Abstract
(-)-Epigallocatechin-3-gallate (EGCG), has been shown to have cancer preventive activity in vitro and in vivo. We have previously shown that EGCG can undergo conjugation to cysteine to form 2′-cysteinyl-EGCG and 2″-cysteinyl-EGCG. Studies of thiol-conjugated metabolites of methamphetamine indicate that such metabolites are not detoxified, but retain biological activity. Here, we examined the growth inhibitory, pro-oxidant, and anti-inflammatory activities of the cysteine metabolites of EGCG. Both compounds dose-dependently inhibited the growth of colon cancer and intestinal cell lines. Both metabolites prevented aberrant arachidonic acid release and nitric oxide production by lipopolysaccharide-stimulated RAW264.7 cells. Under cell culture conditions, 2″-cysteinyl-EGCG produced H2O2 at a faster rate than EGCG. The results of the present study show that cysteine conjugates of EGCG retain the growth inhibitory, anti-inflammatory, and pro-oxidant activities of EGCG in vitro, and may play a role in disease prevention in vivo. These results remain to be confirmed in vivo.
Keywords: green tea, (-)-epigallocatechin-3-gallate, oxidative stress, Phase II metabolism
Introduction
(-)-Epigallocatechin-3-gallate (EGCG) is the most abundant polyphenol in green tea (Camellia sinensis, Theaceae) and has been extensively studied for its chemopreventive effects in in vitro and animal models of carcinogenesis (reviewed in (1, 2)). Based on studies in cancer cell lines and cell-free systems, a number of potential mechanisms of action have been suggested including: inhibition of growth factor signaling, enhancement of antioxidant activity and Phase II metabolism, inhibition of key cellular enzymes (3, 4). More recently there have been reports demonstrating the potential of EGCG to generate oxidative stress in vitro: a more limited number of studies have shown that EGCG can also induce oxidative stress in vivo (5, 6).
Under cell culture conditions, EGCG undergoes auto-oxidation resulting in the formation of dimeric and oligomeric polyphenol oxidation products and hydrogen peroxide (7). Hong et al., have reported that incubation of EGCG in McCoy’s 5 medium at 37°C results in the formation of an approximately 0.5 molar equivalent of hydrogen peroxide (8). These in vitro oxidative effects have been shown to underlie at least some of the biological activity of EGCG. For example, treatment of KYSE150 human esophageal cancer cells with EGCG resulted in decreased levels of epidermal growth factor receptor phosphorylation and protein levels (5). Inclusion of superoxide dismutase, which stabilizes EGCG and prevents is pro-oxidative effects, blocked these effects on epidermal growth factor. Elbling et al., have reported that treatment of RAW264.7 murine macrophage cells and HL-60 human promyelocytic leukemia cells with EGCG results in the formation of hydrogen peroxide and induction of DNA damage (9).
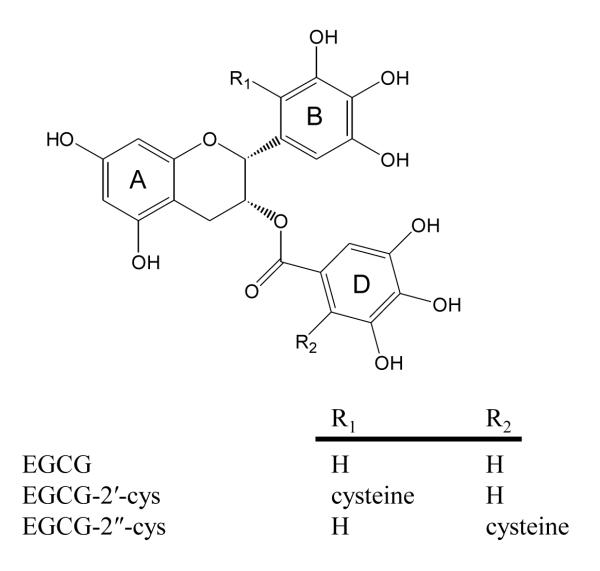
EGCG undergoes extensive biotransformation in vivo resulting in the formation of glucuronidated, sulfated, and methylated metabolites (10, 11). In addition, EGCG undergoes ring-fission metabolism catalyzed by colonic microflora to form the valerolactone-containing metabolites: (-)-5-(3′,4′, 5′- trihydroxyphenyl)-γ-valerolactone (M4) and (-)-5-(3′, 5′- dihydroxyphenyl)-γ-valerolactone (12, 13). More recently, we have demonstrated that oral or intraperitoneal administration of EGCG to mice results in the formation of two cysteine metabolites: 2′-cysteinyl-EGCG and 2″-cysteinyl-EGCG (14, 15). It is hypothesized that these metabolites form after the oxidation of EGCG to a quinone, which results in the activation of the 2′- or 2″- carbons of the B- and D-ring, respectively. Subsequently, a Michael-type reaction occurs between these activated carbons and the thiol group of cysteine. Chemical synthesis of cysteine and other thiolconjugates of EGCG, and studies in cell culture, seem to support this proposed mechanism (14, 16).
