Abstract
Background
Diarrheal illnesses remain a leading cause of morbidity and mortality globally, with increasing recognition of long-term sequelae, including postinfectious irritable bowel syndrome and growth faltering, as well as cognitive deficits in children. Identification of specific etiologic agents is often lacking. In vitro and in vivo data suggest that enterotoxigenic Bacteroides fragilis (ETBF) may contribute to the burden of colonic inflammatory diarrheal disease. The study goal was to investigate the pathogenesis of ETBF diarrheal illnesses.
Methods
We performed an observational study of children and adults with acute diarrheal illnesses in Dhaka, Bangladesh, from January 2004 through November 2005, to define the clinical presentation, intestinal inflammatory responses, and systemic and intestinal antibody responses to ETBF. Other enteric pathogens were also evaluated.
Results
ETBF was identified to cause a clinical syndrome with marked abdominal pain and nonfebrile inflammatory diarrhea in both children (age, >1 year) and adults. Fecal leukocytes, lactoferrin, and proinflammatory cytokines (interleukin 8, tumor necrosis factor–α)—as well as B. fragilis toxin systemic antitoxin responses—increased rapidly in ETBF-infected patients. Evidence of intestinal inflammation often persisted for at least 3 weeks, despite antibiotic therapy.
Conclusions
ETBF infection is a newly recognized cause of inflammatory diarrhea in children and adults. Future studies are needed to evaluate the role of ETBF in persistent colonic inflammation and other morbid sequelae of acute diarrheal disease.
Enterotoxigenic Bacteroides fragilis (ETBF) was described in 1984 as a cause of lamb diarrheal disease [1] and in 1987 as associated with human diarrheal disease [2]. Controlled and cohort studies in both developed and low-resource countries consistently identify ETBF as being associated with acute diarrheal illnesses in young children (age, 1–5 years) [3–8]. In adults, a Swedish study associated ETBF with diarrheal disease in those aged >30 years [9]. Acute, watery diarrhea was reported in ETBF disease, but detailed stool sample studies were not performed. By contrast, experimental infection in rabbits and gnotobiotic piglets suggests that ETBF induces colonic inflammation [10–12]. Consistent with this observation, the only known virulence factor of ETBF—the B. fragilis toxin—stimulates secretion of the proinflammatory cytokine, IL-8, by intestinal epithelial cells in vitro [13–16]. Because available clinical observations on ETBF disease contrasted with experimental results, our study goal was to characterize the clinical characteristics and pathogenesis of ETBF infections. We chose to conduct our study in Bangladeshi children and adults because this is a population in whom ETBF is known to be endemic [5, 6, 17].
METHODS
Recruitment of the study population
Children aged >1 year and adults presenting with acute diarrhea (defined as >3 watery stools per day or any bloody stools) at the hospital of the International Centre for Diarrheal Diseases Research (Dhaka, Bangladesh) or at a community-based clinic in the urban slum Mirpur (Dhaka, Bangladesh) from January 2004 through November 2005 were enrolled for stool screening to identify individuals infected with ETBF; individuals positive for fecal ETBF infection were then enrolled in the 3-week study. Informed consent was obtained from adult patients or from guardians on behalf of study participants who were <18 years of age. Study exclusion criteria were as follows: (1) age <1 year, because ETBF is not associated with diarrheal disease in this age group [6, 8]; (2) ingestion of antibiotics in the previous 2 weeks; (3) current systemic illness, such as pneumonia or meningitis; and (4) malnutrition in children (weight-age z score >2 SDs below the mean). Epidemiologic data on the clinical manifestations and blood and stool specimens were collected at enrollment and 3 weeks after diarrhea onset. Dehydration was defined as none, some, or severe by World Health Organization criteria [18]. Oral rehydration therapy was provided, and the evaluating physician administered antibiotics after enrollment if judged to be clinically warranted. Healthy control individuals—without diarrhea for at least 2 weeks—were recruited from the same populations. The protocol was approved by the Ethical Review Committee of the International Centre for Diarrheal Diseases Research, Bangladesh, and the Western International Review Board in the United States.
Microbiology of stool specimens
Stool specimens were tested for recognized enteropathogens, including enterotoxigenic Escherichia coli, Campylobacter jejuni, Shigella species, Salmonella species, and Vibrio cholerae [19, 20], as well as rotavirus [21]. Stool specimens were tested by direct microscopy for parasites and helminthes. For isolation of B. fragilis, 100 μL of stool (or 1 g of stool mixed in 4 mL of PBS for semisolid stool) was incubated overnight in 8 mL of peptone yeast extract glucose [22] or fastidious anaerobe broth (International Diagnostic Group) under anaerobic conditions (BBL Anaerobic System; GasPak Plus) at 37°C. After centrifugation, the bacterial pellet was inoculated directly on blood agar containing kanamycin (100 mg/L) and vancomycin (7.5 mg/L) at 37°C for 48 h under anaerobic conditions. B. fragilis colonies were identified by mottled appearance under stereomicroscopy and were catalase positive and oxidase negative.
