Abstract
Pro- versus anti-inflammatory cytokine balance is important for successful pregnancy. Chronic hypoxia alters cytokine levels and increases the frequency of fetal growth restriction (FGR). Multigenerational Andean (AND) versus shorter duration European (EUR) high-altitude (HA) residents are protected from altitude-associated FGR. To address whether ancestry group differences in cytokine levels were involved, we conducted serial studies in 56 low-altitude ([LA]; 400 m; n = 29 AND and n = 27 EUR) and 42 HA residents (3600-4100 m; n = 19 ANDs and n = 23 EURs). Pregnancy raised pro- (interleukin 1β [IL-1β]) and anti- (IL-10) inflammatory cytokines and HA lowered IL-6 and tumor necrosis factor-α (TNF-α) near term. There were no ancestry group differences in cytokine levels at any time, but HA reduced IL-1β in ANDs only near term. Higher IL-1β levels correlated with uterine artery (UA) blood flow at 20 weeks in ANDs at HA, suggesting that IL-1β may play a role in AND protection from altitude-associated reductions in fetal growth.
Keywords: cytokines, hypoxia, pregnancy, uterine artery
Introduction
Pregnancy is an immunological state during which the balance between pro- and anti-inflammatory cytokines is important for embryonic implantation,1 placental growth,2 trophoblast differentiation, vascular remodeling,3–5 and the maintenance of pregnancy itself.6 Cytokines can be divided into 2 functional groups on the basis of having pro- versus anti-inflammatory effects due, in turn, to their involvement in cell-mediated immunity via T helper type-1 (Th1) reactions versus humoral responses involving T helper type-2 cells (Th2), respectively. In normal pregnancy, Th2 activity and anti-inflammatory cytokines increase during the first trimester in normal pregnancy, followed by increased Th1 activity and proinflammatory factors near term.6 In contrast, preeclampsia (PE) is marked by an increase in proinflammatory tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) cytokines as well as a decrease in the anti-inflammatory cytokines IL-4 and IL-10.7 In cases of restricted fetal growth, TNF-α is also elevated when compared with normal pregnancy.8,9
Although pregnancy is known to influence maternal cytokine levels, the precise time course of such variation remains unclear. Some reports describe higher IL-6 and TNF-α levels by mid-pregnancy,10 whereas others find that increases in IL-6 were complete after the first trimester and an absence of subsequent changes in IL-4, IL-10, or TNF-α.11 Chronic and acute hypoxia are also known to alter the levels of circulating cytokines both in the nonpregnant12 and in the pregnant condition,13 and to increase the frequency of the pregnancy complications of fetal growth restriction (FGR) and PE. Suggesting that alterations in cytokines might be involved in FGR associated with high-altitude (HA) residence, women living at 3100 versus 1600 m in Colorado had higher proinflammatory (IL-6, TNF-α) relative to anti-inflammatory (IL-10) cytokines during the second and third trimesters.13
Although altitude reduces fetal growth in every population studied to date,14,15 our and other’s studies have shown that longer term (Andean [AND], Tibetan) residents of HA are protected relative to shorter term HA (European [EUR] or Han Chinese) residents from altitude-associated reduction in fetal growth.16 Moreover, such protection is likely due, at least in part, to greater uterine artery (UA) blood flow as well as the delivery of oxygen and other vital nutrients required for fetal development.17–19 Since both acute and chronic hypoxia are known to influence cytokine levels,13,20–22 the current study sought to determine whether cytokine responses to pregnancy and/or altitude differed between AND and EUR women and, if so, whether and how such differences related to fetal growth.18 In particular, we asked whether HA ANDs versus EURs had lesser pregnancy-associated increases in pro- relative to anti-inflammatory cytokines and whether such variation related to the ancestry-associated differences in UA diameter, blood flow, and fetal growth. Findings obtained from studies such as this shed light on the physiological mechanisms contributing to protection against altitude-associated reductions in fetal growth and hopefully lead to improved prediction and, ultimately, prevention of FGR.
Materials and Methods
Participants were 98 women, comprising 29 ANDs and 27 EURs living at high altitude (HA, La Paz or El Alto, 3600-4000 m) and 19 ANDs and 23 EURs living at low altitude (LA, Santa Cruz, 400 m) in Bolivia. Women were recruited through their prenatal care providers. Criteria for inclusion were that the woman was healthy, not more than 20 weeks pregnant, receiving prenatal care, and at no known risk of developing PE as the result of having chronic hypertension, a history of PE, or being obese. All women provided informed written consent to study procedures that had been approved by the human subject review board of the University of Colorado Multiple Institutional Review Board and the Colegio Médico, its Bolivian counterpart.
Each woman completed a questionnaire regarding her ancestry and health history on the first visit. Women self-identified as being either AND (i.e., Aymara or Quechua) or EUR or other LA population origin. Ancestry was verified by parental and grandparental surnames and places of residence and confirmed using a panel of 100 ancestry informative markers (AIMs). Ancestry informative markers were used to assign the proportion of European, Indigenous American, or West African genetic ancestry for each woman, using the maximum likelihood method.18,19 Final assignment of AND ancestry required that a woman have at least 3 Aymara or Quechua surnames and at least 60% Indigenous American AIMs. Women were classified as EUR if they self-identified as being of EUR ancestry and had <50% Indigenous American AIMs or if they had >50% Indigenous American AIMs which we judged to be of LA origin, given their reported Guarani, Nahua (Aztec), or other LA population ancestry. Women who did not meet these criteria were classified as Mestiza and were excluded from the analyses presented here.