It is generally accepted that Phase II metabolism leads to the decreased bioavailability and inactivation of biologically active molecules (17). This appears to be the case for methylated metabolites of EGCG, which have significantly reduced activity to induce tumor cell death, inhibit catechol-O-methyltransferase activity, and inhibit DNA methyltransferase (18-20). Similarly, the microbial metabolite M4 has significantly reduced growth inhibitory activity than EGCG (21). Previous reports with 3,4-methylenedioxymethamphetamine (MDMA), however, have demonstrated that conjugation of catechol metabolites to glutathione or N-acetylcysteine results in the formation of a highly neurotoxic species that is able to redox cycle and may contribute to the neurotoxic effects of MDMA observed in vivo (22, 23).
Since we have demonstrated that structurally analogous metabolites of EGCG are formed following oral administration of high doses of EGCG, we sought to determine whether these EGCG-cysteine metabolites have the potential to contribute to the pool of biologically-active EGCG metabolites. In the present report, we describe the in vitro anti-cancer, anti-inflammatory, and pro-oxidant effects of the two major EGCG-cysteine metabolites, 2′-cysteinyl-EGCG and 2″-cysteinyl-EGCG.
Materials and Methods
Chemicals
EGCG (99% pure) was provided by Mitsui Norin Co. Ltd. (Tokyo, Japan). EGCG cysteine conjugates were prepared as previously described (24). Stock solutions (100 mM) of EGCG and EGCG cysteine conjugates were prepared in DMSO. [5,6,8,9,11,12,14,15-3H](N) arachidonic acid was purchased from NEN Life Science (Boston, MA, USA). All other reagents were of the highest grade available.
Cell Culture
All cell lines were purchased from ATCC (Manassas, VA). HT-29 and HCT-116 human colon cancer cells were maintained in log-phase growth in McCoy’s 5A medium. RAW264.7 murine macrophages were maintained in log-phase growth in Dulbecco’s modified Eagle’s Medium. INT-407 immortalized human intestinal epithelial cells and IEC-6 immortalized rat intestinal epithelial cells were maintained in glutamine-free Basal Medium Eagle (containing Earle’s salts). All medium was supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 0.1 mg/mL streptomycin.
Growth Inhibition
To determine the growth inhibitory activity of EGCG cysteine conjugates cell lines were plated in 96-well plates (10 × 103 cells per well) and allowed to attach for 24 h. The medium was replaced with fresh, serum-free medium containing 0 – 40 μM of 2′-cysteinyl-EGCG or 2″-cysteinyl-EGCG. Cells were incubated for 48 h at 37°C. The medium was removed, the cells were washed once with fresh serum-complete medium to remove residual test compound, and growth inhibition was determined using the 3,[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay (25).
Inhibition of Arachidonic Acid release and NO production
The ability of EGCG-cysteine conjugates to inhibit the release of arachidonic acid and production of NO by lipopolysaccharide (LPS)-stimulated murine macrophages was determined using previously described methods (26). In brief, to determine arachidonic acid release, RAW264.7 cells were incubated overnight with 0.1 μCi/mL [5,6,8,9,11,12,14,15-3H](N) arachidonic acid to allow membrane incorporation. Cells were then washed with PBS containing 0.1% bovine serum albumin. Cells were then stimulated with 2 μg/mL LPS for 1 h, the cells were washed, and fresh medium containing EGCG-cysteine conjugates (0 or 20 μM) was added. Following 18 h incubation, radioactivity in the medium was determined by scintillation counting. To determine inhibition of NO formation, cells were stimulated and treated with test compounds as above. NO levels were determined by measuring nitrite production spectrophotometrically using the Greiss reagent (27).