Detection and characterization of the bft allele
The B. fragilis toxin (bft) gene was detected by PCR (with use of forward primer 5′-CGCGGCATTATTAGCTGCATGTTCTAATG-3′ and reverse primer 5′-GATACATCAGCTGGGTTGTAGACATCCCA-3′), to yield a 1-kilobase DNA band, as described elsewhere [23]. In brief, boiled bacterial DNA (2.5 μL) was added to a reaction mixture that contained 200 mmol 2′-deoxynucleoside-5′-triphosphate, 1.0 U of Taq DNA polymerase (Takara Bio), 2 mM magnesium chloride, and 10 pmol of each primer in a final volume of 25 μL. Reactions underwent 30 cycles of amplification (PT-200 Peltier Thermal Cycler; MJ Research), which consisted of 1-min denaturation at 94°C, 2-min annealing at 62°C, 1-min extension at 72°C, and a final 7-min extension at 72°C. ETBF strain D-134 and B. fragilis J-139 (nontoxigenic B. fragilis strain) served as positive and negative controls, respectively; water, instead of template DNA, served as an additional negative control. PCR products were verified by 1% agarose gel electrophoresis with ethidium bromide staining.
HT29/C1 cell assay
Secretion of biologically active B. fragilis toxin was detected in culture supernatants (fresh or frozen at –20°C until tested) of isolated ETBF strains with use of the cloned human colonic carcinoma epithelial cell line, HT29/C1, as described elsewhere [24]. This cell assay detects as little as 0.5 pM of B. fragilis toxin [25]. Culture supernatants of ETBF strain D-134 and nontoxigenic B. fragilis strain J-139 served as positive and negative controls, respectively.
ELISA for anti– B. fragilis toxin serum and intestinal antibodies
B. fragilis toxin was purified as described elsewhere [26]. We coated 96-well plates (Nunc) with 5 μg/mL of purified B. fragilis toxin diluted in PBS (10 mmol; pH, 7.2) at room temperature overnight. After washing and blocking with 1% bovine serum albumin for 45 min at 37°C followed by additional PBS-Tween washes, serum samples diluted 1:50 in 0.1% bovine serum albumin and 0.05% Tween-20 in PBS were added and incubated for 90 min at 37°C. After washing, peroxidase-conjugated rabbit anti-human immunoglobulins (IgA and IgG; Jackson Immuno Research Laboratories) diluted 1:1000 in 0.1% bovine serum albumin, PBS, and Tween were added, followed by incubation for 90 min at 37°C. After washing, orthophenyl diamine (1 mg/mL; Sigma) in 0.2 mol sodium citrate buffer (pH, 4.5) and 30% hydrogen peroxide solution was added. The optical density was measured kinetically at 450 nm for 5 min, and the results were expressed as the change in milliabsorbance units per minute [27]. The immune responses in patients on different study days were compared with that seen at 1 time point in healthy control individuals; values ≥2-fold higher than the mean ± SEM of the healthy control individuals was defined as a serologic response to B. fragilis toxin.
Other stool and serum assays
Intestinal inflammation was assessed by the following: (1) stool occult blood detected using the modified guaiac acid procedure [28], (2) fecal polymorphonuclear leukocytes detected by microscopy with use of methylene blue staining [29], and (3) sandwich EIAs to detect fecal lactoferrin (Oxis International), IL-8 (BD Biosciences), and TNF-α (BD Biosciences). EIA detection limits were 5, 4, and 7.8 pg/mL for lactoferrin, IL-8, and TNF-α, respectively. Serum C-reactive protein was measured by the Immulite High Sensitivity CRP assay with a detection limit of 0.01 mg/dL (Diagnostic Products), as described by the manufacturer. Blood leukocyte counts were quantitated by differential counts with use of microscopy.
Antibiotic susceptibility testing
Susceptibility testing was performed using the Clinical Laboratory and Standards Institute reference standard agar dilution method [30].
Statistical analyses
Data are expressed as median values with 25th and 75th percentiles and were analyzed by the Wilcoxon signed rank test and the Mann-Whitney U test with use of SigmaStat 3.1 statistical software (Systat Software). P values ≤.05 defined statistically significant differences.
