Abstract
Recirculation of leukocytes is essential for proper immune responses. However, the molecular mechanisms that regulate leukocyte entry into the lymphatics remain unclear. Here we show that plexin-A1, a primary receptor component for class III and class VI semaphorins, is crucially involved in the entry of dendritic cells (DCs) into the lymphatics. Additionally, we show that Sema3A, but not Sema6C or Sema6D, is required for DC transmigration, and that Sema3A produced by the lymphatics promotes actomyosin contraction at the trailing edge of migrating DCs. These findings not only demonstrate that semaphorin-signals are involved in DC trafficking but also provide a novel mechanism that induces actomyosin contraction as these cells pass through narrow gaps.
INTRODUCTION
Dendritic cells (DCs) are highly mobile antigen-presenting cells that use a coordinated trafficking system to ensure that they are present in the right place and at the right time to induce proper immune responses 1. Before foreign antigens (Ag) encounter, DCs reside as sentinels in the peripheral tissues. However, after Ag exposure, DCs migrate from the peripheral tissues to lymphoid organs, where they activate T cells. To exit the periphery, DCs must migrate towards and enter the initial lymphatics 2,3. This is an active process that involves chemokines and adhesion molecules 4–8. Transmission electron microscopic analyses have shown that DCs must undergo dramatic morphological changes in order to pass through endothelial junctions 9,10. Non-muscle myosin II is required to propel DC cellular bodies forward and pass through narrow gaps 11. However, it is still unknown how DCs interact with the lymphatic endothelium during transmigration and whether the lymphatics, in turn, control and affect DC mobilization to coordinate their cytoskeletal dynamics, including contraction and adhesion disassembly.
Semaphorins were initially identified as axonal guidance cues that determine the direction and migration of neurons during neurogenesis 12. In addition, they have diverse and important functions in other physiological processes, including heart development 13, vascular growth 14, tumor progression 15,16 and immune responses 17. Several semaphorins exhibit co-stimulatory molecule-like activities that promote the activation of B cells 18, T cells 19, DCs 20 and macrophages 21 through cell-cell interactions. In the nervous system, semaphorins regulate cell motility and morphology through interaction with receptors of the plexin family 22. A similar role for semaphorins in regulating the migration of immune cells was proposed. Among the plexin family members, plexin-A1 (official gene symbol, Plxna1) represents the primary receptor for class III and class VI semaphorins. Together with ligand-binding neuropilins (Nrps) plexin-A1 transduces repulsive axon guidance signals for soluble class III semaphorins23. Independently of Nrps, plexin-A1 functions in neurogenesis and cardiogenesis as a receptor for class VI transmembrane semaphorins Sema6C and Sema6D13,24. In the immune system, plexin-A1 is specifically expressed in DCs and it may facilitate DC-mediated T-cell stimulation 25. Plexin-A1-deficient (Plxna1−/−) mice have severe defects in Ag-specific T-cell responses 20. However, it is still unclear whether semaphorins physiologically regulate immune cell migration through plexin receptors.
Here we demonstrate that plexin-A1 has a crucial role in DC trafficking, particularly during entry into lymphatics. Specifically, we show that Sema3A produced in the lymphatics functions as a ligand for the plexin-A1-Nrp-1 receptor complex expressed by DCs. Furthermore, we demonstrate that Sema3A acts on the rear side of DCs, where plexin-A1 is localized. Sema3A induces phosphorylation of the myosin light chain (MLC) to promote actomyosin contraction, resulting in increased DC transmigration as these cells pass through narrow gaps.
RESULTS
Plxna1−/− mice have impaired DC trafficking to the draining LNs
Antigen-specific T cells generation is severely impaired in Plxna1−/− mice 20. However, it is unclear how plexin-A1 is involved in Ag-specific T cell priming. To investigate these mechanisms in greater detail, we activated OT-II T cells 26 in wild-type and Plxna1−/− mice. CFSE-labeled OT-II T-cells proliferated extensively after OVA peptides and CFA were subcutaneously administered to wild-type recipient mice (Fig. 1a). In contrast, Ag-specific proliferative responses were markedly reduced when OT-II T cells were transferred into Plxna1−/− recipient mice (Fig. 1a), confirming the importance of plexin-A1 in Ag-specific T-cell responses (Supplementary Fig. 1a).
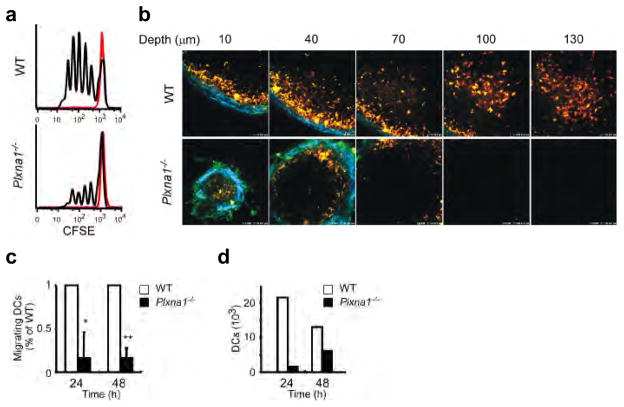
Figure 1. Plxna1−/− mice show impaired T-cell responses due to defects in DC migration into the LNs.