Protocol, Variables, and Instrumentation
Women were studied at 20 and 36 weeks of pregnancy as well as 4 months postpartum for a measurement in the nonpregnant state. Actual times of study were 21.2 ± 0.2 and 36.0 ± 0.3 weeks of pregnancy and 3.4 ± 0.2 months postpartum. Gestational age was calculated from the value estimated at the 20-week ultrasound examination, which in all cases agreed with that obtained using the elapsed weeks from the last menstrual period.
Body weight was determined on the day of study using a balance scale. Height was obtained at the first visit by stadiometer. Blood pressure was averaged from measurements obtained by arm cuff sphygmomanometer from the left and right arm. Women who developed PE, defined as 2 or more blood pressures >140/90 mm Hg accompanied by significant proteinuria (qualitative reading of >1+ by dipstick or 300 mg/L proteinuria in a 24-hour urine collection), were excluded from the analyses reported here.
Blood samples were obtained from the antecubital vein with minimal use of a tourniquet (at the same time of day between 9 and 11 am) in all women. Blood was withdrawn into collection tubes containing citrate, centrifuged, and the plasma stored in aliquots of 1 mL at −80°C. The levels of cytokines IL-1β, IL-4, IL6, IL-10, TNF-α, and IL-1 receptor antagonist (IL-1ra) were assayed in duplicate using a human cytokine 6-plex antibody bead kit (Bio Rad Laboratories, Richmond, California) and a Luminex-100 array assay reader (Luminex Corp, Austin, Texas). Analytes were considered detectable and reported if the levels exceeded the minimal standard concentrations as calculated from standard curves using 5-PL logistic regression and as specified by the manufacturer.
Uterine artery diameter, blood flow velocity, and fetal biometry were measured percutaneously using an obstetrical ATL 3000 Doppler ultrasound with color imaging and a 4-MHz curved linear array probe. The same manufacturer and models of machine were used at both altitudes to avoid instrument variability. The UA was examined at its crossover, with the external iliac artery and UA diameter and flow velocity averaged from values obtained bilaterally as previously described.18 Fetal biparietal diameter, head circumference, abdominal circumference, and femur length were measured after the maternal blood flow measurements, and corrected for actual gestational age at the time of ultrasound. Birth weight, length, gestational age, and infant gender were collected from medical records completed by hospital personnel at the time of delivery. Elective cesarean sections, which occurred approximately 1 week before the estimated due date, were more common in AND women at low than high altitude (67% vs 24%, P < .05), and in the EURs than ANDs at high altitude (62% vs 24%, P < .05). We, therefore, corrected birth weight and ponderal index data for actual gestational age at birth to account for these differences.
Statistics
Data are reported as the mean ± standard error of the mean (SEM) or 95% confidence interval (CI) for proportions. Comparisons between ancestry and altitude groups at single time points were conducted using t tests for continuous variables and χ2 test for nominal or ordinal variables. The effects of pregnancy, ancestry, or altitude on circulating cytokine levels were tested using 1- or 2-way analysis of variance (ANOVA) and differences over time determined with Scheffé post hoc tests. Relationships between pro- or anti-inflammatory factors and UA diameter, UA blood flow, or birth weight were assessed using simple or multiple linear regressions. Actual gestational age and maternal height were used as covariates when comparing the effects of ancestry or altitude on birth weight or ponderal index. All analyses were conducted using SPSS (v 12.0, Chicago, Illinois). Results were considered significant when P values < .05 and reported as trends when .05 < P < .10.
Results
Maternal Characteristics
The LA compared to the HA EUR women had a lower proportion of AIMs attributable to EUR origin due to greater admixture with LA Indigenous American groups (Table 1 ). Low-altitude ANDs also had a somewhat lower proportion of Indigenous American AIMs origin than their HA counterparts, but Indigenous American AIMs comprised the overwhelming majority of AIMs at both altitudes. All women had relatively low percentages of West African AIMs (Table 1).
Table 1.
Maternal Characteristicsa
| Variables | Ancestry | Altitude |
Ancestry |
|||
|---|---|---|---|---|---|---|
| Low | High | Altitude | Low | High | ||
| AIMs % | ||||||
| European | European | 51.6 ± 3.5 (23) | 73.0 ± 4.1 (28) | P < .001 | ||
| Andean | 15.4 ± 2.0 (19) | 4.3 ± 1.4 (29) | P < .001 | P < .001 | P < .001 | |
| Amer-Indian | European | 38.7 ± 2.8 (23) | 20.0 ± 3.8 (28) | P < .001 | ||
| Andean | 74.3 ± 1.9 (19) | 93.6 ± 1.6 (29) | P < .001 | P < .001 | P < .001 | |
| African | European | 9.6 ± 1.2 (23) | 7.1 ± 1.2 (29) | NS | ||
| Andean | 10.4 ± 0.8 (19) | 2.1 ± 0.7 (28) | P < .001 | NS | P < .01 | |
| Age, year | European | 27.4 ± 5.5 (23) | 31.5 ± 0.8 (27) | P < .01 | NS | P < .01 |
| Andean | 25.4 ± 1.4 (19) | 27.2 ± 1.3 (29) | NS | |||
| Parity, no live births | European | 1.7 ± 0.2 (23) | 2.0 ± 0.2 (28) | NS | NS | NS b |
| Andean | 1.6 ± 0.2 (19) | 2.7 ± 0.3 (29) | P < .05 | |||
| Height, cm | European | 159.3 ± 1.1 (23) | 161.3 ± 1.3 (28) | NS | P < .01 | P < .001 |
| Andean | 155.3 ± 0.9 (19) | 150.4 ± 0.9 (29) | P < .001 | |||
| Weight gain (20-36 weeks), kg | European | 9.7 ± 0.7 (21) | 6.9 ± 0.8 (17) | P < .05 | NS | NS |
| Andean | 8.4 ± 1.2 (15) | 6.1 ± 0.6 (27) | NS | |||
| Weight at 36 weeks, kg | European | 76.7 ± 2.7 (21) | 71.9 ± 1.8 (26) | NS | P < .05 | P < .05 |
| Andean | 68.7 ± 2.7 (15) | 66.4 ± 1.8 (29) | NS | |||
| Household income, US$/month | European | 329 ± 64 (19) | 1396 ± 330 (29) | P < .01 | NS | P < .01 |
| Andean | 219 ± 43 (13) | 142 ± 33 (18) | NS | |||
| Residence >2500 m, year | European | --- | 10.2 ± 2.1 (26) | --- | --- | P < .001 |
| Andean | 21.5 ± 1.7 (29) | --- | ||||
| >Secondary education, % | European | 68 (46, 86) (23) | 54 (35,71) (28) | NS | NS | P < .01 |
| Andean | 58 (36, 78) (19) | 21 (9, 38) (29) | P < .05 | |||
Abbreviations: AIMs = ancestry informative markers; NS, not significant.
