Abstract
In general, Fick's law of diffusion describes membrane permeation of hydrophobic or amphiphilic molecules. In contrast to this, Thomae et al. recently identified the volume ratio between barrier and aqueous compartments as important additional determinants of membrane permeability (Pm) [A.V. Thomae, T. Koch, C. Panse, H. Wunderli-Allenspach, and S.D. Kramer, Comparing the lipid membrane affinity and permeation of drug-like acids: the intriguing effects of cholesterol and charged lipids, Pharm. Res. 24 (2007) 1457–1472.]. This new theory was supported by the striking observation that low concentrations of cholesterol increased Pm of salicylic acid. As Fick's law is of fundamental importance to all membrane transport processes, we reinvestigated this phenomenon. We measured the electrophoretic mobility of vesicles and used electrochemical scanning microscopy to study the adsorption of the SA anion to lipid vesicular bilayers and SA transport through planar lipid bilayers, respectively. As predicted by Fick's law, Pm of SA decreased continuously with increasing cholesterol content. Thomae et al. made the contrasting artifactual observation because their kinetic approach lacked the required time resolution and led to an underestimation of Pm by five orders of magnitude. We conclude that there is nothing beyond Fick's law of diffusion. It is still valid.
Keywords: Weak acids, Scanning electrochemical microscopy, Membrane transport, Planar bilayers
1. Introduction
A variety of lipophilic or amphiphilic drugs enter the cell by simple diffusion through the plasma membrane. According to Overtone's rule, membrane permeability (Pm), and thus, drug influx correlates well with membrane partitioning [1,2]. Intracellular drug accumulation is often opposed by efflux transporters like P-glycoprotein. Since uptake by efflux transporters occurs from the lipid phase, the membrane partition coefficient is an important determinant for drug efflux as well. Predicting the balance between influx and efflux from simple physicochemical parameters of the compounds seems feasible [3] if so-called recognition motifs are taken into account which indicate whether the compound may be a substrate of efflux pumps [4].
Thomae et al. [5] now made an alerting observation. They found that membrane permeation did not depend on membrane affinity; i. e. in their hands membrane permeability was not predictable from the rigidity of the bilayer and the electrostatic interactions of charged drug species. They concluded that Fick's law of diffusion:
| (1) |
where J, kp, D, l and Δc denote flux density, partition coefficient, diffusion coefficient, membrane thickness and transmembrane concentration gradient, respectively, does not sufficiently describe the transport process of weak acids, like salicylic acid (SA). Lipid bilayer permeation appeared considerably more complex because volume ratios between barrier and aqueous compartments had to be considered as determinants of the permeation kinetics.
Key experimental observations alluding to the new theory were the following: tightening a phosphatidylcholine bilayer by insertion of 10% cholesterol doubled Pm of SA whereas higher cholesterol concentrations did not affect Pm. Partition of the salicylic anion (SA−) was not energetically disfavored so that SA−:lipid ratios in the order of 1:1 were reachable.
Since the new theory could revolutionize the current view on membrane transport, we monitored SA transport across lipid membranes by means of electrochemical scanning microscopy and SA− insertion into lipid vesicles by particle electrophoresis. Peculiarities in SA transport would be of special interest because salicylate based compounds are widely used (i) as anti-inflammatory drugs [6] or (ii) for the reversal of diet-induced insulin resistance [7].
2. Materials and methods
2.1. Formation of planar membranes
Solvent free planar lipid bilayers were formed by the monolayer apposition technique [8,9] across an aperture (130–180 μm in diameter) in a Teflon septum (thickness 25 μm) separating two aqueous compartments. The septum was pretreated with a 0.5% solution of hexadecane in hexane. The two aqueous phases contained lipid vesicles at a lipid concentration of 1 mg/ml. After lipid monolayers had formed at the air–water-interface, the buffer solution levels in both compartments were raised above the aperture by syringes [10]. Within the aperture the two monolayers combined spontaneously to a bilayer. The buffer solutions were agitated by magnetic bars. The experiments were carried out at room temperature (21–23 °C).