H2O2 Determination
EGCG or 2″-cysteinyl-EGCG (50 μM) was incubated in McCoy’s 5A at 37°C under 5% CO2 atmosphere. At different time points, samples were collected and the concentration of H2O2 in the medium was analyzed using an Amplex Red Hydrogen Peroxide assay kit (Molecular Probes, Eugene, OR) with slight modifications (28).
Statistical Analysis
All data are expressed as the mean of 3 – 18 determinations (depending on the experiment). Error bars represent the standard deviation. Effects of EGCG-cysteine conjugates on NO production and arachidonic acid release compared to LPS-stimulated control cells were tested using the Student’s t test. Differences in the rate of H2O2 production as a function of time were testing by two-way ANOVA with Bonferroni Post-test. Significance was achieved at p < 0.05.
Results and Discussion
EGCG has been shown to have growth inhibitory and pro-apoptotic activity against a number of human cancer cell lines (reviewed in (3, 4)). These effects have been shown to occur via a number of mechanisms including induction of oxidative stress. In vivo, EGCG has been shown undergo extensive Phase II metabolism resulting in the formation of methylated, glucuronidated, and sulfated metabolites, as well as cysteine conjugates (10, 24). Previous studies with glutathione conjugates of MDMA have shown that these metabolites retain the neurotoxic effects of the parent compounds, and have the ability to undergo redox cycling and induce oxidative stress (22). Here we investigated the in vitro anticancer, anti-inflammatory, and pro-oxidative effects of analogous metabolites of EGCG: 2′-cysteinyl-EGCG and 2″-cysteinyl-EGCG.
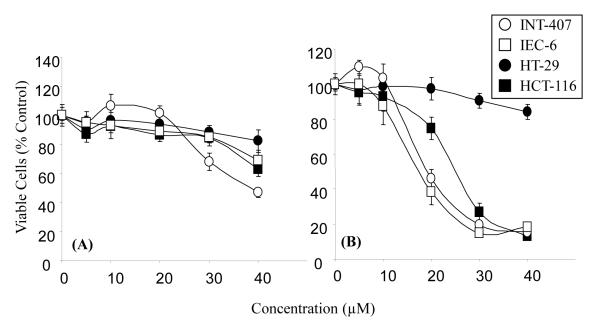
The growth inhibitory effects of both 2′-cysteinyl-EGCG (Fig. 2A) and 2″-cysteinyl-EGCG (Fig. 2B) against HT-29 and HCT-116 human colon cancer cells, as well as INT-407A and IEC-6 immortalized intestinal cells, were determined using the MTT assay. Both compounds dose-dependently inhibited the growth of all cell lines tested. 2″-Cysteinyl-EGCG was significantly more potent than 2′-cysteinyl-EGCG resulting in approximately 80% growth inhibition of HCT-116, IEC-6 and INT-407A cells at 40 μM. HT-29 cells were the least sensitive to the growth inhibitory effects of either 2″-cysteinyl-EGCG or 2′-cysteinyl-EGCG (less than 20% growth inhibition at 40 μM).
Figure 2.
Growth inhibitory effects of 2′-cysteinyl-EGCG (A) and 2″-cysteinyl-EGCG (B) against HT-29, HCT-116, IEC-6 and INT-407A cells. Cells were treated for 48 h and growth inhibition was determined by the MTT assay. Each point represents n = 12 – 18. Error bars represent the standard deviation.
These results are in contrast to those from studies by us and others on the growth inhibitory activity of other EGCG metabolites. For example, M4 had significantly less growth inhibitory activity than EGCG (5, 21). These results suggest that an intact A, C, and D-ring system are necessary for growth inhibitory activity. Likewise, the major methylated metabolites of EGCG, 4′-O-methylEGCG and 4′,4″-di-O-methylEGCG, have much less growth inhibitory activity than EGCG indicating that the intact trihydroxy ring structures are also critical for growth inhibitory activity (18).
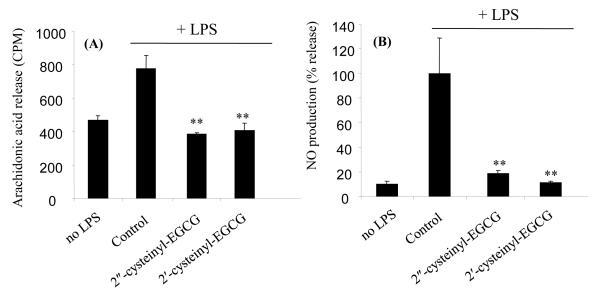
Both 2′-cysteinyl-EGCG and 2″-cysteinyl-EGCG demonstrated significant anti-inflammation related activity in the LPS-stimulated RAW264.7 murine macrophage cell line model. LPS stimulation increased arachidonic acid release and NO production by 1.8 and 6.7-fold, respectively compared to control (Fig 3A and 3B). Both EGCG-cysteine conjugates (20 μM) reduced the aberrant arachidonic acid release and NO production to unstimulated levels.