a Values are shown as mean ± SEM or 95% confidence intervals for proportions with sample sizes in parentheses
b .05 < P < .10 and NS.
Low-altitude EURs compared with ANDs were taller and weighed more at 36 weeks of pregnancy but were similar in other respects (Table 1). High-altitude EURs were older, taller, weighed more at 36 weeks pregnancy, had greater monthly incomes, higher educational levels, and had lived at HA for fewer years than the AND women (Table 1). Comparing altitudes, LA EURs were younger, gained more weight from 20 to 36 weeks of pregnancy, and had lower incomes than their HA counterparts. Low-altitudes versus HA ANDs had lower parity, were taller, and had had more years of schooling (Table 1).
Proinflammatory Cytokines
Pregnancy increased IL-1β levels at 20 weeks in both ancestry groups and at each altitude, with values diminishing near term (Table 2 ). There were no differences between ancestry groups at either altitude, but HA residence lowered IL-1β levels when nonpregnant and near term in ANDs only.
Table 2.
Pro- and Anti-Inflammatory Cytokinesa
| Interacions |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Altitude | Ancestry | Nonpregnant | Week of Pregnancy |
(Time*Ancestry) | (Time*Altitude) | |||
| Week 20 | Week 36 | Time | Ancestry | European | Andean | ||||
| Proinflammatory cytokines | |||||||||
| IL-1B, pg/mL | Low | European | 0.87 ± 0.14 (15) b | 1.36 ± 0.16 (18)c,d | 0.73 ± 0.05 (14) b | P < .01 | NS | NS | NS |
| Low | Andean p-ancestry | 0.87 ± 0.11 (15) b NS | 1.44 ± 0.10 (17) c NS | 1.14 ± 0.19 (13) NS | P < .05 | ||||
| High | European | 0.63 ± 0.06 (14) b | 1.60 ± 0.25 (18)c,d | 0.72 ± 0.10 (17) b | P < .001 | NS | |||
| High | Andean p-ancestry | 0.58 ± 0.06 (20)b,e NS | 1.38 ± 0.12 (20)c,d NS | 0.66 ± 0.04 (17)b,e NS | P < .001 | ||||
| IL-6, pg/mL | Low | European | 7.11 ± 1.07 (15) b | 2.77 ± 0.35 (9)c,d | 7.52 ± 0.97 (15) b | P < .01 | NS | P < 0.01 | P < .001 |
| Low | Andean p-ancestry | 6.85 ± 1.17 (15) b NS | 3.49 ± 0.65 (16)c,d NS | 9.25 ± 1.83 (13) b NS | P < .01 | ||||
| High | European | 3.60 ± 0.53 (19)* | 4.20 ± 0.61 (13) e | 4.18 ± 0.42 (19) e | NS | NS | |||
| High | Andean p-ancestry | 3.92 ± 0.66 (22) e NS | 3.10 ± 0.57 (13)NS | 3.89 ± 0.31 (22) NS e | NS | ||||
| TNF-α, pg/mL | Low | European | 7.78 ± 1.55 (15) | 2.50 ± 0.29 (9)d,e | 12.28 ± 2.79 (16) b | P < .05 | NS | P < .05 | P < .05 |
| Low | Andean p-ancestry | 9.21 ± 1.61 (17) NS | 5.20 ± 1.80 (12) NS | 9.12 ± 1.91 (13) NS | NS | ||||
| High | European | 4.26 ± 0.63 (14) e | 4.38 ± 0.68 (10) e | 4.81 ± 0.88 (16) e | NS | NS | |||
| High | Andean p-ancestry | 4.41 ± 0.85 (19) e NS | 8.84 ± 2.61 (8) NS | 5.00 ± 0.93 (24) e NS | NS | ||||
| Anti-inflammatory cytokines | |||||||||
| IL-1ra, pg/mL | Low | European | 96.00 ± 24.84 (12) | 45.06 ± 9.01 (9) c | 148.59 ± 21.30 (12) b | P < .01 | NS | P < .05 | NS |
| Low | Andean p-ancestry | 109.64 ± 26.49 (12) b NS | 42.60 ± 5.33 (12)c,d NS | 118.82 ± 16.59 (12) b NS | P < .05 | ||||
| High | European | 116.87 ± 33.14 (9) | 98.24 ± 19.92 (13) e | 100.60 ± 14.77 (16) e | NS | NS | |||
| High | Andean p-ancestry | 92.68 ± 19.45 (12)NS | 92.32 ± 23.62 (8) e NS | 91.34 ± 12.30 (15) NS | NS | ||||
| IL-4, pg/mL | Low | European | 1.56 ± 0.35 (11) e | 1.84 ± 0.49 (5) | 1.52 ± 0.36 (14) | NS | NS | NS | NS |
| Low | Andean p-ancestry | 0.97 ± 0.21 (11) NS | 0.94 ± 0.19 (6)NS | 1.29 ± 0.28 (12)NS | NS | ||||
| High | European | 0.74 ± 0.18 (10) e | 1.60 ± 0.61 (8) | 0.66 ± 0.15 (13)* | NS | NS | |||
| High | Andean p-ancestry | 0.68 ± 0.13 (11) NS b | 1.36 ± 0.03 (3) NSc,d | 0.66 ± 0.10 (14)b,e NS | P < .05 | ||||
| IL-10, pg/mL | Low | European | 1.37 ± 0.18 (10) b | 5.09 ± 0.99 (10)c,d | 2.11 ± 0.45 (13) a | P < .01 | NS | NS | NS |
| Low | Andean p-ancestry | 1.82 ± 0.38 (12) b NS | 4.29 ± 0.92 (12) c NS | 2.33 ± 0.48 (13)NS | P < .05 | ||||
| High | European | 1.14 ± 0.30 (14) b | 3.88 ± 0.81 (13)c,d | 1.64 ± 0.25 (19) b | P < .01 | NS | |||
| High | Andean p-ancestry | 1.26 ± 0.23 (19) a NS | 3.43 ± 0.56 (7)c,d NS | 1.52 ± 0.25 (24) b NS | P < .05 | ||||