2.2. Flux measurements
SA transport through membranes is accompanied by chemical reactions in the immediate vicinity [11]. We have monitored proton uptake and release by electrochemical microscopy [12,13]. In brief, scanning pH-sensitive scanning microelectrodes were made of glass capillaries, the tips (1–2 μm in diameter) of which were filled with the hydrogen ionophore II–cocktail A from Fluka (Dreisenhofen, Germany). It contained 6 wt% 4-nonadecylpyridine, 93 wt% 2-nitrophenyl octyl ether, and 1 wt% potassium tetrakis(4-chloropheny)borate. Movement of the electrodes relative to the membrane was realized by a hydraulic stepdrive (Narishige, Japan).
Transmembrane SA flux was derived from fitting an analytical model [2] to the first 50 μm of the experimental pH profiles. This model takes into account all chemical reactions and diffusion processes in the near-membrane unstirred layers [12,13]:
| (2) |
| (3) |
with J, ci and Di being the flux, the concentration of the ith species and the aqueous diffusion coefficient in the cis and trans compartments. The index i denotes the following species: 1 = H+, 2=OH−, 3 = BH (=HEPES), 4 = B−, 5 = SA, 6 = SA−. Ri(c) is the specific local rate of expenditure of the ith species in the chemical reactions:
| (4) |
| (5) |
At the membrane–water interface all fluxes are set to zero (Eq. (6)), except for J5 yielding:
| (6) |
2.3. Membrane concentration of the salicylic anion
Upon binding to large unilamellar vesicles (LUVs), SA− introduces a surface charge to the initially uncharged DPhPC bilayer. In turn, the surface charge increases the electrophoretic mobility of LUVs. The velocity, v, of vesicle movement in an electrical field was measured by a zeta-sizer (model DELSA 440 SX, Coulter Electronics, Inc. Hialeah, FL) with a four-beam electrophoretic laser scattering analyser. It was used to calculate the electrical potential, ζ, at the shear plane. This plane which defines what migrates in the electric field is about 0.2–0.4 nm beyond the charged vesicle surface [14]. The magnitude of the ζ-potential is, thus, less than the surface potential ψs, and it is related to ψs by the Gouy–Chapman theory [15-17].
LUVs were prepared from a thin DPhPC film made by solvent evaporation from a lipid chloroform solution. Buffer addition led to the formation of multilamellar vesicles. Subsequent extrusion [18] via the small-volume apparatus LiposoFast (Avestin Inc., Ottawa, Canada) equipped with filters of 100 nm pore diameter, resulted in unilamellar vesicles. The final lipid concentration was 1 mg/ml.
3. Results
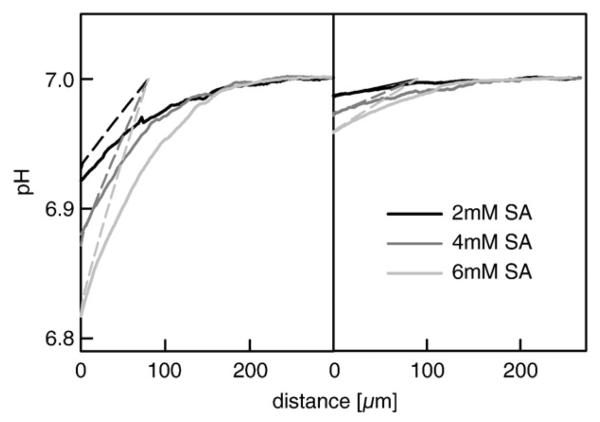
SA was added to the cis compartment of a pure DPhPC bilayer at concentrations of 2, 4 and 6 mM. Its diffusion across the bilayer was indicated by the acidification of the stagnant water layer in the immediate membrane vicinity in the receiving compartment. Larger concentration gradients led to larger SA fluxes, and consequently to more pronounced acidifications (Fig. 1, left panel). Tightening of the lipid bilayer by cholesterol resulted in a less pronounced acidification indicating a decrease of the SA flux (Fig. 1, right panel). According to the analytical model, it corresponds to a drop of Pm from 1.2 cm/s in a cholesterol free membrane to 0.1 cm/s in a membrane that contained 50 mol% cholesterol.