(a) CFSE dilution by CD4+ OT-II T cells intravenously transferred into wild-type or Plxna1−/− mice, subcutaneously injected in the footpads with OVA peptides in CFA. Ag-specific T cell responses were assayed in the draining LNs (black lines) or non-draining LNs (red lines). The data are representative of three independent experiments. (b) Two-photon microscopy imaging of wild-type or Plxna1−/− CMTMR-labeled BMDCs (orange) injected into the footpads of wild-type recipient mice that also received CMFDA-labeled CD4+ OT-II T-cells (green). DC trafficking into the popliteal LNs was observed 24 hours post injection (c) Numbers of wild-type or Plxna1−/− DCs trafficking into the popliteal LNs of wild-type recipient mice following foodpad injections. Donor BMDCs were CFSE-labeled and the following calculation was used: (% of input cells) = (total cell number) x (% of CFSE+ cells)/(input cell number). Mean ± SD, *p<0.01, **p<0.001, by Mann-Whitney’s U test. (d) Absolute numbers of endogenous DCs isolated at the indicated time points the brachial LNs of wild-type and Plxna1−/− mice after epicutanous administration of FITC-isomer to the shoulder skin.
Ag-specific T cells are primed in the draining lymph nodes (LNs) via encounters with Ag-pulsed DCs 27. To examine the impact of Plxna1−/− DCs on DC-T cell interactions in the draining LNs, we injected CMTMR-labeled OVA-pulsed DCs derived from wild-type or Plxna1−/− mice into the footpads of wild-type recipient mice and analyzed the popliteal LNs by two-photon microscopy. When wild-type DCs were injected into the footpads, CMTMR-labeled DCs were abundantly distributed and localized throughout the T cell area of the draining LNs (Fig.1b upper). By contrast, when Plxna1−/− DCs were injected, DCs were only minimally observed in the draining LNs of recipient mice (Fig. 1b lower), indicating that Plxna1−/− DCs had impaired migratory capabilities. To quantitatively compare the in vivo migratory activities of wild-type and Plxna1−/− DCs, we injected CFSE-labeled DCs into the footpads of wild-type mice and examined their arrival in the draining LNs. Compared to wild-type DCs, Plxna1−/− DCs exhibited impaired migratory activities (Fig. 1c, Supplementary Fig. 1b). The migration of endogenous DCs was analyzed under steady-state conditions. The number of CD11c+MHC IIhi and CD11c+CD4−CD8− migratory DC subsets in the skin-draining LNs 6,28 was reduced in Plxna1−/− mice (Supplementary Fig. 1c). Because the expression of plexin-A1 is increased during DC maturation (Supplementary Fig. 2b), we investigated endogenous DC trafficking to the draining LNs under inflammatory conditions. FITC was epicutaneously applied to an area of skin that drains to the brachial LNs and the number of FITC-positive DCs in the draining LNs was determined 29,30. Significantly reduced number of FITC-positive DCs accumulated in brachial LNs in Plxna1−/− mice compared to wild-type mice (Fig. 1d). Collectively, these findings indicate that plexin-A1 is critically involved in DC trafficking and imply that impaired DC migration is the primary reason for defective T cell priming in Plxna1−/− mice.
Plxna1−/− DCs exhibit impaired transmigration
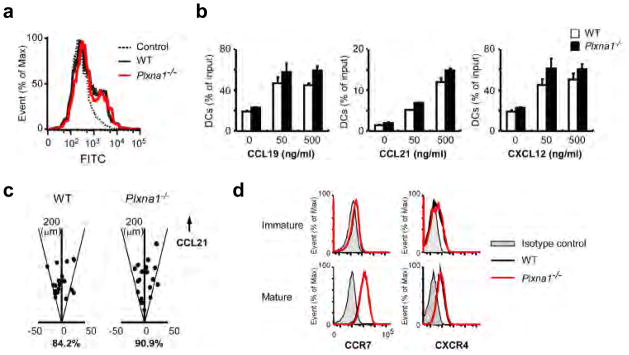
To determine which step during DCs trafficking from he peripheral tissues to the lymphatics3 requires plexin-A1, we studied Ag uptake, interstitial migration toward the lymphatics in response to chemokines, and transmigration across the lymphatics in greater detail (Supplementary Fig. 3). We did not find differences between wild-type and Plxna1−/− DCs in Ag uptake (Fig. 2a). In addition, there were no differences in the ability of DCs to migrate in response to CCL19, CCL21 or CXCL12 in transwell assays (Fig. 2b) or to sense direction in a chemokine gradient assay (Fig. 2c, Supplementary Movie 1). Consistent with these findings, CCR7 and CXCR4 were comparably expressed in wild-type and Plxna1−/− DCs (Fig. 2d). These results indicate that plexin-A1 is not required for Ag uptake or chemokine responsiveness during migration.