Figure 3.
Effect of 2′-cysteinyl-EGCG, and 2″-cysteinyl-EGCG on NO production (A) and aberrant arachidonic acid release (B) by LPS-stimulated RAW264.7 murine macrophage cells. Data represent the mean of n = 3. Error bars represent the standard deviation. ** = p < 0.01 compared to LPS-stimulated control.
By contrast, we have previously reported that EGCG had no significant effect on LPS-mediated NO production and arachidonic acid release by RAW264.7 cells (29). M4 and M6 have differential effects on NO production by LPS-stimulated macrophages. M4, which has an intact gallate ring, was shown to inhibit the production of NO by activated RAW264.7 cells, whereas M6 was not (21). Overall our new findings with 2′-cysteinyl-EGCG and 2″-cysteinyl-EGCG demonstrate the importance of an intact trihydroxy ring structure for the inhibition of NO production.
Previous studies with EGCG and EGCG-glucuronides have demonstrated that glucuronidation of EGCG on the B- or D-ring has no effect on the ability of the compound to prevention aberrant arachidonic acid release by HT-29 cells, however, glucuronidation of EGCG on the 7-OH position on the A-ring significantly reduced the inhibitory activity of EGCG (30). These results suggest that the A-ring is more important for this effect than the B- or D-ring.
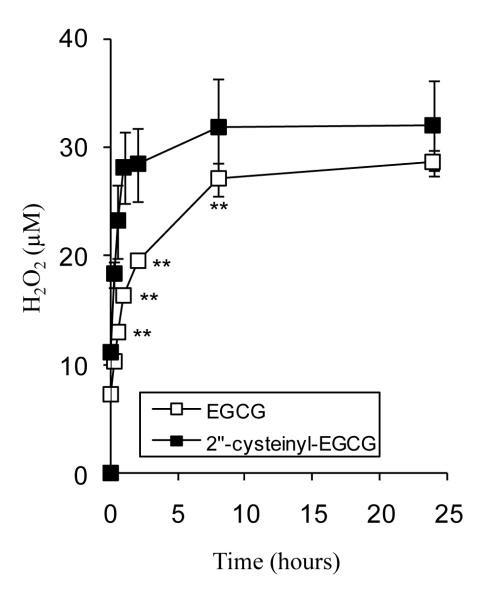
We and others have reported that EGCG is unstable under cell culture conditions and forms oligomers and H2O2. The ability of 2″-cysteinyl-EGCG to produce H2O2 was compared to that of EGCG (Fig. 4). Incubation of equimolar concentrations of EGCG and 2″-cysteinyl-EGCG (50 μM) produced similar concentrations of H2O2 after 24 h incubation. The kinetics of H2O2 formation, however, were accelerated for 2″-cysteinyl-EGCG compared to EGCG. The time to half-maximal H2O2 level were 15 and 60 min for 2″-cysteinyl-EGCG and EGCG, respectively. These results are similar to those reported by Monks, et al. on the pro-oxidant activity of glutathione-conjugated catechol metabolites of MDMA (22). Those authors suggest that the neurotoxic effects of MDMA are at least in part due to the enhanced redox cycling activity of these glutathione-conjugated metabolites, and that in the case of MDMA, glutathione conjugation represents a maladaptive metabolic response.
Figure 4.
H2O2 generation by EGCG and 2″-cysteinyl-EGCG under cell culture conditions. EGCG or 2″-cysteinyl-EGCG (50 μM) were incubated in serum-free cell culture medium at 37°C under 5% CO2 atmosphere. Each point represents the mean of n = 6. Error bars represent that standard deviation. ** = p < 0.01.
In summary, the present results demonstrate that, in contrast to other Phase II metabolites of EGCG, 2′-cysteinyl-EGCG and 2″-cysteinyl-EGCG retain the growth inhibitory and anti-inflammatory capacity of EGCG in vitro, and are more pro-oxidative. Whether these effects are observed in vivo, and by extension whether these metabolites contribute to the cancer preventive effects of EGCG, remains to be determined.
Figure 1.
Structure of EGCG, 2′-cysteinyl-EGCG, and 2″-cysteinyl-EGCG.