Fig. 1.
pH profiles in the trans unstirred water layer for three different concentrations of sodium salicylate added, as indicated, in the cis side of the chamber. The left panel shows measured profiles for bilayers folded from pure DPhPC, whereas the right one displays profiles for bilayers formed from a mixture of DPhPC and Cholesterol (1:1). The dotted lines represent model simulations for trans side with the following relevant parameter set: pKHepes = 7.5, DHepes = 5.1 × 10−6 cm2s−1, pKSalicylate = 2.75, DSalicylate = 5.5 × 10−6 cm2s−1, kinetic association rates kHepes = 2 × 1010 M−1s and kSalicylate = 5 × 108 M−1s, size of the unstirred layer δ = 90 μm, PDPhPC = 1.2 cm/s and PDPhPC/Chol = 0.1 cm/s, respectively. The bulk solution contained 200 mM NaCl, 3 mM Hepes, 3 mM β-Alanin adjusted to pH = 7.
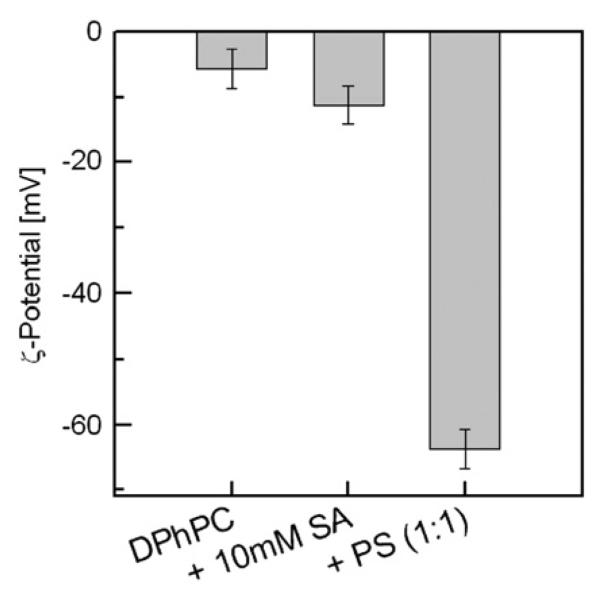
To test the putative partitioning of SA− into bilayers [5], we determined the electrophoretic mobility (the zeta potential, ζ) of vesicles in the presence and absence of SA− (Fig. 2). As a positive control, we compared our result to ζ of vesicles which contained uncharged (DPhPC) and charged lipids (diphytanyl phosphatidylserine, DPhPS) at 1:1 ratio. Pure DPhPC vesicles exhibited a small negative ζ at the polar surface of about −6 mV, which is consistent with previously published data [19]. The surface charge density of DPhPC vesicles in the presence of 10 mM SA increased slightly if compared to DPhPC liposomes in SA free buffer. The decrease of ζ from −6 mV to −12 mV is consistent with the observation that SA− inserted into the bilayer, acts as an uncoupler [20] at physiological pH and moderately increases bilayer conductivity [2,20]. From the corresponding ψs of about −15 mV, a SA−:lipid ratio of about 1:26 is estimated (compare [16]). A 1:1 ratio would have resulted in ζ=−63 mV as in the case of equimolar reconstitution of DPhPS (Fig. 2).
Fig. 2.
Zeta-potential of lipid vesicles for the following conditions: DPhPC + buffer, DPhPC + buffer + 10 mM SA and DPhPC/PS (1:1 lipid ratio) + buffer. The buffering solution contained 50 mM KCl, 2 mM Tris (pH = 7.3) and 0.5 mM EDTA. The final lipid concentration was 1 mg per 1 ml buffer solution.
4. Discussion
We observed that cholesterol decreases Pm of SA by more than one order of magnitude. This finding is in agreement with the expected decrease of D due to an increase in membrane viscosity. However, it contrasts with the invariability of Pm at high cholesterol concentrations reported by Thomae et al. [5]. The reason for the discrepancy between the experimental results becomes clear considering the time resolution of the underlying kinetic experiments. The uptake of SA into lipid vesicles of diameter, d =200 nm [5,21] and Pm = 1.2 cm/s proceeds with an absolute time constant, τ, of:
| (7) |
where V and A denote liposome volume and surface area, respectively. Thomae et al. [5,21] have mixed a suspension with Tb3+-loaded vesicles and an aqueous solution with SA by a conventional stopped flow apparatus. After mixing was completed and after the dead time, τd ~ 1 ms, of the stopped flow device had passed, the light emitted due to Tb3+-ligation by SA was observed [5,21]. Since τd >> τ, the kinetics of SA entry into vesicles cannot be monitored by stopped flow. Even for cholesterol rich membranes, the entry is two orders of magnitude too fast to be observed. Caution is also required when using the Tb3+-ligation method to study peptide permeation processes [22,23]. The conclusions may be equally invalid.