Figure 2. FITC-dextran uptake and responses to chemokines are not affected in Plxna1−/− DCs.
(a) FITC-dextran uptake by wild-type and Plxna1−/− BMDCs at 37°C for 30 min. Cells cultured with FITC-dextran on ice were used as controls. (b) Chemotaxis of wild-type and Plxna1−/− DCs toward CCL19, CCL21 or CXCL12 gradients in transwell migration assays (pore size: 5 μm). (c) Directional sensing in wild-type or Plxna1−/− DCs in response to a CCL21 gradient in a Zigmond chamber. Scatter plots show the position of wild-type and Plxna1−/− DCs relative to their original positions after 60 min of chemokine gradient. The percentages of cells that ended up within a 30° arc facing the CCL21 source are shown. (d) CCR7 and CXCR4 expression in wild-type and Plxna1−/− DCs.
To assess the roles of plexin-A1 in the transmigration process in vivo, we adoptively transferred wild-type or Plxna1−/− CFSE-labeled DCs into the dermis of oxazolone-treated mice and assessed the fate of emigrating DCs 7. Large numbers of Plxna1−/− DCs were retained along the LYVE-1-positive lymphatics in the dermis of recipient mice 24 h after adoptive transfer (Fig. 3a), a behavior not observed with wild-type DC, indicating that Plxna1−/− DCs have impaired transmigration across the lymphatics.
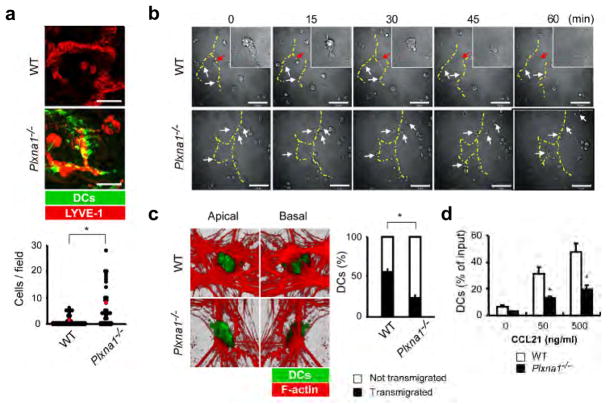
Figure 3. Plxna1−/− DCs exhibit impaired transmigration across the lymphatics.
(a) Upper: Confocal Z-stack imaging of wild-type or Plxna1−/− CFSE-labeled BMDCs (green) intradermally injected into the ear tissues of oxazolone-sensitized mice. Whole-mount staining was performed 24 h post transfer using biotinylated anti-LYVE-1 with streptavidin-Cy3 (red). Scale bars, 50 μm (upper). Lower: Quantification of the number of retained DCs in the fields. Red circles indicate the mean number of cells. *p<0.001, by Mann-Whitney’s U test. (b) Transmigration of wild-type and Plxna1−/− BMDCs across a lymphatic EC monolayer. Interactions between DCs and the lymphatic ECs were recorded every 30 sec by a time-lapse video microscope. The yellow dotted lines show the cellular junctions of the ECs. White arrows indicate DCs that were contacting the lymphatic ECs. Red arrows indicate the transmigration process that was observed in wild-type DCs. Scale bars, 50 μm. (c) Left: Confocal microscopy of wild-type and Plxna1−/− CFSE-labeled DCs added to EC monolayers, incubated for 45 min, fixed, and then stained with Alexa 546-conjugated phalloidin. Images were obtained with an optical section separation (Z-interval) of 0.22 μm. Right: Quantification of DC transmigration determined by confocal microscopy and displayed as percentage of transmigrated DCs relative to the total number of DCs. Mean ± SD. *p<0.001, by Student’s t-test. (d) Chemotaxis of wild-type or Plxna1−/− DC across transwells (pore size: 5 μm) layered with lymphatic EC in response to a CCL21 gradient. Mean ± SD. *p<0.001, by Student’s t test.
By time-lapse imaging we investigated whether plexin-A1 deficiency in DCs affected the initial contacts between DCs and lymphatic epithelial cells (ECs). Wild-type DCs interacted with ECs at the cell-cell junctions of lymphatic ECs and transmigrated across the ECs (Fig. 3b upper, Supplementary Movie 2). Although Plxna1−/− DCs actively moved, extended their dendrites and contacted the lymphatic ECs to the same degree as the wild-type DCs, they did not transmigrate across the lymphatic ECs (Fig. 3b lower, Supplementary Movie 2), To confirm this observation, we added CFSE-labeled DCs onto monolayers of lymphatic ECs stained with the F-actin marker phalloidin and observed these cells by confocal Z-stack imaging. Wild-type DCs were observed from the top to the bottom of the ECs. In contrast, although Plxna1−/− DCs were able to attach to the lymphatic ECs, they could not transmigrate across these cells (Fig. 3c, Supplementary Movie 3). Additionally, Plxna1−/− DCs showed significantly impaired chemokine-induced transmigration across EC monolayers in transwell experiments (Fig. 3d). Taken together, these results strongly suggest that plexin-A1 plays an important role in the transmigration of DCs across the lymphatics.
