roy M. Oxidation of methionine residues in lutropin. Can J Biochem. 1980 Sep;58(9):745–748. doi: 10.1139/o80-105. [DOI] [PubMed] [Google Scholar]
- DeGrado W. F., Musso G. F., Lieber M., Kaiser E. T., Kézdy F. J. Kinetics and mechanism of hemolysis induced by melittin and by a synthetic melittin analogue. Biophys J. 1982 Jan;37(1):329–338. doi: 10.1016/S0006-3495(82)84681-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Pock R., Neubauer A., Catt K. J. In vitro bioassay of LH in human serum: the rat interstitial cell testosterone (RICT) assay. J Clin Endocrinol Metab. 1976 May;42(5):958–969. doi: 10.1210/jcem-42-5-958. [DOI] [PubMed] [Google Scholar]
- Faiman C., Ryan R. J. Radioimmunoassay for human follicle stimulating hormone. J Clin Endocrinol Metab. 1967 Mar;27(3):444–447. doi: 10.1210/jcem-27-3-444. [DOI] [PubMed] [Google Scholar]
- Goverman J. M., Pierce J. G. Differential effects of alkylation of methionine residues on the activities of pituitary thyrotropin and lutropin. J Biol Chem. 1981 Sep 25;256(18):9431–9435. [PubMed] [Google Scholar]
- Grasso P., Crisp T. M. Prolactin-mediated progesterone secretion by cultured rat granulosa cells: regulation by purified human glycoprotein hormones and their subunits. Endocrinology. 1985 Jan;116(1):319–327. doi: 10.1210/endo-116-1-319. [DOI] [PubMed] [Google Scholar]
- Hammonds R. G., Jr, Hammonds A. S., Ling N., Puett D. beta-Endorphin and deletion peptides. A correlation of opiate receptor affinity with helix potential. J Biol Chem. 1982 Mar 25;257(6):2990–2995. [PubMed] [Google Scholar]
- Hilgenfeldt U., Merz W. E., Brossmer R. Circular dichroism of human chorionic gonadotropin. A study of the structural characteristics of the hormone and its subunits under various conditions. Hoppe Seylers Z Physiol Chem. 1974 Aug;355(8):1051–1057. doi: 10.1515/bchm2.1974.355.2.1051. [DOI] [PubMed] [Google Scholar]
- Hruby V. J., Mosberg H. I. Conformational and dynamic considerations in peptide structure-function studies. Peptides. 1982 May-Jun;3(3):329–336. doi: 10.1016/0196-9781(82)90095-x. [DOI] [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Amphiphilic secondary structure: design of peptide hormones. Science. 1984 Jan 20;223(4633):249–255. doi: 10.1126/science.6322295. [DOI] [PubMed] [Google Scholar]
- Kalyan N. K., Bahl O. P. Role of carbohydrate in human chorionic gonadotropin. Effect of deglycosylation on the subunit interaction and on its in vitro and in vivo biological properties. J Biol Chem. 1983 Jan 10;258(1):67–74. [PubMed] [Google Scholar]
- Keutmann H. T., McIlroy P. J., Bergert E. R., Ryan R. J. Chemically deglycosylated human chorionic gonadotropin subunits: characterization and biological properties. Biochemistry. 1983 Jun 21;22(13):3067–3072. doi: 10.1021/bi00282a007. [DOI] [PubMed] [Google Scholar]
- Keutmann H. T., Ratanabanangkoon K., Pierce M. W., Kitzmann K., Ryan R. J. Phosphorylation of human choriogonadotropin. Stoichiometry and sites of phosphate incorporation. J Biol Chem. 1983 Dec 10;258(23):14521–14526. [PubMed] [Google Scholar]
- Lee C. Y., Ryan R. J. Interaction of ovarian receptors with human luteinizing hormone and human chorionic gonadotropin. Biochemistry. 1973 Nov 6;12(23):4609–4615. doi: 10.1021/bi00747a011. [DOI] [PubMed] [Google Scholar]
- Mock E. J., Niswender G. D. Differences in the rates of internalization of 125I-labeled human chorionic gonadotropin, luteinizing hormone, and epidermal growth factor by ovine luteal cells. Endocrinology. 1983 Jul;113(1):259–264. doi: 10.1210/endo-113-1-259. [DOI] [PubMed] [Google Scholar]
- Moudgal N. R., Li C. H. Beta subunits of human choriogonadotropin and ovine lutropin are biologically active. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2500–2503. doi: 10.1073/pnas.79.8.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallai P. V., Mabilia M., Goodman M., Vale W., Rivier J. Structural homology of corticotropin-releasing factor, sauvagine, and urotensin I: circular dichroism and prediction studies. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6770–6774. doi: 10.1073/pnas.80.22.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Ratanabanangkoon K., Keutmann H. T., Kitzmann K., Ryan R. J. Properties of the phosphorylated beta subunit of human choriogonadotropin. J Biol Chem. 1983 Dec 10;258(23):14527–14531. [PubMed] [Google Scholar]
- Rosenblatt M., Segre G. V., Tyler G. A., Shepard G. L., Nussbaum S. R., Potts J. T., Jr Identification of a receptor-binding region in parathyroid hormone. Endocrinology. 1980 Aug;107(2):545–550. doi: 10.1210/endo-107-2-545. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Subramanian S. Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry. 1981 May 26;20(11):3096–3102. doi: 10.1021/bi00514a017. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Morrisett J. D., Gotto A. M., Jr A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 1974 Jan 15;38(3):247–258. doi: 10.1016/0014-5793(74)80064-5. [DOI] [PubMed] [Google Scholar]
- Sluss P. M., Krystek S. R., Jr, Andersen T. T., Melson B. E., Huston J. S., Ridge R., Reichert L. E., Jr Inhibition of iodine-125-labeled human follitropin binding to testicular receptor by epidermal growth factor and synthetic peptides. Biochemistry. 1986 May 6;25(9):2644–2649. doi: 10.1021/bi00357a053. [DOI] [PubMed] [Google Scholar]
- Stevens V. C., Chou W. S., Powell J. E., Lee A. C., Smoot J. The identification of peptide sequences of human chorionic gonadotropin containing a conformational epitope. Immunol Lett. 1986 Jan;12(1):11–18. [PubMed] [Google Scholar]
- Tregear G. W., van Rietschoten J., Sauer R., Niall H. D., Keutmann H. T., Potts J. T., Jr Synthesis, purification, and chemical characterization of the amino-terminal 1-34 fragment of bovine parathyroid hormone synthesized by the solid-phase procedure. Biochemistry. 1977 Jun 28;16(13):2817–2823. doi: 10.1021/bi00632a002. [DOI] [PubMed] [Google Scholar]
- Ward D. N., Reichert L. E., Jr, Liu W. K., Nahm H. S., Hsia J., Lamkin W. M., Jones N. Chemical studies of luteinizing hormone from human and ovine pituitaries. Recent Prog Horm Res. 1973;29:533–561. doi: 10.1016/b978-0-12-571129-6.50018-x. [DOI] [PubMed] [Google Scholar]