RESULTS
Isolation of B. fragilis, ETBF, and other enteropathogens
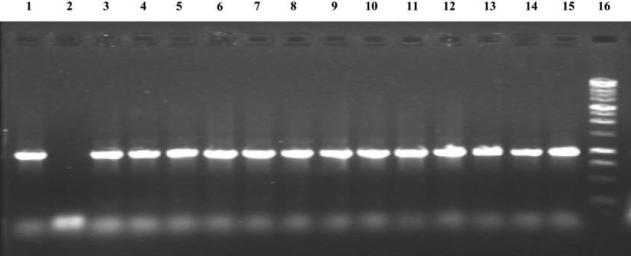
A total of 1209 patients with diarrhea were screened for carriage of B. fragilis and ETBF. The first 714 stool samples were enriched using peptone yeast glucose medium, yielding B. fragilis in 227 (31.8%); subsequently, stool enrichment with use of fastidious anaerobe broth media yielded isolation of B. fragilis in 190 positive stool samples (38.4%) of the 495 screened (P = .021), which indicates that fastidious anaerobe broth is superior as an enrichment medium for B. fragilis recovery. Overall, 417 (34.5%) and 86 (7.0%) of the 1209 stool samples yielded B. fragilis and ETBF, respectively. Of B. fragilis strains isolated, 86 (20.6%) of 417 were ETBF; all ETBF isolates were confirmed by PCR for the bft gene (figure 1) and by detection of biologically active B. fragilis toxin in culture supernatants of the ETBF strains in the HT29/C1 cell assay (data not shown). There are 3 reported bft alleles [31]. All ETBF isolates that were available for analysis (79 isolates) possessed the bft-1 allele except 2 isolates that contained the bft-2 allele. Only enterotoxigenic E. coli isolates (5 isolates positive for the heat-stable enterotoxin, 1 for the heat-labile toxin, and 1 for both) were identified as copathogens in 7 (8.1%) of 86 study participants.
Figure 1.
PCR products of the bft gene. Amplicons were separated on a 1.0% agarose gel. Lane 1, The bft gene from positive control strain D-134. Lane 2, Negative control strain J-139. Lanes 3–15, Amplified bft from Bacteroides fragilis strains isolated from study participants. Lane 16, One-kilobase DNA ladder (Promega).
Epidemiology of ETBF infection
Of the 86 individuals from whom ETBF was isolated, 73 consented to participate in the 3-week study. ETBF infections were evenly distributed by sex (38 male [52%] and 35 female [48%]). Table 1 shows the rates of ETBF isolation by the age of the study participants. Overall, 43 (59%) of the 73 ETBF-positive study participants were children aged <15 years (P < .047). ETBF isolation was not more common among children 1–5 years of age than among older age groups (P = .955). Most (59 [81%] of 73) of the ETBF-infected study participants were identified in the Mirpur community; the remaining individuals were identified at the International Centre for Diarrheal Diseases Research Hospital (Dhaka, Bangladesh) (P < .001).
Table 1.
Enterotoxigenic Bacteroides fragilis (ETBF) isolation by age of study participants.
| Age group, years | No. of positive isolates |
Percentage of ETBF-positive isolates | |
|---|---|---|---|
| B. fragilis | ETBF | ||
| 1–5 | 113 | 25a | 22.1 |
| 5–15 | 131 | 18 | 13.7 |
| >15 | 173 | 30 | 17.3 |
| Total | 417 | 73b | 17.5 |
P = .955 for children aged 1–5 years versus older age groups.
Forty-three (59%) of 73 enterotoxigenic B. fragilis–positive patients were aged <15 years versus 30 (41%) of 73 who were older (P< .047).
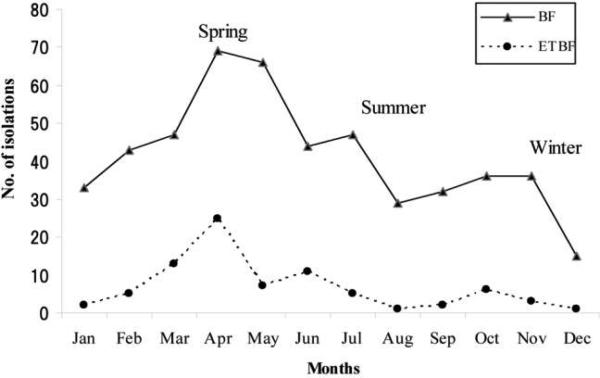
Figure 2 shows the monthly and seasonal isolation of B. fragilis and ETBF during the study. Both nontoxigenic B. fragilis and ETBF were isolated in diarrheal specimens throughout the year. However, isolation rates for ETBF (59%) were significantly higher during the hot, dry spring months (March–May) than during either the summer months (June–October; ETBF isolation, 27%; P < .002) or winter months (November–February; ETBF isolation, 14%; P < .001). In contrast, 40% and 35% of the B. fragilis was isolated during the spring and summer seasons, respectively (P = .559).
Figure 2.
Mean monthly isolation of Bacteroides fragilis (BF) and enterotoxigenic B. fragilis (ETBF) during the study. Data for the month of December are from 1 year only (2004). B. fragilis and ETBF isolations were from 350 and 859 of a total of 1209 patients with diarrhea who were evaluated at the International Centre for Diarrheal Diseases Research (Dhaka, Bangladesh) and Mirpur sites (Dhaka, Bangladesh), respectively, during the study. At the International Centre for Diarrheal Diseases Research and Mirpur sites, 16–44 and 34–123 patients with diarrhea, respectively, were evaluated monthly, with the lowest number evaluated during November and the highest number evaluated during March.