Sema3A is responsible for plexin-A1-dependent DC trafficking
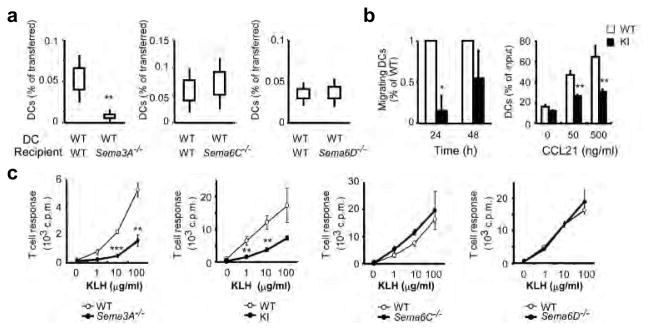
Plexin-A1 is a receptor component for two types of semaphorins: a secreted class III semaphorin, Sema3A, and class VI transmembrane semaphorins, Sema6C and Sema6D (Supplementary Fig. 2a). Notably, Sema3A, Sema6C and Sema6D are all expressed in the lymphatic ECs (Supplementary Fig. 2b). To determine which interaction was responsible for the defects in Plxna1−/− DCs, we adoptively transferred DCs from wild-type mice into Sema3A−/− 31, Sema6C−/− (Supplementary Fig. 4) and Sema6D−/− (Supplementary Fig. 5) mice. DCs from wild-type mice exhibited impaired migration to the draining LNs when transferred into Sema3A−/− recipient mice (Fig. 4a). However, there were no defects in migration of wild-type DCs in Sema6C−/− or Sema6D−/− recipient mice (Fig. 4a). These results suggest that Sema3A in the lymphatics is indispensable for DC trafficking. Consistently, the Sema3A receptor component, Nrp1, was expressed in DCs (Supplementary Fig. 2b). In addition, DCs from Nrp1sema- knock-in mice 32, in which the Sema3A-binding sites are defective, showed impaired trafficking to the draining LNs when transferred into wild-type recipient mice (Fig. 4b left). We confirmed these findings by performing in vitro transmigration assays in a lymphatic EC monolayer and found that DCs from Nrp1sema- knock-in mice displayed impaired transmigration (Fig. 4b right), similar to that of DCs from Plxna1−/− mice (Fig. 3d). Although DCs express Sema6D (Supplementary Fig. 2b), DCs from Sema6D−/− mice did not show any defects (data not shown). These results imply that Nrp1 expression in DCs is required for DC trafficking. Consistent with these results, Sema3A−/− and Nrp1sema- knock-in mice had defects in T-cell priming (Fig. 4c) that were comparable to Plxna1−/− mice (Supplementary Fig. 1a), whereas neither Sema6C−/− nor Sema6D−/− mice had any defects in Ag-specific T-cell priming (Fig. 4c). Collectively, these results indicate that Sema3A, but not Sema6C or Sema6D, is a functional ligand for plexin-A1 during DC trafficking into the LNs.
Figure 4. Sema3A-Nrp1-plexin-A1 interactions are responsible for DC trafficking.
(a) Wild-type DC trafficking in the lymphatics after adoptive transferred into wild-type and Sema3A−/−, Sema6C−/− or Sema6D−/− mice. Data are pooled from three independent experiments. Standard error ± 95% confidence interval, **p<0.01, by Mann-Whitney’s U test. (b) Left: DCs trafficking into the lymphatics following adoptive transferred of wild-type and Nrp1sema--knock-in (KI) DCs into wild-type recipients. Mean ± SE, *p<0.05, by Mann-Whitney’s U test. Right: Chemotaxis of wild-type and Nrp1sema--knock-in (KI) DCs through transwells (pore size: 5 μm) layered with lymphatic ECs in response to a CCL21 gradient. Mean ± SD, **p<0.01, by Student’s t test. (d) In vitro CD4+ T cell proliferation in response to KLH following KLH in CFA immunization of Sema3A−/−, Nrp1sema--knock-in (KI), Sema6C−/−, Sema6D−/− and wild-type mice. Mean ± SD. **p<0.01, ***p<0.001, by Student’s t test.