Our steady state measurements agree very well with predictions from Overtone's rule: the SA octanol/water partition coefficient of 300 [21] suggests Pm ~ 1 cm/s [2]. They are also in line with Pm = 0.7 cm/s obtained by monitoring tracer accumulation [11].
Thomae et al. have found that the anion partition coefficient into the bilayer is equal to 7, i.e. it exceeds the one into octanol by at least 350 fold [5]. We have tested this result by measuring the electrophoretic mobility of DPhPC/SA− vesicles. Assuming that the lipid concentration in the bilayer is 1 M, a SA−:lipid ratio of 1:14 was expected. The experiment agreed reasonable well with the prediction. Calculation of the surface charge density revealed that SA− is 26 fold less abundant than the accommodating lipid (Fig. 2). In a certain sense, the term anion partition coefficient is misleading. The charge of the respective molecules is not buried into the membrane interior, but protrudes into the external aqueous solution. Consequently, the anion partition coefficient into the bilayer is useless for the prediction of anion membrane permeability.
In contrast to the membrane partition coefficient, the octanol partition coefficient accounts for the energy that is required to bury the charge into a hydrophobic environment. In agreement with Overtone's rule, the ratio of the octanol partition coefficients of the charged (<0.02) and neutral (300) forms is in reasonable agreement with the ratio of the corresponding membrane permeabilities of, respectively, 4×10−7 cm/s and 1 cm/s (compare also [2]).
Due to electrostatic repulsion of SA−, any increase in membrane surface charge density should have a negative effect on SA permeation. The Gouy–Chapman theory predicts, for example, that the SA− concentration drops by an order of magnitude in the immediate membrane vicinity of our DPhPC/DPhPS vesicles due to their surface potential of −63 mV (Fig. 2). The neglect of these electrostatic effects would result in an underestimation of Pm since Δc is smaller than the difference of the respective bulk concentrations at both sides of the membrane (Eq. (1)). The effect of the charge introduced by 10 mM SA− is less dramatic. Neglect of the increment in surface potential of −6 mV (Fig. 2) would have led to an error in the calculation of Pm well below 20%. It is important to note, that the flux measurements itself are not affected by changes in surface potential. The effect that a charged surface may have on the concentration of adjacent protons at physiological salt concentrations decays within a few nanometers. The scanning microelectrode, however, senses proton concentration in micrometer distance from the membrane.
Since the permeability measurements of Thomae et al. are based on an artefactual approach and since our steady state measurements confirmed that cholesterol decreases SA permeability, we conclude that the criticism of Fick's law [5] is not justified. Weak acid partition follows Overtone's rule and its transmembrane diffusion obeys Fick's law.
Acknowledgments
We thank Quentina Beatty for editorial help. This work was supported by the Austrian Science Fund (FWF W1201-N13) and the Upper Austrian state government (Wi-213970).
References
- 1.Al-Awqati Q. One hundred years of membrane permeability: does Overton still rule? Nat. Cell Biol. 1999;1:E201–E202. doi: 10.1038/70230. [DOI] [PubMed] [Google Scholar]
- 2.Saparov SM, Antonenko YN, Pohl P. A new model of weak acid permeation through membranes revisited: does Overton still rule? Biophys. J. 2006;90:L86–L88. doi: 10.1529/biophysj.106.084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seelig A. The role of size and charge for blood-brain barrier permeation of drugs and fatty acids. J. Mol. Neurosci. 2007;33:32–41. doi: 10.1007/s12031-007-0055-y. [DOI] [PubMed] [Google Scholar]
- 4.Seelig A, Gerebtzoff G. Enhancement of drug absorption by noncharged detergents through membrane and P-glycoprotein binding, Expert Opin. Drug Metab. Toxicol. 2006;2:733–752. doi: 10.1517/17425255.2.5.733. [DOI] [PubMed] [Google Scholar]
- 5.Thomae AV, Koch T, Panse C, Wunderli-Allenspach H, Kramer SD. Comparing the lipid membrane affinity and permeation of drug-like acids: the intriguing effects of cholesterol and charged lipids. Pharm. Res. 2007;24:1457–1472. doi: 10.1007/s11095-007-9263-y. [DOI] [PubMed] [Google Scholar]