Clinical presentation of ETBF infection
ETBF-infected individuals reported substantial abdominal pain (64 patients [88%]), tenesmus (48 [66%]), and nocturnal diarrhea (58 [79%]). In contrast, fever (temperature, >37.8°C; 5 patients [7%]), leukocytosis (median leukocyte count, 8.5 × 103/mm3; range, 7.0–9.8 × 103/mm3), and fecal occult blood (6 patients [8%]) were identified infrequently. ETBF was associated with acute diarrheal illnesses that lasted a median of 3 days (range, 2–11 days) and resulted in dehydration in 14 individuals (19%); no individual experienced severe dehydration. Because the clinical symptoms of abdominal pain and tenesmus were reported as being severe, the clinician chose to treat 61 (84%) of the patients with antibiotics (metronidazole, 400 mg 3 times daily for 5 days [56 adults]; metronidazole, 20 mg/kg daily for 5 days [3 children]; nalidixic acid [1 child]; and cotrimoxazole [1 child]); 12 patients (16%) received no antibiotics. On day 21, B. fragilis and ETBF were identified by stool culture in 16 patients (22%; 14 of them had received metronidazole) and 1 patient (1%; who had received metronidazole), respectively, of 73 patients evaluated. Of 58 initial ETBF isolates tested, 97% were sensitive to ampicillin, 90% were sensitive to clindamycin, 93% were sensitive to metronidazole, and 74% were sensitive to tetracycline.
Stimulation of intestinal inflammation by ETBF infection
Table 2 gives the quantitation of fecal polymorphonuclear leukocytes during the ETBF illness. Notably, >70% of ETBF-infected patients had excess fecal polymorphonuclear leukocytes (>10 per high-power field) at the onset of the diarrheal illness, with evidence of persistence of intestinal inflammation in ~55% on day 21 after illness onset. Of the 39 patients with >10 polymorphonuclear leukocytes per high-power field in stool samples on day 21, 34 (87%) had received treatment with antibiotics (33 with metronidazole and 1 with nalidixic acid).
Table 2.
Stool polymorphonuclear leukocytes in enterotoxigenic Bacteroides fragilis infection.
| Stool polymorphonuclear leukocyte count range per high-power field | No. (%) of patients with stool polymorphonuclear leukocytes |
||
|---|---|---|---|
| Days 1–3 | Days 5–11 | Day 21 | |
| 0–10 | 15 (20.5) | 58 (79.4) | 31 (42.5) |
| 10–20 | 44 (60.3) | 14 (19.2) | 38 (52) |
| 20–50 | 10 (13.7) | 1 (1.4) | 1 (1.4) |
| Not done | 4 (5.5) | 0 (0) | 3 (4.1) |
To further assess intestinal inflammation in ETBF-infected patients, fecal lactoferrin (a protein released from the granules of polymorphonuclear leukocytes) and 2 proinflammatory cytokines (TNF-α and IL-8) were assessed in the stool samples collected at the time of ETBF diagnosis (table 3). Lactoferrin, TNF-α, and IL-8 were significantly increased in the stool samples of ETBF-infected patients, compared with nondiarrheal control stool samples obtained from individuals from the same population and socioeconomic strata as the ETBF-infected patients. In contrast, serum C-reactive protein, a marker of systemic inflammation, was not significantly different between ETBF-infected patients and healthy control individuals (median values, 0.8 vs. 0.3 mg/L for ETBF-infected patients [73 patients] vs. healthy control individuals [18 individuals]; P = .192). Because the diagnosis of ETBF usually required a minimum of 5 days to complete stool collection, culture, and PCR testing for the bft gene, the mean delay of collection of serum samples was 7 days (range, 3–10 days).
Table 3.
Intestinal inflammation in enterotoxigenic Bacteroides fragilis infection.
| Mediator | Patients at enrollment (n = 73) | Healthy control individuals (n = 18) | P |
|---|---|---|---|
| Lactoferrin, ng/mL | 58 (21–161) | 12 (0–27.5) | < .001 |
| IL-8, pg/mL | 174 (19.5–205) | 0 | < .001 |
| TNF-α, pg/mL | 17 (8–43) | 0 | <.001 |
NOTE. All data are median and range (25th–75th percentiles), unless otherwise indicated. The Mann-Whitney U test was used for statistical analyses.
Stimulation of systemic and mucosal immune responses by ETBF infection
Table 4 gives the results of assessment of serum and fecal IgG and/or IgA for anti–B. fragilis toxin antibodies by ELISA. Analysis of serum at days 7 and 21 after onset of ETBF infection identified significant increases in systemic IgG and IgA anti–B. fragilis toxin antibody titers, compared with healthy control serum samples (P < .005 for both IgG and IgA titers, patients vs. control individuals). Overall, 64 (96%) of 67 and 54 (79%) of 68 ETBF-infected patients developed serum IgG and IgA responses, respectively, to B. fragilis toxin by day 7, compared with 2 (12.5%) of 16 and 3 (30%) of 10 among healthy control individuals, respectively. In contrast, fecal anti–B. fragilis toxin IgA responses did not differ significantly between control individuals and ETBF-infected patients, although anti–B. fragilis toxin IgA increased over time in the ETBF-infected population (median geometric titer, 18.3 for control individuals and 18.3 for ETBF-infected patients on day 1, and 37 for ETBF-infected patients on day 7; P < .06, day 1 compared with day 7 responses). By day 7, fecal IgA was detected in 29 (49%) of 59 ETBF-infected patients and in 3 (30%) of 10 healthy control individuals (P = .17); fecal IgA was not tested on day 21.