Sema3A acts on the rear side of DCs
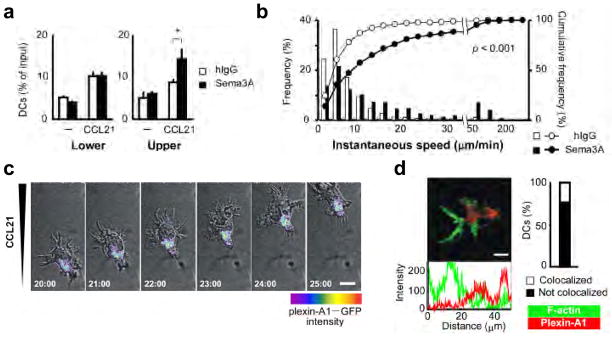
Sema3A was identified as a chemorepellent factor that guides the direction of neurons 12., while here we show it promote DC trafficking. Therefore, we hypothesized that cell polarity generated by chemokines during DC migration is critical for the effects induced by Sema3A. To determine the mechanism by which Sema3A regulates DC migration, we performed chemotaxis assays by adding Sema3A to the lower or upper chambers of transwells in the absence or presence of chemokines. In the absence of chemokines, Sema3A did not show an effect on spontaneous DC migration. By contrast, in the presence of chemokines, DC chemotaxis was enhanced in transwell assays when Sema3A was added to the upper chamber, where Sema3A acted on the rear side of DCs, but not to the lower chamber (Fig. 5a). In two-dimensional directional migration assays evaluated by EZ-TAXIScan, Sema3A increased the motilities and velocities of DCs when it was applied against a chemokine gradient (Fig. 5b, Supplementary Movie 4). Consistent with these findings, plexin-A1 localized at the trailing edge but not at the leading edge of migrating DCs, where actin polymerization was readily observed (Fig. 5c,d, Supplementary Movie 5). These results suggest that the effects of Sema3A depend on the polarity of migrating DCs.
Figure 5. Sema3A acts on the rear side of DCs.
(a) Chemotaxis of DCs in the presence of human IgG or recombinant Sema3A proteins in the lower (left) or upper (right) chambers of transwells, while CCL21 was absent or present in the lower chambers. Mean ± SD. *p<0.01, by Student’s t test. (b) DC velocities in two-dimensional DC chemotaxis assays using EZ-TAXIScan in which Sema3A or human IgG was added to the opposite side of the CCL21. The frequency distribution (bar chart) and cumulative frequency distribution (line chart) of the instantaneous speed were determined. p<0.001, by Mann-Whitney’s U test. Data are representative of three independent experiments. (c) Confocal time-lapse video-microscopy of plexin-A1-EGFP expressing BMDCs treated with LPS, suspended in type I collagen gels and placed into a Zigmond chamber with chemokine gradients. DC locomotion was examined at 1-min intervals. (d) Left: Confocal Z-stack imaging showing localization and intensity of plexin-A1 (anti-plexin-A1 polyclonal antibody plus anti-rabbit IgG-Cy3-red) and F-actin (Alexa 488-conjugated phalloidin-green) in DC. Scale bars, 10 μm. Right: Percentage of cells with no co-localization of signals. Data are representative of three independent experiments.
Sema3A induces actomyosin contraction through myosin II activity
Myosin II, which is regulated by phosphorylation of MLC 33 and ROCK, is involved in DC trafficking 11. The localization of these molecules resembles that of plexin-A1 (Fig. 5c, Supplementary Movie 5) 11. Myosin II is believed to be required to squeeze the cell body and induce actomyosin contraction when cells pass through narrow gaps or constricted areas 11. In addition, myosin II is implicated in Sema3A-mediated axon retraction 34,35. Considering these findings, we investigated whether Sema3A affects the function of myosin II in DCs during their mobilization. As expected, MLC phosphorylation was enhanced in DCs that were stimulated with recombinant Sema3A (Fig. 6a), and this effect was not observed in Plxna1−/− DCs (Supplementary Fig. 6), indicating that Sema3A promotes MLC phosphorylation through plexin-A1. In 3D collagen matrices, which have been used to model passage through constricted areas in vitro, Sema3A increased the velocities of DCs and the motile DC fraction. (Fig. 6b, Supplementary Movie 6) and significantly enhanced DC transmigration, and this effect was abolished in Plxna1−/− DCs (Fig. 6c). In addition, Sema3A-induced DC transmigration in collagen matrices (Fig. 6d left) or across lymphatic EC monolayers (Fig. 6d right) was abolished when the DCs were treated with either a myosin II inhibitor, blebbistatin, or a ROCK inhibitor, Y-27632. Collectively, these results suggest that Sema3A induces actomyosin contraction in DCs during plexin-A1-mediated transmigration (Supplementary Fig. 7).
Figure 6. Sema3A induces phosphorylation of MLC and promotes actomyosin contraction.