Abbbreviations: ANOVA, analysis of variance; IL, interleukin; TNF, tumor necrosis factor; NS, not significant;.
a Effects of time and ancestry were evaluated by 1-(time, altitude) or 2-way ANOVA (interaction between time and altitude within ancestry group).Values are shown as mean ± SEM with sample sizes in parentheses and = .05 < P < .10.
Significant differences between time points within ancestry and altitude groups are designated as follows:
b Significantly different from week 20.
c Significantly different from nonpregnant value.
d Significantly different from week 36.
e P < .05 for altitude comparisons within ancestry groups and week of pregnancy.
Interleukin 6 levels decreased at 20 weeks of pregnancy at LA, with values rising by 36 weeks in both ancestry groups (Table 2). There were no changes during pregnancy at HA in either ancestry group. Interleukin 6 levels were higher at LA versus HA across all time points in both ancestry groups.
Tumor necrosis factor-α levels fell by 20 weeks at LA, with values rising near term in the EUR women only. At HA, there was no change in TNF-α levels among EURs. Values tended to rise in ANDs by 20 weeks and then decrease near term. Both ancestry groups had generally lower TNF-α levels at HA versus LA (Table 2).
Anti-Inflammatory Cytokines
At LA, IL-1ra levels were lower at 20 weeks of pregnancy than when nonpregnant, and then rose near term. IL-1ra values were unchanged by pregnancy at HA. Low-altitude versus HA EURs and ANDs had lower IL-1ra levels at 20 weeks (Table 2).
Interleukin 4 levels were unchanged by pregnancy at LA but rose by 20 weeks in HA ANDs (Table 2). There were no differences between ancestry groups at a given altitude or between altitudes.
Interleukin 10 rose by 20 weeks in both ancestry groups at LA, with values decreasing near term (Table 2). At HA, IL-10 levels rose above nonpregnant levels at 20 weeks and decreased by 36 weeks in both ancestry groups. There were no differences between ancestry groups at a given altitude or between altitudes.
Relationship Between Cytokines, UA Blood Flow, and Fetal Growth
Pregnancy increased UA diameter by 20 weeks in all groups (Table 3 ). Although the increment in UA diameter, velocity, and blood flow was similar in ANDs and EURs at LA, the pregnancy-associated increases in UA diameter, velocity, and blood flow were greater in ANDs versus EURs at HA (Table 3).
Table 3.
Uterine Artery Characteristics and Fetal Biometrya
| Variable | Altitude | Ancestry Group | Nonpregnant | Week of Pregnancy |
P Time | |
|---|---|---|---|---|---|---|
| Week 20 | Week 36 | |||||
| UA diameter, cm | Low | European | 0.26 ± 0.01 (22) | 0.50 ± 0.02 (23) | 0.50 ± 0.02 (22) | P < .001 |
| Andean p-ancestry | 0.20 ± 0.01 (16) P < .05 | 0.47 ± 0.01 (18) NS | 0.46 ± 0.02 (16) NS | P < .001 | ||
| High | European | 0.42 ± 0.05 (21) b | 0.54 ± 0.51 (18) | 0.54 ± 0.01 (26) | P < .001 | |
| Andean p- ancestry | 0.39 ± 0.01 (11)* NS | 0.63 ± 0.01 (27) b P < .001 | 0.64 ± 0.01 (28) b P < .001 | P < .001 | ||
| UA blood flow, mL/min | Low | European | 23.45 ± 3.39 (22) | 288.66 ± 24.46 (23) | 398.33 ± 39.16 (22) | P < .001 |
| Andean p-ancestry | 12.50 ± 2.77 (16) P < 0.05 | 285.58 ± 31.05 (18) NS | 345.63 ± 35.41 (16) NS | P < .001 | ||
| High | European | 78.18 ± 17.50 (15)* | 343.82 ± 43.50 (17) | 401.95 ± 36.39 (24) | P < .001 | |
| Andean p- ancestry | 69.27 ± 24.52 (3) NS | 494.80 ± 70.57 (11) b | 678.15 ± 72.75 (19) b NS P < .01 | P < .001 | ||
| UA flow velocity, | Low | European | 7.05 ± 0.68 (22) | 24.26 ± 1.45 (23) | 31.22 ± 2.16 (22) | P < .001 |
| Andean p-ancestry | 5.48 ± 0.46 (16) NS | 26.13 ± 2.53 (18) NS | 33.60 ± 2.56 (16) NS | P < .001 | ||
| High | European | 11.30 ± 2.42 (15) | 24.12 ± 2.45 (17) | 28.24 ± 2.01 (24) | P < .05 | |
| Andean p- ancestry | 10.25 ± 4.13 (3) NS | 25.36 ± 3.31 (11) NS | 37.30 ± 4.19 (20) P < .05 | P < .05 | ||
| Biparietal diameter, cm | Low | European | --- | 4.87 ± 0.16 (22) | 8.88 ± 0.08 (22) | P < .001 |
| Andean p-ancestry | --- | 5.26 ± 0.17 (18) NS | 8.85 ± 0.07 (16) NS | P < 0.001 | ||
| High | European | --- | 5.40 ± 0.14 (18) b | 9.05 ± 0.07 (26) | P < .001 | |
