Abstract
Background
Venous thromboembolism (VTE) is common following lung transplantation. Enoxaparin is an approved therapy for VTE and anti-factor Xa (AXAL) can be used to monitor enoxaparin activity. Some studies have demonstrated elevated AXALs are associated with an increased risk of hemorrhage. Having identified a high incidence of supratherapeutic AXALs in lung transplant recipients, we aimed to elucidate the relationship between enoxaparin dose and AXAL in this patient population.
Methods
We identified post-lung transplantation patients with VTE receiving therapeutic enoxaparin who had AXAL measured. Standard enoxaparin dosing was defined as 0.9–1.1 mg/kg. After identifying a high incidence of supratherapeutic AXALs, we implemented “non-standard” dosing of 0.8 mg/kg. Multivariable linear regression analysis was used to examine the association between enoxaparin dose and AXAL; age, BMI, and creatinine clearance were included as covariates.
Results
In the cohort, 18 patients received standard and 8 patients received non-standard enoxaparin dosing. 12 of 18 patients (67%; 95% CI 43% to 91%) receiving standard dosing had supratherapeutic AXALs versus 0 of 8 patients (0%; 95% CI 0 to 37%) receiving lower non-standard dosing (p = 0.002). AXALs were significantly different between the two groups; the mean AXAL was 1.3 IU/ml (95% CI 1.06 to 1.53) in the standard group versus 0.79 IU/mL (95%CI 0.67 to 0.91) in the non-standard group (p = 0.008). After controlling for covariates, for each 0.1mg/kg increase in enoxaparin, the mean AXAL increased by 0.18 IU/mL (95% CI: 0.05 to 0.31; p = 0.011; model r2 = 0.53).
Conclusions
Standard dosing of enoxaparin in lung transplant recipients is associated with a high incidence of supratherapeutic anti-Xa levels. To correlate this finding with risk of hemorrhage requires further study.
INTRODUCTION
Venous thromboembolism (VTE) is a common post-operative complication in lung transplantation recipients.1–5 For lung transplantation recipients with VTE, enoxaparin represents an attractive therapeutic option and is consistent with the American College of Chest Physicians recommendations for the treatment of VTE.6 Due to its predictable pharmacology, enoxaparin does not warrant monitoring except in certain patient subpopulations.7, 8 Supratherapeutic enoxaparin, quantified by anti-factor Xa levels, is associated with increased risk of hemorrhage9 but this association has not been consistently observed.10
After noting cases of hemorrhage in lung transplant recipients anticoagulated with enoxaparin we implemented a mandatory anti-factor Xa monitoring program. During the program, we identified a high incidence of elevated anti-factor Xa levels and, in response, empirically reduced the initial dosing below the standard weight-based algorithm. This analysis delineates the relationship between enoxaparin and anti-factor Xa levels in a series of lung transplant recipients.
METHODS
Inclusion and Exclusion Criteria
All lung post-operative transplant recipients at the University of California, San Francisco ≥ 18 years of age between March 2006 and December 2009 were eligible for study entry. Inclusion criteria were patients with documented VTE who had received twice-daily therapeutic doses of subcutaneous enoxaparin and had anti-factor Xa activity levels measured. We excluded patients not treated with twice-daily therapeutic doses of enoxaparin or who did not have anti-factor Xa activity levels. Patient’s eligibility for study entry was based on a review of computerized medical records.
Study population
Until identifying several cases of hemorrhage in 2005 and early 2006, records systematically identifying patients on enoxaparin anticoagulation were not maintained. As a result, details on the number of patients who received enoxaparin without anti-Xa activity levels are not known. Once concern for hemorrhage was identified in March 2006, we implemented mandatory monitoring of anti-factor Xa levels on all patients treated with therapeutic doses of enoxaparin and uniform tracking was initiated. This subgroup of transplant recipients treated with enoxaparin between March 2006 and December 2009 comprises our study population.
Diagnosis of DVT
To diagnose suspected DVT, standard duplex ultrasonography was performed and interpreted by clinical radiologists trained in vascular ultrasonography. To diagnose suspected PE, standard contrast-enhanced PE protocols were performed using 16- or 64-slice multirow detector computed tomography imaging of the chest with 1.25 mm sections and interpreted by clinical chest radiologists. Repeat imaging studies to assess for resolution of clot burden after anticoagulation is not performed at our center.