Table 4.
Systemic and mucosal antibody responses to Bacteroides fragilis toxin.
| Serum response and test day | Patients (n = 71) | Healthy control individuals (n = 16) | P |
|---|---|---|---|
| IgG | |||
| Day 7 | 100.33 (72.5–145.8) | 27.66 (21.5–38.0) | <.001 |
| Day 21 | 102.33 (66.4–142.5) | NA | <.001a |
| IgA | |||
| Day 7 | 11.17 (7.5–17.5) | 5.17 (4–10) | .005 |
| Day 21 | 11.41 (8.3–15.8) | NA | .002a |
NOTE. Data are median (range) milliabsorbance units per minute, unless otherwise indicated. NA, not applicable.
P values compared patient data with control data; healthy control individuals were assessed 1 time.
DISCUSSION
This study defines ETBF, to our knowledge for the first time, as an inflammatory enteric pathogen in humans. ETBF was proposed to be a human diarrheal disease agent in 1987 [2], and the first controlled study associating ETBF with diarrheal disease was conducted in the Apache population in 1992, where acute, watery diarrheal disease in children aged >1 year was observed [7]. Subsequent human studies confirmed the association of ETBF with diarrheal disease and extended the pathogenicity of ETBF to adults [9, 32]. However, because bloody diarrhea was not observed, ETBF infection was presumed to cause noninflammatory diarrhea. Our detailed studies indicate that ETBF induces intestinal, likely colonic (on the basis of the known intestinal niche of B. fragilis), inflammation in most infected symptomatic individuals. These clinical data are supported by laboratory studies that identify ETBF and B. fragilis toxin as potent inducers of IL-8 synthesis and secretion by intestinal epithelial cells and colitis in mice, rabbits, and gnotobiotic piglets [10–16, 33]. Similarly, ETBF and B. fragilis toxin stimulate fluid secretion and mixed cellularity inflammation when tested in ligated small bowel and colonic segments in animal models [34–36]. When compared with data reported on intestinal inflammation detected in the stool samples of Bangladeshi patients infected with enterotoxigenic E. coli, V. cholerae, or Shigella species, ETBF infection stimulates a greater stool inflammatory response than does enterotoxigenic E. coli or V. cholerae but generally causes less marked inflammation than does Shigella infection (data not shown) [37–39]. Our results are a reminder that the origin and pathogenesis of most diarrheal illnesses cannot be predicted at the bedside without specific microbiology and other laboratory studies.
Another striking and previously unreported feature of symptomatic ETBF infection is its association in our study with substantial abdominal pain and tenesmus, symptoms also consistent with colonic inflammatory disease. The severity of the abdominal discomfort led the clinicians evaluating the patients to empirically treat patients with metronidazole when or even before ETBF infection was diagnosed. It is unknown whether antibiotic therapy modifies the clinical course and is of clinical benefit to those with ETBF infection; a randomized controlled therapeutic trial would be required to address this issue. The frequent use of antibiotic therapy in our population limited our ability to define the natural history of ETBF infection. This study combined with prior studies and additional data from the International Centre for Diarrheal Diseases Research (Bangladesh) (F.Q. and R.B.S., unpublished data) suggest that ETBF infections are usually acute, mildly dehydrating diarrheal illnesses but that significant abdominal pain may occur. Exceptions of ETBF infection associated with prolonged diarrheal illnesses are reported [6, 7]. Consistent with frequent antibiotic use in this population, 7% of ETBF strains isolated were resistant to metronidazole, a frequency higher than noted elsewhere [40].
Nontoxigenic B. fragilis has recently been reported as a symbiont that may modulate systemic T cell–dependent immunity [41]. Our data demonstrate that symptomatic ETBF infection stimulates systemic and mucosal antibody responses to B. fragilis toxin, the only identified virulence factor of ETBF. Additional data confirm the detection of anti–B. fragilis toxin antibodies by Western blot analysis (C.L.S., S.I., and F.Q., unpublished results). Detected anti–B. fragilis toxin antibody responses were significantly greater than in control serum samples obtained from individuals in the same community and of similar socioeconomic background. The age-dependent seroprevalence of antibodies to B. fragilis toxin is unknown but deserves further study. Asymptomatic ETBF infection identified in control populations of published studies is not uncommon and has a prevalence range of 4%–20% [8]. In 1 endoscopy-based study, ETBF was recovered from 35% of stool samples of control patients without diarrhea [42]. It is unknown whether colonic carriage of ETBF is associated with development of anti–B. fragilis toxin serum antibodies similar to the age-dependent increase in prevalence of antibodies to the Clostridium difficile toxins A and B [43]. One limitation of our observations is that we could not determine whether infected patients were seronegative for B. fragilis toxin antibodies at the onset of their illnesses. Initial serum sample collection was delayed an average of 7 days, until the diagnosis of ETBF infection was made by anaerobic stool culture followed by specific detection of B. fragilis toxin by PCR and/or a tissue culture assay (HT29/C1 cell assay). Our data also do not ascertain whether induction of antibody responses to B. fragilis toxin is T cell dependent, similar to immune responses to the polysaccharide capsule of B. fragilis [44].