| Andean p- ancestry | --- | 5.68 ± 0.12 (26) NS | 8.89 ± 0.07 (26) NS | P < .001 | ||
| Head circum, cm | Low | European | --- | 17.10 ± 0.53 (22) | 30.37 ± 0.72 (22) | P < .001 |
| Andean p-ancestry | --- | 18.92 ± 0.61 (17) P < .05 | 31.22 ± 0.85 (16) NS | P < .001 | ||
| High | European | --- | 19.19 ± 0.51 (18) b | 31.82 ± 0.59 (26) | P < .001 | |
| Andean p- ancestry | --- | 20.50 ± 0.43 (25) NS | 31.01 ± 0.59 (26) NS | P < .001 | ||
| Abdominal circum, cm | Low | European | --- | 15.74 ± 0.53 (22) | 31.71 ± 0.36 (22) | P < .001 |
| Andean p-ancestry | --- | 16.92 ± 0.58 (18) NS | 31.44 ± 0.43 (16) NS | P < .001 | ||
| High | European | --- | 18.05 ± 0.51 (17) b | 32.16 ± 0.33 (26) | P < .001 | |
| Andean p- ancestry | --- | 19.05 ± 0.42 (26) NS | 31.55 ± 0.33 (26) NS | P < .001 | ||
| Femur length, cm | Low | European | --- | 3.41 ± 0.13 (22) | 7.00 ± 0.05 (22) | P < .001 |
| Andean p-ancestry | --- | 3.75 ± 0.14 (18) NS | 6.95 ± 0.06 (16) NS | P < .001 | ||
| High | European | --- | 3.69 ± 0.12 (18) | 7.00 ± 0.78 (26) | P < .001 | |
| Andean p- ancestry | --- | 4.11 ± 0.10 (26) P < .05 | 8.08 ± 0.78 (26) NS | P < .01 | ||
Abbreviations: circum, circumference; SEM, standard error of mean; NS, not significant; UA, uterine artery.
a Values are shown as mean ± SEM, with sample sizes in parentheses and = .05< P < .10. Corrected for actual gestational age.
b P < .05 for altitude comparisons within ancestry groups and week of pregnancy.
AND compared with EUR babies at 20 weeks had greater femur lengths at HA and greater head circumferences at LA (Table 3). All fetal biometry measures were similar in ANDs at HA versus LA, but EUR LA babies had smaller biparietal diameters, abdominal, and head circumferences than their HA counterparts.
Low-altitude EURs who delivered by cesarean section delivered earlier than ANDs at LA and earlier than either group at HA. This was due to the practice of scheduling cesarean sections at LA approximately 1 week before the due date. For this reason, we adjusted birth weight for actual gestational age and maternal height, another known determinant of birth weight. After correcting for such covariates, EUR babies at HA weighed less than their LA counterparts and also less than HA ANDs (Table 4 ). Ponderal index was also lower at HA versus LA among EURs, irrespective of whether the data were corrected for variation in gestational age and maternal height. Ponderal index was also lower at HA versus LA among EURs, irrespective of whether the data were corrected for variation in gestational age but similar in HA vs. LA ANDs.
Table 4.
Newborn and Delivery Characteristicsa
| Variable | Ancestry | Altitude |
P Altitude | |
|---|---|---|---|---|
| Low | High | |||
| Head circumference, cm | European | 35.1 ± 0.3 (22) | 34.6 ± 0.3 (24) | NS |
| Andean p-ancestry | 35.4 ± 0.2 (18) NS | 34.2 ± 0.3 (24) NS | P < .01 | |
| Gestational age, wks | European | 37.7 ± 0.4 (22) | 39.3 ± 0.3 (28) | P < .01 |
| Andean p-ancestry | 39.5 ± 0.5 (18) P < .01 | 39.2 ± 0.3 (28) NS | NS | |
| Cesarean section, % | European | 59 (38, 78) (22) | 62 (47, 80) (24) | NS |
| Andean p-ancestry | 67 (44, 85) (18) NS | 24 (11, 42) (29) P < .05 | P < .05 | |
| Birth weight, g | European | 3423 ± 91 (22) | 3197 ± 62 (28) | P < .05 |
| Andean p-ancestry | 3301 ± 112 (18) NS | 3198 ± 63 (29) NS | NS | |
| Adjusted birth weight, g | European | 3411 ± 108 (22) | 3087 ± 69 (28) | P < .05 |
| Andean p-ancestry | 3315 ± 122 (18) NS | 3322 ± 69 (28) P < .05 | NS | |
| Ponderal index, kg/m3 | European | 28.1 ± 0.5 (22) | 25.7 ± 0.7 (27) | P < .05 |
| Andean p-ancestry | 26.9 ± 0.7 (18) NS | 27.1 ± 0.7 (26) NS | NS | |
| Adjusted ponderal index, kg/m3 | European | 27.6 ± 0.7 (22) | 25.1 ± 0.8 (27) | P < .05 |
| Andean p-ancestry | 27.4 ± 0.6 (18) NS | 27.9 ± 0.9 (25) P < .05 | NS | |
Abbreviations: NS, not significant; SEM, standard error of mean;
a Values are shown as mean ± SEM or 95% confidence intervals for proportions with sample sizes in parenthenses; = 0.05 < P < .10 and NS. Adjusted values are corrected for gestational age and maternal height, and displayed for comparisons between ancestry groups within altitude.