Treatment algorithm
Prior to January 1st, 2009, patients maintained on therapeutic anticoagulation with enoxaparin were prescribed a “standard” dose of 1mg/kg (rounded to the nearest 10 mg) administered subcutaneously twice-daily. For the purposes of this analysis, we have defined standard enoxaparin dosing as 0.9–1.1mg/kg. After identifying a high incidence of supratherapeutic anti-factor Xa levels (>1.0 U/mL), the UCSF Lung Transplantation Service uniformly lowered the initial enoxaparin dosing to 0.8mg/kg twice-daily. This new dose was defined as “lower dose” administration.
Venous blood samples for anti-factor Xa activity level measurement were collected by trained clinical phlebotomists at UCSF. Samples were collected 4 hours after the third or fourth dose of enoxaparin. Peak levels best reflect the pharmacokinetic exposure to enoxaparin and steady state is estimated to be achieved at 21 hours post initiation of therapy.
For patients with supratherapeutic anti-factor Xa levels, enoxaparin doses were reduced by 10mg and anti-factor Xa levels were rechecked as described above. Doses were serially reduced, as necessary, to achieve anti-factor Xa levels between 0.6 – 1.0 IU/ml.
A volume of 2.7 mL of venous blood was collected in sodium citrate blue top tubes (BD vacutainer, Becton Dickinson) containing 0.3 mL 3.2% buffered sodium citrate, with a resultant 9:1 ratio of blood to anticoagulant. Specimens were centrifuged for 8 minutes at 5000 RPM to obtain platelet poor plasma within 4 hours of collection. For samples not tested immediately, the plasma aliquots were frozen at −30° centigrade. All anti-factor Xa analyses were performed in a single clinical laboratory using STA®-Rotachrom® Heparin kits (Diagnostica Stago, Parsippany, NJ). As part of quality assurance, the clinical laboratory tests two control samples twice daily. To test precision, each control is run five times in series every ten days. Linearity is tested by running 5-fold serial dilutions on the controls.
Primary outcome
The primary study outcome was defined as the peak plasma anti-factor Xa level recorded after the third or fourth dose of enoxaparin. The STA®-Rotachrom® Heparin kits reference range for therapeutic anti-Xa activity levels is 0.5–1.0 IU/mL. This reference range is also the recommended therapeutic window for clinical dosing of enoxaparin.6, 11 We, therefore, defined supratherapeutic anti-factor Xa activity as >1.0 IU/mL.
Covariates
Enoxaparin is renally excreted. Tacrolimus, which is part of the standard immunosuppressive regimen for post-lung transplant recipients at our institution was administered to all but one patient in this series. It causes impaired renal function via a number of postulated mechanisms, including arteriolar vasoconstriction, decreased endothelin-1, and decreased nitric oxide production.12 For this reason, in addition to quantifying anti-factor Xa levels, we also investigated the relationships among creatinine clearance, tacrolimus serum levels, and anti-Xa activity levels. Creatinine clearance was calculated based on ideal body weight using the Cockcroft-Gault equation.13 The tacrolimus level utilized for this study was a serum level drawn within 36 hours of the levels used for creatinine clearance and anti-Xa activity levels.
Statistical analysis
Statistical analysis was performed using STATA 11.0 (StataCorp, College Station, Texas). Bivariate analyses were conducted using the Fisher exact test for categorical variables and the Students t-test for normally distributed continuous variables. Comparisons of proportions were tested using the Fishers exact test. Spearman’s rank correlation was used to examine the association between creatinine clearance and anti-Xa activity levels and between plasma tacrolimus level and anti-factor Xa activity levels.
We selected additional covariates that might confound the relationship between the predictor and outcome measures of study interest. These included age, body mass index, creatinine clearance, tacrolimus level, and prothrombin time. Multiple linear regression analysis was used to examine the association between enoxaparin dose as the independent predictor and anti-factor Xa activity as the dependent variable. For the multivariable analysis, an Allen-Cady backward elimination procedure was used to retain covariates associated with the dependent variable at p ≤ 0.2. Creatinine clearance, identified in previous studies as an important covariate, was retained in the model on an a priori basis. All covariates were included in the model as continuous variables.