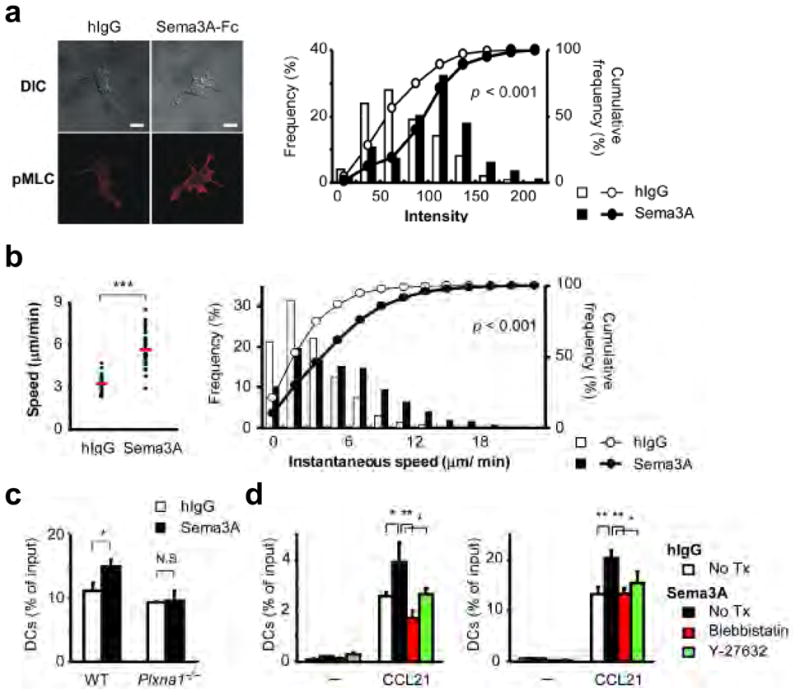
(a) Left: Confocal Z-stack imaging of DCs on fibronectin-coated coverslips treated with human IgG or Sema3A-Fc and stained with an anti-pMLC antibody plus an anti-rabbit-IgG-Cy3 (lower). Eight Z-stack images with an optical section separation (Z-interval) of 0.36 μm were were projected onto one single image. Scale bars, 10 μm (left). Right: Relative frequency distribution (bar chart) and cumulative frequency distribution (line chart) of the average intensities of dendrite regions in DCs that were stimulated with human IgG or Sema3A-Fc. p<0.001, by Mann-Whitney’s U test. Data are representative of three independent experiments. (b) Mean velocities (left) and relative frequency distribution (bar chart) or cumulative frequency distribution (line chart) of the instantaneous speed (right) of a single DC in response to chemokines in the presence of human IgG or Sema3A-Fc in type I collagen matrices analyzed by time-lapse microscopy imaging and MetaMorph software. ***p<0.001, by Mann-Whitney’s U test. Data are representative of three independent experiments. (c) Chemotaxis of wild-type or Plxna1−/− DCs in response to CCL21 in the presence of human IgG or Sema3A-Fc. DCs were added to the upper chambers of transwell assays with type I collagen. An overall difference between the groups was determined by one-way ANOVA. Post hoc multiple comparisons were made using Tukey’s test. *p<0.05. (d) Chemotaxis of DCs treated with 50 μM blebbistatin or 30 μM Y-27632 for 30 min at 37°C and added to the upper chambers of transwells (pore size: 5 μm) layered with 3 mg/ml type I collagen (left) and a HMVEC-dLy monolayer (right) in response to CCL21 in the presence of human IgG or Sema3A-Fc in the upper chambers. An overall difference between the groups was determined by one-way ANOVA. Post hoc multiple comparisons were made using Tukey’s test. *p<0.05, **p<0.01.
DISCUSSION
Although semaphorins were originally identified as axonal guidance cues that regulate cell motility and morphology, accumulating evidence indicates that they also function as immune-regulatory molecules. To date, most functional studies on semaphorins and their receptors have focused on their co-stimulatory effects on immune cells 17. Although the nervous and immune systems have considerable crosstalk and overlap in their molecular repertories and machineries 36, it is still unknown whether semaphorins function as guidance cues that physiologically regulate immune cell movement. Here we demonstrate that semaphorin signals are crucial for DC trafficking, particularly for the entry of DCs into the lymphatics. Furthermore, we highlighted a novel mechanism for DC transmigration across the lymphatics, in which Sema3A promotes actomyosin contraction at the trailing edge of migrating DCs so they can pass through narrow gaps.
To exit peripheral tissues, DCs must migrate towards and enter the initial lymphatics. Although it has been assumed that the migration of DCs into the lymphatics is an indolent process, we provide evidence that Sema3A expressed in lymphatics is crucially involved in DC transmigration. Furthermore, we found that plexin-A1 localizes at the trailing edge of migrating DCs, which is responsible for actomyosin contraction. Numerous factors, including chemokines 6,8, inflammatory molecules 5,37,38 and adhesion molecules 7, have been reported to participate in DC transmigration. However, in our study, neither chemokine responsiveness nor the expression of chemokine receptors was affected in the absence of plexin-A1. The adhesion of DCs to extracellular matrix (ECM) proteins or to lymphatic ECs was comparable between wild-type and Plxna1−/− DCs and the expression levels of integrins were similar between wild-type and Plxna1−/− DCs (data not shown). In addition, there were no differences in the secretion of pro-MMP9 and TNF-α between wild-type and Plxna1−/− DCs (data not shown). Thus, these results not only indicate that DC transmigration is regulated by active mechanisms but also provide a novel mechanism for this process.