- 6.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 7.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 8.Montal M, Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc. Natl. Acad. Sci. U. S. A. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krylov AV, Pohl P, Zeidel ML, Hill WG. Water permeability of asymmetric planar lipid bilayers: leaflets of different composition offer independent and additive resistances to permeation. J. Gen. Physiol. 2001;118:333–340. doi: 10.1085/jgp.118.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pohl P, Saparov SM, Borgnia MJ, Agre P. High selectivity of water channel activity measured by voltage clamp: analysis of planar lipid bilayers reconstituted with purified AqpZ. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9624–9629. doi: 10.1073/pnas.161299398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutknecht J, Tosteson DC. Diffusion of weak acids across lipid bilayer membranes: effects of chemical reactions in the unstirred layers. Science. 1973;182:1258–1261. doi: 10.1126/science.182.4118.1258. [DOI] [PubMed] [Google Scholar]
- 12.Antonenko YN, Denisov GA, Pohl P. Weak acid transport across bilayer lipid membrane in the presence of buffers — theoretical and experimental pH profiles in the unstirred layers. Biophys. J. 1993;64:1701–1710. doi: 10.1016/S0006-3495(93)81542-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonenko YN, Pohl P, Denisov GA. Permeation of ammonia across bilayer lipid membranes studied by ammonium ion selective microelectrodes. Biophys. J. 1997;72:2187–2195. doi: 10.1016/S0006-3495(97)78862-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carnie S, McLaughlin S. Large divalent cations and electrostatic potentials adjacent to membranes. A theoretical calculation. Biophys. J. 1983;44:325–332. doi: 10.1016/S0006-3495(83)84306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkov AG, Deamer DW, Tanelian DL, Markin VS. Electrical double layers at the oil/water interface. Prog. Surf. Sci. 1996;53:1–134. doi: 10.1016/s0079-6816(97)82876-6. [DOI] [PubMed] [Google Scholar]
- 16.Rokitskaya TI, Block M, Antonenko YN, Kotova EA, Pohl P. Photosensitizer binding to lipid bilayers as a precondition for the photoinactivation of membrane channels. Biophys. J. 2000;78:2572–2580. doi: 10.1016/S0006-3495(00)76801-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pohl EE, Peterson U, Sun J, Pohl P. Changes of intrinsic membrane potentials induced by flip–flop of long-chain fatty acids. Biochemistry. 2000;39:1834–1839. doi: 10.1021/bi9919549. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald RC, MacDonald RI, Menco B.Ph.M., Takeshita K, Subbarao NK, Hu LR. Small-volume extrusion apparatus for preparation of large, unilammellar vesicles. Biochim. Biophys. Acta. 1991;1061:297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- 19.Cevc G. Membrane electrostatics. Biochim. Biophys. Acta. 1990;1031:311–382. doi: 10.1016/0304-4157(90)90015-5. [DOI] [PubMed] [Google Scholar]
- 20.Gutknecht J. Salicylates and proton transport through lipid bilayer membranes: a model for salicylate-induced uncoupling and swelling in mitochondria. J. Membr. Biol. 1990;115:253–260. doi: 10.1007/BF01868640. [DOI] [PubMed] [Google Scholar]
- 21.Thomae AV, Wunderli-Allenspach H, Kramer SD. Permeation of aromatic carboxylic acids across lipid bilayers: the pH-partition hypothesis revisited. Biophys. J. 2005;89:1802–1811. doi: 10.1529/biophysj.105.060871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer SD, Wunderli-Allenspach H. No entry for TAT(44–57) into liposomes and intact MDCK cells: novel approach to study membrane permeation of cell-penetrating peptides. Biochim. Biophys. Acta. 2003;1609:161–169. doi: 10.1016/s0005-2736(02)00683-1. [DOI] [PubMed] [Google Scholar]
- 23.Shimanouchi T, Walde P, Gardiner J, Mahajan YR, Seebach D, Thomae A, Kramer SD, Voser M, Kuboi R. Permeation of a beta-heptapeptide derivative across phospholipid bilayers. Biochimica Et Biophysica Acta-Biomembranes. 2007;1768:2726–2736. doi: 10.1016/j.bbamem.2007.07.011. [DOI] [PubMed] [Google Scholar]