RESULTS
We identified 26 patients who received therapeutic anti-coagulation with enoxaparin and had plasma anti-factor Xa activity levels measured between March 2006 and December 2009. Of this group, seven were diagnosed with PE. Eighteen patients received standard enoxaparin dosing and 8 patients (all treated in 2009) received lower, non-standard dosing. The median time from the date of transplant to the diagnosis of VTE was 30 days (interquartile range: 19 to 340). Aside from a higher baseline prothrombin time in the low dose enoxaparin dosing group versus the standard enoxaparin dosing group (18.5 sec ± 8.3 vs. 14.1 sec ± 1.2, p = 0.03), there were no significant pre-treatment differences in demographic, laboratory, or medication measurements. Patient characteristics are presented in Table 1.
Table 1.
Baseline subject characteristics, laboratory features, and immunosuppressive regimen
| Characteristic | Standard (1mg/kg) enoxaparin dose n = 18 |
Non-standard (0.8mg/kg) enoxaparin dose n = 8 |
p-value for comparison |
|---|---|---|---|
| Age (years) | 57.7 (11.2) | 51.4 (10.2) | 0.19 |
| Sex (female) | 6 (24) | 3 (38) | 0.46 |
| Body mass index (kg/m2) | 26.7 (5.5) | 26.4 (3.9) | 0.91 |
| Serum creatinine (mg/dL) | 1.01 (0.33) | 1.23 (0.51) | 0.20 |
| Creatinine clearance (ml/min) | 88.3 (26.1) | 90.9 (16.2) | 0.80 |
| Prothrombin time (seconds) | 14.1 (1.2) | 18.5 (8.3) | 0.03* |
| Partial thromboplastin time (seconds) | 33.8 (14.1) | 30.7 (5.9) | 0.61 |
| AST (IU/L) | 27.5 (22.3) | 28.9 (14.6) | 0.87 |
| ALT (IU/L) | 32.0 (19.6) | 31.6 (23.3) | 0.97 |
| Serum tacrolimus level (mg/L) | 8.9 (2.9) | 9.5 (4.1) | 0.67 |
| Immunosuppressive Regimen | |||
| Tacrolimus | 18 (100) | 6 (88) | 0.13 |
| Mycophenolate mofetil | 15 (83) | 6 (75) | 0.62 |
| Prednisone | 18 (100) | 8 (100) | N/A |
| Voriconazole | 14 (78) | 6 (75) | 0.88 |
Format is n (%) for categorical variables or mean (sd) for continuous variables
In this study, 12 of 18 patients (67%; 95% CI: 43% - 91%) receiving standard enoxaparin dosing had supratherapeutic anti-factor Xa activity levels versus 0 of 8 (0%; 95% CI 0 – 37%) patients receiving lower non-standard dosing (p = 0.002). The mean post-enoxaparin treatment anti-factor Xa activity level was also significantly different between the two groups; it was 1.3 IU/ml (95% CI 1.06 – 1.53) in the standard dosing group versus 0.79 IU/mL (95%CI 0.67 – 0.91) in the lower, non-standard dose group (p = 0.008) (Figure 1.) There was no difference in mean anti-factor Xa levels in patients diagnosed with PE versus those diagnosed with DVT (p = 0.70). There was one case of subtherapeutic (<0.5 IU/ml) anti-factor Xa activity levels in the standard dosing group and none in the lower dosing group (p = 1.0). There was no difference in the mean anti-factor Xa levels drawn after the third dose compared to the fourth dose (p = 0.58)
Figure 1.