Plexin-A1 is a primary receptor component not only for soluble semaphorin Sema3A, in association with the Nrp1 receptor, but also for transmembrane-type semaphorins Sema6C and Sema6D. We here determine that Sema3A produced in the lymphatics functions as a ligand for the plexin-A1-Nrp1 receptor complex expressed in DCs, indicating that the Sema3A-Nrp1-plexin-A1 pathways play important roles in DC migration by mediating interactions between DCs and lymphatic ECs. However, it is still unclear why Sema3A, but not Sema6C or Sema6D, is involved in this process. One possible explanation is that signaling downstream plexin-A1 is modified by the presence of Nrp1 in the receptor. Alternatively, differential effect may be due to the fact that Sema3A is a soluble protein. In contrast, Sema6C and Sema6D are membrane-bound semaphorins, which may prevent them from functioning at critical interaction sites between transmigrating DCs and ECs.
Members of the Rho family of small GTPases, Rac1, Cdc42 and RhoA, regulate cell movement by altering actin assembly, adhesion and actomyosin contraction 39. Among these molecules, Rac1 is required to generate actin-rich lamellipodial protrusions and integrin-mediated adhesion 40. In contrast, RhoA activates ROCK and subsequently activates non-muscle myosin II, which promotes an actomyosin contractile force 41. Mesenchymal cell movement depends on integrin-mediated traction forces. In contrast, amoeboid cell movement, particularly in three-dimensional environments does not require integrins,. Instead, ROCK and myosin II- dependent contraction, is crucially required for passage through narrow gaps 11. Consistent with this, we found that Sema3A promoted actomyosin contraction by inducing MLC phosphorylation. Furthermore, this effect was attenuated by blocking ROCK activity, indicating that the Sema3A-induced effects on myosin II activity require RhoA-ROCK-mediated signals. In neurons, Sema3A induces the local translation of RhoA in neuronal dendrites 42, and siRNA-mediated knockdown of RhoA blocked Sema3A-mediated growth cone collapse 43. In addition, Sema3A induces MLC phosphorylation, and inhibition of myosin II activity blocks Sema3A-mediated axon retraction 34,35. These findings indicate that Sema3A-mediated signals promote actomyosin contractile force through RhoA-dependent myosin II activation in both immune and neuronal cells.
DCs have to pass through different environments. In tissues such as fibroblastic reticular tissues and inner vessel walls, DCs use integrin-mediated attachment and contractile force for cell movement. By contrast, in constricted areas, DCs use myosin II-mediated actomyosin contractile force to move forward because such tissues confine and mechanically anchor cell bodies 44,45. During DC transmigration, at least three sequential mechanisms may be required. First, DCs have to form a lamellipodial protrusion at the leading edge in response to chemokines, which are the driving signal for forward movement. Second, DCs must contract and squeeze their bodies by actomyosin contraction to pass through narrow gaps. Third, after DCs are exposed to the lumenal side, the trailing edge has to detach from ECs in order for these cells to enter the circulation. Here, we show that Sema3A acts on the rear side of DCs through plexin-A1 to promote DC migration. These findings indicate that Sema3A-mediated signals are involved not only in actomyosin contraction but also in disassembling adhesive components at the trailing edge during DC transmigration. Indeed, myosin II promotes a traction stress that facilitates detachment at the trailing edge 46,47, which suggests that detachment can be induced by Sema3A-mediated actomyosin contraction. On the other hand, Sema3A can inhibit integrin-mediated adhesion by inducing the sequestration of phosphatidylinositol phosphate kinase type I isoform PIPKIγ661 from talin, a major component of focal adhesion 48. In this context, it is plausible that Sema3A plays dual or integral roles in regulating actomyosin contraction and adhesion disassembly at the trailing edge during the course of DC transmigration.
In conclusion, our study not only shows the importance of Sema3A-mediated signal in DC trafficking, particularly in the passage through the lymphatics, but also provides a novel mechanism that promotes actomyosin contraction at the trailing edge of migrating cells. Since semaphorins are also expressed in vascular endothelial cells 14,16, it is plausible that they play a role in leukocyte extravasation or cancer metastasis. Additional detailed studies are required to gain insight into these mechanisms which have the potential to regulate immune reactions in order to treat autoimmune, allergic and infectious diseases and inhibit cancer metastasis.
Supplementary Material
Acknowledgments
We thank D. D. Ginty and A. L. Kolodkin for the Neuropilin-1 mutant mice, and T. Yazawa for technical support. This study was supported by research grants from JSPS Research Fellowships for Young Scientists (H.T.), NIH grant RO1NS065048 (Y.Y.), the Ministry of Education, Culture, Sports, Science and Technology of Japan, grants-in-aid from the Ministry of Health, Labour and Welfare, the program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (A.K.), the Target Protein Research Program of the Japan Science and Technology Agency (T.T and A.K.), Uehara memorial foundation (A.K.) and Takeda Scientific Foundation (T.T. N. T. and A.K.). The authors have no conflicting financial interests.