Acknowledgements
This work was supported in part by NIH grant AT004678 (to JDL) and NIH grants CA120915, CA122474, and CA133021 (to CSY).
Abbreviations
- EGCG
(-)-epigallocatechin-3-gallate
- LPS
lipopolysaccharide
- M4
(-)-5-(3′, 4′, 5′-trihydroxyphenyl)-γ-valerolactone
- MDMA
3,4-methylenedioxymethamphetamine
- MTT
3,[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide
Footnotes
Safety No issues to report.
Literature Cited
- (1).Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–39. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Bode AM, Dong Z. Epigallocatechin 3-gallate and green tea catechins: United they work, divided they fail. Cancer Prev Res (Phila Pa) 2009;2:514–7. doi: 10.1158/1940-6207.CAPR-09-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dou QP. Molecular mechanisms of green tea polyphenols. Nutr Cancer. 2009;61:827–35. doi: 10.1080/01635580903285049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Yang CS, Sang S, Lambert JD, Hou Z, Ju J, Lu G. Possible mechanisms of the cancer-preventive activities of green tea. Mol Nutr Food Res. 2006;50:170–5. doi: 10.1002/mnfr.200500105. [DOI] [PubMed] [Google Scholar]
- (5).Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV, Yang CS. Mechanism of Action of (-)-Epigallocatechin-3-Gallate: Auto-oxidation-Dependent Inactivation of Epidermal Growth Factor Receptor and Direct Effects on Growth Inhibition in Human Esophageal Cancer KYSE 150 Cells. Cancer Res. 2005;65:8049–56. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- (6).Li GX, Chen YK, Hou Z, Xiao H, Jin H, Lu G, Lee MJ, Liu B, Guan F, Yang Z, Yu A, Yang CS. Pro-oxidative Activities and Dose-response Relationship of (-)-Epigallocatechin-3-gallate in the Inhibition of Lung Cancer Cell Growth: A Comparative Study in vivo and in vitro. Carcinogenesis. 2010 doi: 10.1093/carcin/bgq039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Sang S, Lee MJ, Hou Z, Ho CT, Yang CS. Stability of Tea Polyphenol (-)-Epigallocatechin-3-gallate and Formation of Dimers and Epimers under Common Experimental Conditions. J Agric Food Chem. 2005;53:9478–84. doi: 10.1021/jf0519055. [DOI] [PubMed] [Google Scholar]
- (8).Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–6. [PubMed] [Google Scholar]
- (9).Elbling L, Weiss RM, Teufelhofer O, Uhl M, Knasmueller S, Schulte-Hermann R, Berger W, Micksche M. Green tea extract and (-)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. Faseb J. 2005;19:807–9. doi: 10.1096/fj.04-2915fje. [DOI] [PubMed] [Google Scholar]
- (10).Lambert JD, Sang S, Yang CS. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol Pharm. 2007;4:819–25. doi: 10.1021/mp700075m. [DOI] [PubMed] [Google Scholar]
- (11).Lambert JD, Sang S, Lu AY, Yang CS. Metabolism of dietary polyphenols and possible interactions with drugs. Curr Drug Metab. 2007;8:499–507. doi: 10.2174/138920007780866870. [DOI] [PubMed] [Google Scholar]
- (12).Wang LQ, Meselhy MR, Li Y, Nakamura N, Min BS, Qin GW, Hattori M. The heterocyclic ring fission and dehydroxylation of catechins and related compounds by Eubacterium sp. strain SDG-2, a human intestinal bacterium. Chem Pharm Bull (Tokyo) 2001;49:1640–3. doi: 10.1248/cpb.49.1640. [DOI] [PubMed] [Google Scholar]
- (13).Li C, Lee MJ, Sheng S, Meng X, Prabhu S, Winnik B, Huang B, Chung JY, Yan S, Ho CT, Yang CS. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem Res Toxicol. 2000;13:177–84. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- (14).Sang SM, Lambert JD, Hong J, Tian SY, Lee MJ, Stark RE, Ho CT, Yang CS. Synthesis and structure identification of thiol conjugates of (-)-epigallocatechin gallate and their urinary levels in mice. Chem Res Toxicol. 2005;18:1762–69. doi: 10.1021/tx050151l. [DOI] [PubMed] [Google Scholar]