Classic microbiologic approaches indicate that B. fragilis comprises a small percentage of the fecal flora but emerges as the leading anaerobe in human disease [45–47]. On the basis of stool cultures, 40%–70% of humans are estimated to be colonized with B. fragilis. The accuracy of these prevalence estimates has not been verified using molecular approaches to detect B. fragilis in stool samples, and the durability over time of fecal carriage of specific B. fragilis strains is not known. Our data suggest that seasonal variations in B. fragilis carriage occur; alternatively, seasonal variations in fecal flora facilitate the recovery of B. fragilis. Limited data suggest that ETBF may comprise a greater proportion of the fecal flora during diarrheal illnesses and that long-term carriage of ETBF after a diarrheal illness may occur [7]. Because persistent moderate inflammation, as assessed by fecal polymorphonuclear leukocytes, was noted in our study population despite apparent eradication of ETBF by metronidazole therapy in most patients, an important question that emerges from our data is whether asymptomatic carriage of ETBF is associated with ongoing intestinal inflammation and is associated with other intestinal diseases, such as postinfectious diarrhea-predominant irritable bowel syndrome [48]. In this regard, preliminary observations suggest an association between active inflammatory bowel disease and colorectal carcinoma and ETBF infection [42, 49, 50]. The ongoing intestinal inflammation detected at 21 days is likely not unique to ETBF infection, because a prior study of Shigella infections in the same population also suggested a protracted colon inflammatory response [51].
Our observations that symptomatic ETBF infection stimulates intestinal inflammation in most patients alters our understanding of the pathogenesis of ETBF disease and raises pertinent new questions about the epidemiology of ETBF infection and the role of ETBF in colonic disease. Currently, the diagnosis of ETBF infection is technically difficult and is restricted to research settings. Development of rapid, molecular approaches for diagnosis of ETBF infection will allow these areas of concern to be addressed.
Acknowledgments
We acknowledge the contribution of staff at the Mirpur field site for their active support.
Financial support. National Institutes of Health (RO1 DK/AI 59655 to C.L.S.) and the International Centre for Diarrheal Disease Research, Bangladesh. The Centre is supported by agencies and countries that share its concern for the health problems of the developing countries.
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Myers LL, Firehammer BD, Shoop DS, Border MM. Bacteroides fragilis: a possible cause of acute diarrheal disease in newborn lambs. Infect Immun. 1984;44:241–4. doi: 10.1128/iai.44.2.241-244.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myers LL, Shoop DS, Stackhouse LL, et al. Isolation of enterotoxigenic Bacteroides fragilis from humans with diarrhea. J Clin Microbiol. 1987;25:2330–3. doi: 10.1128/jcm.25.12.2330-2333.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasan KZ, Pathela P, Alam K, et al. Aetiology of diarrhoea in a birth cohort of children aged 0–2 year(s) in rural Mirzapur, Bangladesh. J Health Popul Nutr. 2006;24:25–35. [PubMed] [Google Scholar]
- 4.Nguyen TV, Van PL, Huy CL, Weintraub A. Diarrrhea caused by enterotoxigenic Bacteroides fragilis in children less than 5 years of age in Hanoi, Vietnam. Anaerobe. 2005;11:109–14. doi: 10.1016/j.anaerobe.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Pathela P, Hasan KZ, Roy E, et al. Enterotoxigenic Bacteroides fragilis–associated diarrhea in children 0–2 years of age in rural Bangladesh. J Infect Dis. 2005;191:1245–52. doi: 10.1086/428947. [DOI] [PubMed] [Google Scholar]
- 6.Sack RB, Albert MJ, Alam K, Neogi PKB, Akbar MS. Isolation of enterotoxigenic Bacteroides fragilis from Bangladeshi children with diarrhea: a controlled study. J Clin Microbiol. 1994;32:960–3. doi: 10.1128/jcm.32.4.960-963.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sack RB, Myers LL, Almeido-Hill J, et al. Enterotoxigenic Bacteroides fragilis: epidemiologic studies of its role as a human diarrhoeal pathogen. J Diarrhoeal Dis Res. 1992;10:4–9. [PubMed] [Google Scholar]
- 8.Sears C, Franco A, Wu S. Bacteroides fragilis toxins. In: Alouf J, Popoff M, editors. Bacterial protein toxins. 3rd ed. Academic Press; Oxford, United Kingdom: 2005. [Google Scholar]
- 9.Zhang G, Svenungsson B, Karnell A, Weintraub A. Prevalence of enterotoxigenic Bacteroides fragilis in adult patients with diarrhea and healthy controls. Clin Infect Dis. 1999;29:590–4. doi: 10.1086/598639. [DOI] [PubMed] [Google Scholar]