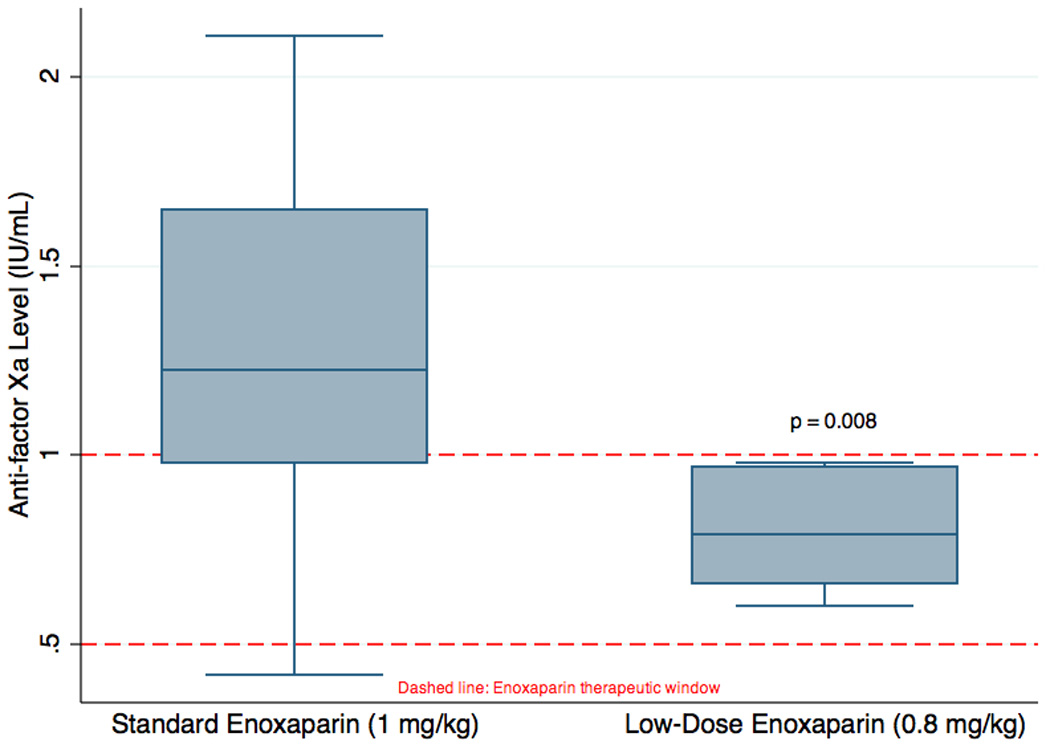
Anti-factor Xa levels for standard and non-standard low-dose enoxaparin.
For the 12 patients receiving standard enoxaparin dosing with supratherapeutic anti-factor Xa activity levels, their enoxaparin doses were reduced to a mean of 0.73mg/kg (SD ± 0.11) with a final anti-factor Xa level of 0.82 (SD ± 0.14). The mean enoxaparin dose and final anti-factor Xa level in this group were not different compared to the low-dose enoxaparin group (p = 0.13 and p = 0.88, respectively).
One patient in each group developed clinically significant hemorrhage while on enoxaparin therapy. In the standard dose group, one patient developed a subscapular hematoma with a 1.2 gm/dl fall in hemoglobin that required temporary cessation of enoxaparin therapy. In the low-dose group, one patient developed a large abdominal wall hematoma with 1.0 gm/dl fall in hemoglobin that also required temporary cessation of enoxparin therapy. Neither patient required blood transfusion. There were no cases of recurrent VTE in either group.
We found no association between creatinine clearance and anti-Xa activity levels (rs = 0.15, p = 0.46) or between plasma tacrolimus levels and anti-factor Xa activity levels (rs = 0.21, p = 0.31). There was a moderate negative correlation between plasma tacrolimus level and creatinine clearance (rs = −0.47, p = 0.02).
All 26 patients were included in the regression analysis. In unadjusted analysis, for every 0.1 mg/kg increase in enoxaparin dose, the mean anti-factor Xa level increased by 0.19 IU/ml ((95% CI: 0.02 to 0.35; p = 0.027; model r2 = 0.16). After adjusting for age, BMI, serum tacrolimus level, and creatinine clearance, for every 0.1 mg/kg increase in enoxaparin dose, the mean anti-factor Xa activity level increased by 0.18 IU/mL (95% CI: 0.05 to 0.31; p = 0.011; model r2 = 0.53) (Figure 2.).
Figure 2.