Footnotes
AUTHOR CONTRIBUTIONS
A.K. and H.T. designed the study and wrote the manuscript. H.T. performed most experiments and analysed the data with N.Y., T.O., M.M., S.J. and S.N.. N.T. produced Sema6D−/− mice, recombinant Sema6D protein and anti-Sema6D antibody. M.T. performed two-photon microscopic experiments. M.T. produced Sema3A−/− mice. R.H.F., H.R. and M. TL. produced Sema6C−/− mice. Y.Y. produced anti-plexin-A1 antibody. T.T. performed histological analysis. Y.N., I.K., T.T. and H.K. provided critical collaborative suggestion to promote this study.
References
- 1.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–26. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–28. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–42. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forster R, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 5.MartIn-Fontecha A, et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–21. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohl L, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–88. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Johnson LA, et al. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–77. doi: 10.1084/jem.20051759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabashima K, et al. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am J Pathol. 2007;171:1249–57. doi: 10.2353/ajpath.2007.070225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoitzner P, Pfaller K, Stossel H, Romani N. A close-up view of migrating Langerhans cells in the skin. J Invest Dermatol. 2002;118:117–25. doi: 10.1046/j.0022-202x.2001.01631.x. [DOI] [PubMed] [Google Scholar]
- 10.Baluk P, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–62. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lammermann T, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–5. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 12.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–99. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 13.Toyofuku T, et al. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435–47. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serini G, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–7. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- 15.Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632–45. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- 16.Capparuccia L, Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment--two sides of a coin. J Cell Sci. 2009;122:1723–36. doi: 10.1242/jcs.030197. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- 18.Kumanogoh A, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13:621–31. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- 19.Kumanogoh A, et al. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity. 2005;22:305–16. doi: 10.1016/j.immuni.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Takegahara N, et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol. 2006;8:615–22. doi: 10.1038/ncb1416. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki K, et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature. 2007;446:680–4. doi: 10.1038/nature05652. [DOI] [PubMed] [Google Scholar]
- 22.Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi T, et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida Y, Han B, Mendelsohn M, Jessell TM. PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron. 2006;52:775–88. doi: 10.1016/j.neuron.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong AW, et al. CIITA-regulated plexin-A1 affects T-cell-dendritic cell interactions. Nat Immunol. 2003;4:891–8. doi: 10.1038/ni960. [DOI] [PubMed] [Google Scholar]
- 26.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 27.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–9. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 28.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 29.Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987;166:1654–67. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angeli V, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24:203–15. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Taniguchi M, et al. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron. 1997;19:519–30. doi: 10.1016/s0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
- 32.Gu C, et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45–57. doi: 10.1016/s1534-5807(03)00169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol. 2007;17:178–86. doi: 10.1016/j.tcb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Gallo G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J Cell Sci. 2006;119:3413–23. doi: 10.1242/jcs.03084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown JA, Wysolmerski RB, Bridgman PC. Dorsal root ganglion neurons react to semaphorin 3A application through a biphasic response that requires multiple myosin II isoforms. Mol Biol Cell. 2009;20:1167–79. doi: 10.1091/mbc.E08-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Cumberbatch M, Kimber I. Tumour necrosis factor-alpha is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology. 1995;84:31–5. [PMC free article] [PubMed] [Google Scholar]
- 38.Ratzinger G, et al. Matrix metalloproteinases 9 and 2 are necessary for the migration of Langerhans cells and dermal dendritic cells from human and murine skin. J Immunol. 2002;168:4361–71. doi: 10.4049/jimmunol.168.9.4361. [DOI] [PubMed] [Google Scholar]
- 39.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–69. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 40.Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–21. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- 41.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–90. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu KY, et al. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–4. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hengst U, Jaffrey SR. Function and translational regulation of mRNA in developing axons. Semin Cell Dev Biol. 2007;18:209–15. doi: 10.1016/j.semcdb.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–9. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 45.Lammermann T, Sixt M. Mechanical modes of ‘amoeboid’ cell migration. Curr Opin Cell Biol. 2009;21:636–44. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Lombardi ML, Knecht DA, Dembo M, Lee J. Traction force microscopy in Dictyostelium reveals distinct roles for myosin II motor and actin-crosslinking activity in polarized cell movement. J Cell Sci. 2007;120:1624–34. doi: 10.1242/jcs.002527. [DOI] [PubMed] [Google Scholar]
- 47.Smith LA, Aranda-Espinoza H, Haun JB, Dembo M, Hammer DA. Neutrophil traction stresses are concentrated in the uropod during migration. Biophys J. 2007;92:L58–60. doi: 10.1529/biophysj.106.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toyofuku T, et al. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci. 2005;8:1712–9. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.