- (15).Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (-)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48:409–416. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Galati G, Lin A, Sultan AM, O’Brien P,J. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med. 2006;40:570–80. doi: 10.1016/j.freeradbiomed.2005.09.014. [DOI] [PubMed] [Google Scholar]
- (17).Klaassen CD. Casarett & Doull’s Toxicology: The Basic Science of Poisons. 5th ed McGraw-Hill; New York, NY: 1996. p. 1111. [Google Scholar]
- (18).Landis-Piwowar KR, Wan SB, Wiegand RA, Kuhn DJ, Chan TH, Dou QP. Methylation suppresses the proteasome-inhibitory function of green tea polyphenols. J Cell Physiol. 2007 doi: 10.1002/jcp.21124. [DOI] [PubMed] [Google Scholar]
- (19).Chen D, Wang CY, Lambert JD, Ai N, Welsh WJ, Yang CS. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: structure-activity relationship and molecular-modeling studies. Biochem Pharmacol. 2005;69:1523–31. doi: 10.1016/j.bcp.2005.01.024. [DOI] [PubMed] [Google Scholar]
- (20).Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–70. [PubMed] [Google Scholar]
- (21).Lambert JD, Rice JE, Hong J, Hou Z, Yang CS. Synthesis and biological activity of the tea catechin metabolites, M4 and M6 and their methoxy-derivatives. Bioorg Med Chem Lett. 2005;15:873–6. doi: 10.1016/j.bmcl.2004.12.070. [DOI] [PubMed] [Google Scholar]
- (22).Monks TJ, Jones DC, Bai F, Lau SS. The role of metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-methylenedioxymethamphetamine (ecstasy) toxicity. Ther Drug Monit. 2004;26:132–6. doi: 10.1097/00007691-200404000-00008. [DOI] [PubMed] [Google Scholar]
- (23).Bai F, Lau SS, Monks TJ. Glutathione and N-acetylcysteine conjugates of alpha-methyldopamine produce serotonergic neurotoxicity: possible role in methylenedioxyamphetamine-mediated neurotoxicity. Chem Res Toxicol. 1999;12:1150–7. doi: 10.1021/tx990084t. [DOI] [PubMed] [Google Scholar]
- (24).Sang S, Lambert JD, Hong J, Tian S, Lee MJ, Stark RE, Ho CT, Yang CS. Synthesis and structure identification of thiol conjugates of (-)-epigallocatechin gallate and their urinary levels in mice. Chem Res Toxicol. 2005;18:1762–9. doi: 10.1021/tx050151l. [DOI] [PubMed] [Google Scholar]
- (25).Bold RJ, Virudachalam S, McConkey DJ. Chemosensitization of pancreatic cancer by inhibition of the 26S proteasome. J Surg Res. 2001;100:11–7. doi: 10.1006/jsre.2001.6194. [DOI] [PubMed] [Google Scholar]
- (26).Sang S, Lambert JD, Tian S, Hong J, Hou Z, Ryu JH, Stark RE, Rosen RT, Huang MT, Yang CS, Ho CT. Enzymatic synthesis of tea theaflavin derivatives and their anti-inflammatory and cytotoxic activities. Bioorg Med Chem. 2004;12:459–67. doi: 10.1016/j.bmc.2003.10.024. [DOI] [PubMed] [Google Scholar]
- (27).Ryu JH, Ahn H, Lee H. Jin. Inhibition of nitric oxide production on LPS-activated macrophages by kazinol B from Broussonetia kazinoki. Fitoterapia. 2003;74:350–4. doi: 10.1016/s0367-326x(03)00062-5. [DOI] [PubMed] [Google Scholar]
- (28).Lambert JD, Kwon SJ, Hong J, Yang CS. Salivary hydrogen peroxide produced by holding or chewing green tea in the oral cavity. Free Radic Res. 2007;41:850–3. doi: 10.1080/10715760601091659. [DOI] [PubMed] [Google Scholar]
- (29).Lambert JD, Sang S, Hong J, Kwon SJ, Lee MJ, Ho CT, Yang CS. Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate. Drug Metab Dispos. 2006;34:2111–6. doi: 10.1124/dmd.106.011460. [DOI] [PubMed] [Google Scholar]
- (30).Lu H, Meng X, Li C, Sang S, Patten C, Sheng S, Hong J, Bai N, Winnik B, Ho CT, Yang CS. Glucuronides of tea catechins: enzymology of biosynthesis and biological activities. Drug Metab Dispos. 2003;31:452–61. doi: 10.1124/dmd.31.4.452. [DOI] [PubMed] [Google Scholar]