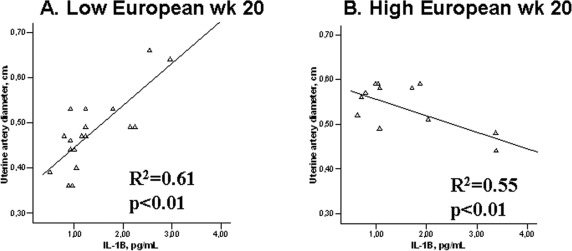
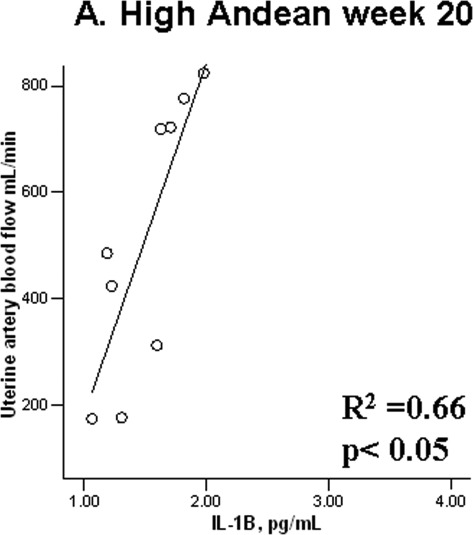
Among EURs, higher levels of the proinflammatory cytokine IL-1β at 20 weeks were associated with greater UA diameters at LA (Figure 1A) and a trend toward greater UA blood flow (R 2 = .25, P = .09). At HA, the opposite pattern was observed, with higher IL-1β levels associated with smaller UA diameters (Figure 1B). Among ANDs, there was a strong positive association between IL-1β and UA blood flow at HA (R 2 = .66, P < .05; Figure 2 ).
Figure 1.
A, Low-altitude European women with higher IL-1B levels, have greater uterine artery diameters at 20 weeks of pregnancy. B, European women with higher IL-1B levels at 20 weeks of pregnancy have smaller uterine artery diameters at high altitude. IL indicates interleukin.
Figure 2.
Andean women with higher IL-1B levels at 20 weeks of pregnancy have greater uterine artery blood flows at high altitude. IL indicates interleukin.
Discussion
This is the first study to compare pro- and anti-inflammatory cytokines in AND versus EUR residents of HA, groups known to differ in their susceptibility to altitude-associated reductions in fetal growth. Our results are novel insofar as they demonstrate time-dependent alterations in the levels of both pro- and anti-inflammatory substances during pregnancy. Specifically, proinflammatory IL-1β and anti-inflammatory IL-10 increased by mid-pregnancy, whereas a late-pregnancy rise was seen for IL-1ra, and proinflammatory IL-6 and TNF-α. As opposed to previous reports,11,13 our study also demonstrated that HA influenced cytokine levels by reducing pro- and anti-inflammatory factors in the nonpregnant state and at individual time points during pregnancy. These observations support the idea that cytokine production behaves differently when the challenges of pregnancy are combined with those of exposure to HA. We did not find that cytokine levels were different between ancestry groups at any given time. However, we did find that IL-1β, a cytokine known to promote vasodilation23 and to be involved in pregnancy-associated UA changes,4 was positively associated with UA blood flow in ANDs and negatively associated with UA diameter in EUR women at HA. Thus, we speculate that this differences in IL-1β associations between AND versus EUR residents of HA, may contribute to the ANDs's greater UA enlargement, rise in UA blood flow, and hence protection from altitude-associated in fetal growth.
Interpretation of our study results should consider limitations posed by the nature of our study design. First, the relationships between cytokine levels and birth weight or UA blood flow characteristics are associational and do not suggest a cause and effect relationship. Second, although we recruited the same number of participants at both altitudes, sample sizes available for analysis were reduced by the necessity to exclude women with greater admixture, limitations in the assay which prevented the detection of very low values and the lack of complete blood flow data in all participants. Third, we were not able to study the women before they became pregnant but nonpregnant values were obtained ~4 months postpartum, which is well after any labor-associated changes in cytokine production would be expected to be present. Finally, since only maternal blood samples could be obtained, we were not able to distinguish between the contributions of maternal versus fetal sources of production and/or metabolism to differences in circulating levels, nor was the information in this or previous studies able to tell what differences may have been present at the cellular level. Study strengths were its longitudinal nature and our ability to define ancestry groups with a high degree of resolution by the use of a large number of gene markers and detailed 3-generation genealogical histories.