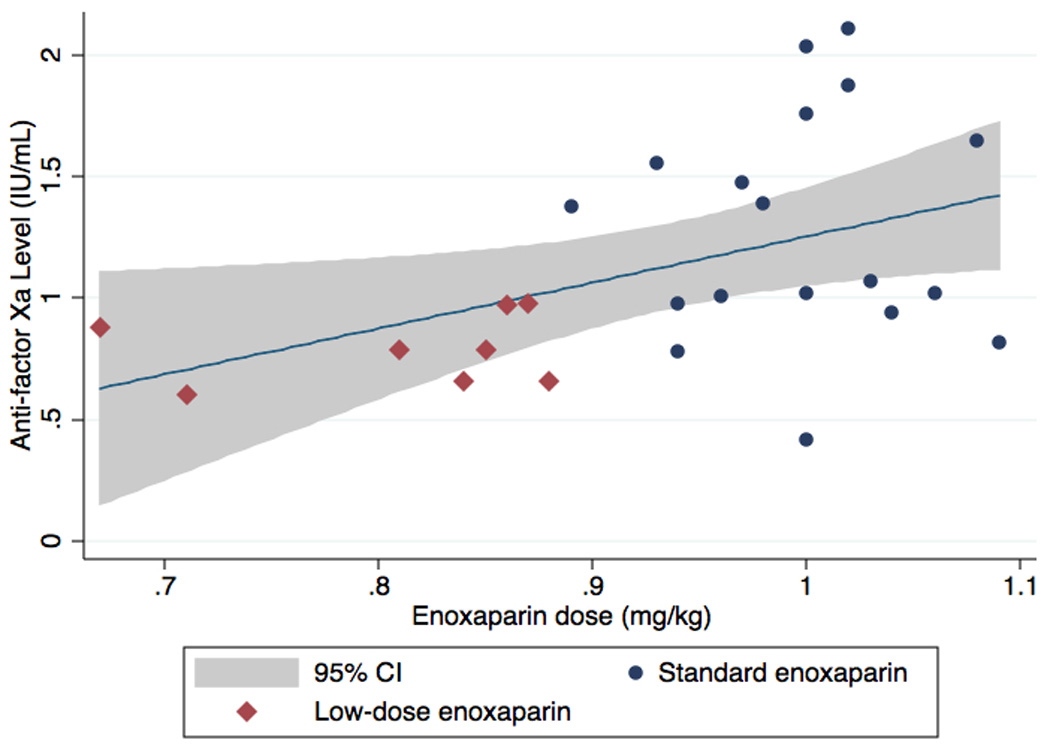
Relationship between enoxaparin dose and anti-Xa level adjusted for age, body mass index, serum tacrolimus level, and creatinine clearance. Shaded area: 95% confidence interval; red diamonds: low-dose enoxaparin; blue circles: standard enoxapar
DISCUSSION
We found that two-thirds of lung transplant patients treated with standard enoxaparin had supratherapeutic anti-factor Xa levels. Furthermore, when the dose of enoxaparin was lowered to 0.8 mg/kg twice-daily, there were no further cases of supratherapeutic anti-Xa levels and all patients were within the recommended therapeutic range [Absolute Risk Reduction: 67%, (95% CI: 45% to 88%)].
Enoxaparin is recommended as first-line therapy for the treatment of acute DVT and PE, collectively referred to as VTE.6 Its pharmacokinetic profile is based on its activity against coagulation factor Xa (anti-factor Xa).11 The pharmacokinetics of enoxaparin are linear and predictable permitting its standard dosing regimen.14 While elevated anti-Xa levels (> 1IU/ml) are associated with increased risk of hemorrhage, due to the predictable pharmacokinetics of enoxaparin routine monitoring of anti-factor Xa levels has been discouraged6 except for in certain patient populations including severe renal insufficiency, morbid obesity, and pregnancy.7, 8, 15 Our findings identify lung transplant recipients as an additional patient population where anti-factor Xa monitoring and/or dose adjustment may be important, especially considering that within the first year following transplantation, 8–29% of patients experience symptomatic VTE.2–4, 16
Additionally, anticoagulation after transplantation presents unique challenges. In the first several months after transplantation, patients commonly undergo invasive procedures. The need to frequently interrupt therapy makes anticoagulation with warfarin unwieldy. Therefore, enoxaparin represents a practical choice for anticoagulation in the early post-operative period.
We posited calcineurin inhibitor use might explain the supratherapeutic anti-factor Xa levels through its renal effects but we found no correlation between tacrolimus levels and anti-factor Xa levels. Presently, the etiology of the apparently altered pharmacodynamics of enoxaparin in lung transplant recipients remains uncertain.
Our study is subject to limitations. First, based on these limited data it is premature to argue for wide adoption of modified enoxaparin dosing based on established clinical benefit. While elevated anti-Xa levels have been associated with increased risk of hemorrhage, the data are inconsistent. Although differences were identified, our findings represent a single-center experience with a limited sample-size. Second, our sample size did not afford sufficient power to evaluate incident hemorrhage or recurrent VTE, nor do we have data from other solid-organ transplant recipients from our institution. Third, we do not have anti-factor Xa levels on patients who suffered hemorrhage prior to the study period nor on the use of prophylactic doses of enoxaparin. Fourth, we do not have data on patients exposed to enoxaparin for reasons other than VTE. Finally, larger studies are needed to confirm our findings and to determine whether increased anti-Xa levels are associated with increased risk of hemorrhage so that any potential clinical benefit might be quantified.