- 10.Duimstra JR, Myers LL, Collins JE, Benfield DA, Shoop DS, Bradbury WC. Enterovirulence of enterotoxigenic Bacteroides fragilis in gnoto-biotic pigs. Vet Pathol. 1991;28:514–8. doi: 10.1177/030098589102800608. [DOI] [PubMed] [Google Scholar]
- 11.Myers LL, Shoop DS. Association of enterotoxigenic Bacteroides fragilis with diarrheal disease in young pigs. Am J Vet Res. 1987;48:774–5. [PubMed] [Google Scholar]
- 12.Myers LL, Shoop DS, Collins JE. Rabbit model to evaluate enterovirulence of Bacteroides fragilis. J Clin Microbiol. 1990;28:1658–60. doi: 10.1128/jcm.28.7.1658-1660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JM, Cho SJ, Oh YK, Jung HY, Kim YJ, Kim N. Nuclear factor-kappa B activation pathway in intestinal epithelial cells is a major regulator of chemokine gene expression and neutrophil migration induced by Bacteroides fragilis enterotoxin. Clin Exp Immunol. 2002;130:59–66. doi: 10.1046/j.1365-2249.2002.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JM, Oh YK, Kim YJ, Oh HB, Cho YJ. Polarized secretion of CXC chemokines by human intestinal epithelial cells in response to Bacteroides fragilis enterotoxin: NF-κ B plays a major role in the regulation of IL-8 expression. Clin Exp Immunol. 2001;123:421–7. doi: 10.1046/j.1365-2249.2001.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanfilippo L, Li CK, Seth R, Balwin TJ, Menozzi MG, Mahida YR. Bacteroides fragilis enterotoxin induces the expression of IL-8 and transforming growth factor-beta (TGF-β) by human colonic epithelial cells. Clin Exp Immunol. 2000;119:456–63. doi: 10.1046/j.1365-2249.2000.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu S, Powell J, Mathioudakis N, Kane S, Fernandez E, Sears CL. Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-κB pathway. Infect Immun. 2004;72:5832–9. doi: 10.1128/IAI.72.10.5832-5839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albert MJ, Faruque AS, Faruque SM, Sack RB, Mahalanabis D. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J Clin Microbiol. 1999;37:3458–64. doi: 10.1128/jcm.37.11.3458-3464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization [1 May 2008];The treatment of diarrhoea: a manual for physicians and other senior health workers. 2005 Available at: http://whqlibdoc.who.int/publications/2005/9241593180.pdf.
- 19.Qadri F, Das SK, Faruque AS, et al. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J Clin Microbiol. 2000;38:27–31. doi: 10.1128/jcm.38.1.27-31.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . Programme for control of diarrhoeal dis ease (CDD/93.3 Rev 1) World Health Organization; Geneva, Switzerland: 1987. pp. 9–20. [Google Scholar]
- 21.Unicomb LE, Podder G, Gentsch JR, et al. Evidence of high-frequency genomic reassortment of group A rotavirus strains in Bangladesh: emergence of type G9 in 1995. J Clin Microbiol. 1999;37:1885–91. doi: 10.1128/jcm.37.6.1885-1891.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang G, Weintraub A. Rapid and sensitive assay for detection of enterotoxigenic Bacteroides fragilis. J Clin Microbiol. 1998;36:3545–8. doi: 10.1128/jcm.36.12.3545-3548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franco AA, Buckwold S, Shin JW, Ascon M, Sears CL. Mutation of the zinc-binding metalloprotease motif affects Bacteroides fragilis toxin activity without affecting propeptide processing. Infect Immun. 2005;73:5273–7. doi: 10.1128/IAI.73.8.5273-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mundy LM, Sears CL. Detection of toxin production by Bacteroides fragilis: assay development and screening of extraintestinal clinical isolates. Clin Infect Dis. 1996;23:269–76. doi: 10.1093/clinids/23.2.269. [DOI] [PubMed] [Google Scholar]
- 25.Saidi RF, Sears CL. Bacteroides fragilis toxin rapidly intoxicates human intestinal epithelial cells (HT29/C1) in vitro. Infect Immun. 1996;64:5029–34. doi: 10.1128/iai.64.12.5029-5034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S, Dreyfus LA, Tzianabos AO, Hayashi C, Sears CL. Diversity of the metalloprotease toxin produced by enterotoxigenic Bacteroides fragilis. Infect Immun. 2002;70:2463–71. doi: 10.1128/IAI.70.5.2463-2471.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qadri F, Ryan ET, Faruque AS, et al. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect Immun. 2003;71:4808–14. doi: 10.1128/IAI.71.8.4808-4814.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huicho L, Sanchez D, Contreras M, et al. Occult blood and fecal leukocytes as screening tests in childhood infectious diarrhea: an old problem revisited. Pediatr Infect Dis J. 1993;12:474–7. doi: 10.1097/00006454-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Korzeniowski OM, Barada FA, Rouse JD, Guerrant RL. Value of examination for fecal leukocytes in the early diagnosis of shigellosis. Am J Trop Med Hyg. 1979;28:1031–5. doi: 10.4269/ajtmh.1979.28.1031. [DOI] [PubMed] [Google Scholar]