Previous studies at LA have described an increase in the proinflammatory IL-6 and TNF-α by mid-pregnancy,10 with elevations in IL-6 levels being maintained until labor. We found a somewhat different pattern in which IL-6 and TNF-α levels as well as those of the anti-inflammatory IL-1ra were below nonpregnant values at 20 weeks and then all 3 cytokines returned to or exceeded the nonpregnant values at 36 weeks. In addition, unlike prior reports in which higher IL-10 levels were only present during labor, we found higher levels at mid-gestation, which then tapered off toward nonpregnant values by term.11 We consider that cytokine profile variation between this and other studies may be due to differences in sampling intervals10 and ethnicity of the participants included for the study.10,11,24
At HA, Coussons et al found lower IL-10 values during the second and third trimesters in Colorado residents of 3100 versus 1600 m,13 which is qualitatively similar to the results reported here. However, IL-6 and TNF-α levels increased more near term in the HA versus LA Colorado women, which was not the pattern observed in this report perhaps as a result of the higher altitudes of the current study. Not only were IL-6 and TNF-α lower at HA than LA but AND’s IL-1β, EUR’s IL-1ra, and IL-4 levels in both ancestry groups were also lower near term at HA. One factor that may contribute to such an altitude-associated decline in cytokine levels is the previously described reduction in CD4+ T lymphocytes seen under conditions of chronic hypoxia,20 given the role of CD4+ lymphocytes in regulating Th1- or Th2-mediated cytokine production.6
Another factor that is likely important is the time point at which altitude-associated influences on cytokine levels occur. We found that the pro- (IL-1β) and anti-inflammatory (IL-10) cytokine levels were above nonpregnant levels at 20 weeks. We also observed higher Placental growth factor (PlGF) levels at HA than LA by week 20 (Davila 2010, personal communication). Such findings are consistent with the hypothesized 2-stage model in which hypoxia is postulated to exert a stimulatory effect on angiogenic factors early in pregnancy,13 followed by reductions in the levels of such substances. These effects may influence the likelihood of reduced fetal growth.25
Consistent with our and other’s studies, AND women in the current study gave birth to heavier birth-weight babies than EUR residents of HA. In addition, unlike the EURs, the ANDs had babies whose birth weights were the same as their LA counterparts, after adjusting for the known influences of gestational age and maternal height.16–18 Similar group differences were evident when ponderal index rather than birth weight was compared, which indicated that protection from altitude-associated reductions in fetal growth were chiefly responsible for the AND’s heavier birth weights rather than the ANDs simply having larger babies at any altitude. As we have argued elsewhere, the AND’s larger UA diameters and higher blood flow were present by mid-gestation, or perhaps even earlier as suggested by the presence of fetal-biometry differences at 20 weeks, suggesting that the higher UA blood flows were an important contributor to the birth weight differences observed. Consistent with this, is the strong positive relationship between IL-1β and higher UA blood flow observed at mid-pregnancy (Figure 2), the same time point at which the increase of UA diameter and blood flow in HA AND versus EUR women were observed. Among LA EURs, higher IL-1β levels were also associated with greater UA diameters, whereas their counterparts at HA had smaller diameters when higher IL-1β levels were observed (Figure 1). Whether the cause of these differing associations between IL-1β levels and UA diameter are unknown, one possibility is that other factors (eg, vasoconstrictors) may be influencing and/or antagonizing the vasodilatory effects of IL-1β in EUR at HA. Consistent with the positive association between IL-1β and UA diameter, both IL-1α and IL-1β are known to have angiogenic effects.26,27 Moreover, the UA is known to express IL-1 and to demonstrate increased IL-1 activity and concentrations during pregnancy.4 A mechanism by which such an association could occur is IL-1β-induced activation of cyclic guanosine monophosphate (cGMP) and stimulation of nitric oxide activity,23 which could then be expected to result in greater UA vasodilation. Thus, it is possible that the lower levels of this proinflammatory cytokine by week 36 and their strong association with UA blood flow by mid-pregnancy may have contributed to the ANDs protection from altitude-associated reductions in fetal growth.
In summary, our study demonstrated that both pregnancy stage and altitude influenced cytokine levels. In particular, hypoxia diminished the proinflammatory IL-1β, IL-6, and TNF-α cytokines late in gestation, whereas ancestry did not affect them overall. Contrary to our hypothesis, ANDs compared to EURs did not have a lesser pregnancy-associated rise in pro- relative to anti-inflammatory substances at HA. Yet consistent with our hypothesis was that HA ANDs experienced a greater reduction than HA EURs in the proinflammatory IL-1β cytokine late in pregnancy. The strong positive association between IL-1β and UA blood flow by week 20 observed in HA AND alone suggests that this cytokine might be important in relation to the ANDs' greater UA blood flow and protection from altitude-associated FGR. Needed are direct measurements of UA IL-1β activity as well as evaluation of whether ANDs are characterized by genetic variation affecting cytokine production or activity.
Acknowledgments
We thank all the women who kindly agreed to participate in this study as well as the many technicians for their invaluable support. In particular, we thank Martha Aguilar, Ana-María Alarcón, Jose Luis Casanova, Dolly Condori, Cristina Gonzáles, Jennifer Hageman, Freddy Limachi, Lourdes Mabrich, Zaida Martínez, Gene and Rosann McCullough, Julio Roca, Armando Rodriguez, and Wilmar Velásquez. We also express our appreciation to the several physicians who helped with participant recruitment and study conduct, including Drs J. Fernando Armaza, María del Pilar García, Jessica Pardo, Jessica Schaymann, Marco Vargas, and Elizabeth Zelada.
Footnotes
The author(s) declared no conflicts of interests with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: the National Institutes of Health (HL60131, HL07171, and HL079647), a National Sciences Foundation predoctoral fellowship (MJW) and Graduate Research support (CGJ), and an American Heart Association predoctoral fellowship (CGJ) (0610129Z).