In summary, lung transplant recipients are at increased risk for supratherapeutic anti-Xa levels based on standard dosing of enoxaparin although an associated increased risk of hemorrhage has yet to be established.
Footnotes
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Burns KE, Iacono AT. Pulmonary embolism on postmortem examination: an under-recognized complication in lung-transplant recipients? Transplantation. 2004 Mar 15;77(5):692–698. doi: 10.1097/01.tp.0000114308.94880.2a. [DOI] [PubMed] [Google Scholar]
- 2.Izbicki G, Bairey O, Shitrit D, Lahav J, Kramer MR. Increased thromboembolic events after lung transplantation. Chest. 2006 Feb;129(2):412–416. doi: 10.1378/chest.129.2.412. [DOI] [PubMed] [Google Scholar]
- 3.Kahan ES, Petersen G, Gaughan JP, Criner GJ. High incidence of venous thromboembolic events in lung transplant recipients. J Heart Lung Transplant. 2007 Apr;26(4):339–344. doi: 10.1016/j.healun.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Kroshus TJ, Kshettry VR, Hertz MI, Bolman RM., 3rd Deep venous thrombosis and pulmonary embolism after lung transplantation. J Thorac Cardiovasc Surg. 1995 Aug;110(2):540–544. doi: 10.1016/S0022-5223(95)70252-0. [DOI] [PubMed] [Google Scholar]
- 5.Nathan SD, Barnett SD, Urban BA, Nowalk C, Moran BR, Burton N. Pulmonary embolism in idiopathic pulmonary fibrosis transplant recipients. Chest. 2003 May;123(5):1758–1763. doi: 10.1378/chest.123.5.1758. [DOI] [PubMed] [Google Scholar]
- 6.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008 Jun;133(6 Suppl):454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 7.Bazinet A, Almanric K, Brunet C, et al. Dosage of enoxaparin among obese and renal impairment patients. Thromb Res. 2005;116(1):41–50. doi: 10.1016/j.thromres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Friedrich E, Hameed AB. Fluctuations in anti-factor Xa levels with therapeutic enoxaparin anticoagulation in pregnancy. J Perinatol. 2010 Apr;30(4):253–257. doi: 10.1038/jp.2009.164. [DOI] [PubMed] [Google Scholar]
- 9.Levine MN, Planes A, Hirsh J, Goodyear M, Vochelle N, Gent M. The relationship between anti-factor Xa level and clinical outcome in patients receiving enoxaparine low molecular weight heparin to prevent deep vein thrombosis after hip replacement. Thromb Haemost. 1989 Nov 24;62(3):940–944. [PubMed] [Google Scholar]
- 10.Bara L, Leizorovicz A, Picolet H, Samama M. Correlation between anti-Xa and occurrence of thrombosis and haemorrhage in post-surgical patients treated with either Logiparin (LMWH) or unfractionated heparin. Post-surgery Logiparin Study Group. Thromb Res. 1992 Mar 1;65(4–5):641–650. doi: 10.1016/0049-3848(92)90213-t. [DOI] [PubMed] [Google Scholar]
- 11.Samama MM, Poller L. Contemporary laboratory monitoring of low molecular weight heparins. Clin Lab Med. 1995 Mar;15(1):119–123. [PubMed] [Google Scholar]
- 12.Baran DA, Galin ID, Gass AL. Calcineurin inhibitor-associated early renal insufficiency in cardiac transplant recipients: risk factors and strategies for prevention and treatment. Am J Cardiovasc Drugs. 2004;4(1):21–29. doi: 10.2165/00129784-200404010-00003. [DOI] [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 14.Hirsh J, Levine MN. Low molecular weight heparin. Blood. 1992 Jan 1;79(1):1–17. [PubMed] [Google Scholar]
- 15.Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008 Jun;133(6 Suppl):141S–159S. doi: 10.1378/chest.08-0689. [DOI] [PubMed] [Google Scholar]
- 16.Yegen HA, Lederer DJ, Barr RG, et al. Risk factors for venous thromboembolism after lung transplantation. Chest. 2007 Aug;132(2):547–553. doi: 10.1378/chest.07-0035. [DOI] [PubMed] [Google Scholar]