- 30.Hecht DW, Citron DM, Cox M, et al. Methods for antimicrobial susceptibility testing of anaerobic bacteria: approved standard—seventh edition. Clinical Laboratory and Standards Institute; Wayne, Pennsylvania: 2007. [1 March 2008]. Available at: http://www.clsi.org/source/orders/free/m11a7.pdf. [Google Scholar]
- 31.Chung GT, Franco AA, Wu S, et al. Identification of a third metal-loprotease toxin gene in extraintestinal isolates of Bacteroides fragilis. Infect Immun. 1999;67:4945–9. doi: 10.1128/iai.67.9.4945-4949.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sears CL. The toxins of Bacteroides fragilis. Toxicon. 2001;39:1737–46. doi: 10.1016/s0041-0101(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 33.Rabizadeh S, Rhee KJ, Wu S, et al. Enterotoxigenic Bacteroides fragilis: a potential instigator of colitis. Inflamm Bowel Dis. 2007;13:1475–83. doi: 10.1002/ibd.20265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JM, Jung HY, Lee JY, Youn J, Lee CH, Kim KH. Mitogen-activated protein kinase and activator protein-1 dependent signals are essential for Bacteroides fragilis enterotoxin-induced enteritis. Eur J Immunol. 2005;35:2648–57. doi: 10.1002/eji.200526321. [DOI] [PubMed] [Google Scholar]
- 35.Myers LL, Shoop DS, Collins JE, Bradbury WC. Diarrheal disease caused by enterotoxigenic Bacteroides fragilis in infant rabbits. J Clin Microbiol. 1989;27:2025–30. doi: 10.1128/jcm.27.9.2025-2030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obiso RJ, Jr, Lyerly DM, Van Tassell RL, Wilkins TD. Proteolytic activity of the Bacteroides fragilis enterotoxin causes fluid secretion and intestinal damage in vivo. Infect Immun. 1995;63:3820–6. doi: 10.1128/iai.63.10.3820-3826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qadri F, Bhuiyan TR, Dutta KK, et al. Acute dehydrating disease caused by Vibrio cholerae serogroups O1 and O139 induce increases in innate cells and inflammatory mediators at the mucosal surface of the gut. Gut. 2004;53:62–9. doi: 10.1136/gut.53.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raqib R, Mia SM, Qadri F, et al. Innate immune responses in children and adults with shigellosis. Infect Immun. 2000;68:3620–9. doi: 10.1128/iai.68.6.3620-3629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raqib R, Moly PK, Sarker P, et al. Persistence of mucosal mast cells and eosinophils in Shigella-infected children. Infect Immun. 2003;71:2684–92. doi: 10.1128/IAI.71.5.2684-2692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snydman DR, Jacobus NV, McDermott LA, et al. National survey on the susceptibility of Bacteroides fragilis group: report and analysis of trends in the United States from 1997 to 2004. Antimicrob Agents Chemother. 2007;51:1649–55. doi: 10.1128/AAC.01435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–18. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Basset C, Holton J, Bazeos A, Vaira D, Bloom S. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig Dis Sci. 2004;49:1425–32. doi: 10.1023/b:ddas.0000042241.13489.88. [DOI] [PubMed] [Google Scholar]
- 43.Viscidi R, Laughon BE, Yolken R, et al. Serum antibody response to toxins A and B of Clostridium difficile. J Infect Dis. 1983;148:93–100. doi: 10.1093/infdis/148.1.93. [DOI] [PubMed] [Google Scholar]
- 44.Tzianabos AO, Finberg RW, Wang Y, et al. T cells activated by zwitterionic molecules prevent abscesses induced by pathogenic bacteria. J Biol Chem. 2000;275:6733–40. doi: 10.1074/jbc.275.10.6733. [DOI] [PubMed] [Google Scholar]
- 45.Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007;44:895–900. doi: 10.1086/512197. [DOI] [PubMed] [Google Scholar]
- 46.Polk FB, Kasper DL. Bacteroides fragilis subspecies in clinical isolates. Ann Intern Med. 1977;86:569–71. doi: 10.7326/0003-4819-86-5-569. [DOI] [PubMed] [Google Scholar]
- 47.Redondo MC, Arbo MD, Grindlinger J, Snydman DR. Attributable mortality of bacteremia associated with the Bacteroides fragilis group. Clin Infect Dis. 1995;20:1492–6. doi: 10.1093/clinids/20.6.1492. [DOI] [PubMed] [Google Scholar]
- 48.Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662–71. doi: 10.1016/s0016-5085(03)00324-x. [DOI] [PubMed] [Google Scholar]
- 49.Prindiville TP, Sheikh RA, Cohen SH, Tang YJ, Cantrell MC, Silva J., Jr Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg Infect Dis. 2000;6:171–4. doi: 10.3201/eid0602.000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toprak NU, Yagci A, Gulluoglu BM, et al. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect. 2006;12:782–6. doi: 10.1111/j.1469-0691.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 51.Raqib R, Lindberg AA, Wretlind B, Bardhan PK, Andersson U, Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun. 1995;63:289–96. doi: 10.1128/iai.63.1.289-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]