References
- 1. Salamonsen LA, Hannan NJ, Dimitriadis E. Cytokines and chemokines during human embryo implantation: roles in implantation and early placentation. Semin Reprod Med. 2007;25(6):437–444 [DOI] [PubMed] [Google Scholar]
- 2. Krussel JS, Bielfeld P, Polan ML, Simon C. Regulation of embryonic implantation. Eur J Obstet Gynecol Reprod Biol. 22 2003;110(suppl 1):S2–S9 [DOI] [PubMed] [Google Scholar]
- 3. Karmakar S, Das C. Regulation of trophoblast invasion by IL-1beta and TGF-beta1. Am J Reprod Immunol. 2002;48(4):210–219 [DOI] [PubMed] [Google Scholar]
- 4. Huleihel M, Leiberman JR, Yohay D, Holcberg G, Katz M, Mazor M. IL-1 activity is expressed differently during pregnancy in the rat uterine artery than in aortic or uterine tissues. Am J Reprod Immunol. 2002;48(3):163–169 [DOI] [PubMed] [Google Scholar]
- 5. Librach CL, Feigenbaum SL, Bass KE, et al. Interleukin-1 beta regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. J Biol Chem. 1994;269(25):17125–17131 [PubMed] [Google Scholar]
- 6. Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16(2):206–215 [DOI] [PubMed] [Google Scholar]
- 7. Teran E, Escudero C, Moya W, Flores M, Vallance P, Lopez-Jaramillo P. Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. Int J Gynaecol Obstet. 2001;75(3):243–249 [DOI] [PubMed] [Google Scholar]
- 8. Bartha JL, Romero-Carmona R, Comino-Delgado R. Inflammatory cytokines in intrauterine growth retardation. Acta Obstet Gynecol Scand. 2003;82(12):1099–1102 [DOI] [PubMed] [Google Scholar]
- 9. Laskowska M, Leszczynska-Gorzelak B, Laskowska K, Oleszczuk J. Evaluation of maternal and umbilical serum TNFalpha levels in preeclamptic pregnancies in the intrauterine normal and growth-restricted fetus. J Matern Fetal Neonatal Med. 2006;19(6):347–351 [DOI] [PubMed] [Google Scholar]
- 10. Curry AE, Vogel I, Skogstrand K, et al. Maternal plasma cytokines in early- and mid-gestation of normal human pregnancy and their association with maternal factors. J Reprod Immunol. 2008;77(2):152–160 [DOI] [PubMed] [Google Scholar]
- 11. Vassiliadis S, Ranella A, Papadimitriou L, Makrygiannakis A, Athanassakis I. Serum levels of pro- and anti-inflammatory cytokines in non-pregnant women, during pregnancy, labour and abortion. Mediators Inflamm. 1998;7(2):69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mazzeo RS, Donovan D, Fleshner M, et al. Interleukin-6 response to exercise and high-altitude exposure: influence of alpha-adrenergic blockade. J Appl Physiol. 2001;91(5):2143–2149 [DOI] [PubMed] [Google Scholar]
- 13. Coussons-Read ME, Mazzeo RS, Whitford MH, Schmitt M, Moore LG, Zamudio S. High altitude residence during pregnancy alters cytokine and catecholamine levels. Am J Reprod Immunol. 2002;48(5):344–354 [DOI] [PubMed] [Google Scholar]
- 14. Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res. 2003;54(1):20–25 [DOI] [PubMed] [Google Scholar]
- 15. Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Public Health. 1997;87(6):1003–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moore LG, Young D, McCullough RE, Droma T, Zamudio S. Tibetan protection from intrauterine growth restriction (IUGR) and reproductive loss at high altitude. Am J Hum Biol. 2001;13(5):635–644 [DOI] [PubMed] [Google Scholar]
- 17. Zamudio S, Postigo L, Illsley NP, et al. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol. 2007;582(pt 2):883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed. 2007;92(5):F372–F377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Julian CG, Wilson MJ, Lopez M, et al. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1564–R1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Facco M, Zilli C, Siviero M, et al. Modulation of immune response by the acute and chronic exposure to high altitude. Med Sci Sports Exerc. 2005;37(5):768–774 [DOI] [PubMed] [Google Scholar]
- 21. Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82(5):1582–1588 [DOI] [PubMed] [Google Scholar]
- 22. Klokker M, Kharazmi A, Galbo H, Bygbjerg I, Pedersen BK. Influence of in vivo hypobaric hypoxia on function of lymphocytes, neutrocytes, natural killer cells, and cytokines. J Appl Physiol. 1993;74(3):1100–1106 [DOI] [PubMed] [Google Scholar]
- 23. Takizawa S, Ozaki H, Karaki H. Interleukin-1beta-induced, nitric oxide-dependent and -independent inhibition of vascular smooth muscle contraction. Eur J Pharmacol. 1997;330(2-3):143–150 [DOI] [PubMed] [Google Scholar]
- 24. Velez DR, Fortunato SJ, Morgan N, et al. Patterns of cytokine profiles differ with pregnancy outcome and ethnicity. Hum Reprod. 2008;23(8):1902–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krampl E, Lees C, Bland JM, Espinoza Dorado J, Moscoso G, Campbell S. Fetal biometry at 4300 m compared to sea level in Peru. Ultrasound Obstet Gynecol. 2000;16(1):9–18 [DOI] [PubMed] [Google Scholar]
- 26. Voronov E, Shouval DS, Krelin Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A. 2003;100(5):2645–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. El Awad B, Kreft B, Wolber EM, et al. Hypoxia and interleukin-1beta stimulate vascular endothelial growth factor production in human proximal tubular cells. Kidney Int. 2000;58(1):43–50 [DOI] [PubMed] [Google Scholar]