Abstract
Background
The presence of subclinical myocardial necrosis as a prodrome to longer term adverse cardiac event risk has been debated. The debate has focused predominantly within patients with acute coronary syndrome, and on issues of troponin assay variability and accuracy of detection, rather than the clinical significance of the presence of subclinical myocardial necrosis (i.e. “troponin leak”) within stable cardiac patients. Herein we examine the relationship between different degrees of subclinical myocardial necrosis and long-term adverse clinical outcomes within a stable cardiac patient population with essentially normal renal function.
Methods and Results
Sequential consenting patients (N=3,828; median creatinine clearance 100 ml/min/1.73m2) undergoing elective diagnostic coronary angiography with cardiac troponin I (cTnI) levels below the diagnostic cutoff for defining myocardial infarction (<0.03 ng/mL) were evaluated. The relationship of subclinical myocardial necrosis with incident major adverse cardiovascular events (MACE, defined as any death, myocardial infarction, or stroke) over 3-year follow-up was examined. “Probable” (cTnI 0.001–0.008 ng/mL) and “definite” (cTnI 0.009 –0.029 ng/mL) subclinical myocardial necrosis were observed frequently within the cohort (34% and 18%, respectively). A linear relationship was observed between the magnitude of subclinical myocardial necrosis and risk of 3-year incident MACE, particularly in those with cTnI 0.009 ng/mL or higher (Hazard Ratio 3.00, 95% confidence interval 2.4–3.8), even following adjustment for traditional risk factors, C-reactive protein (CRP), and creatinine clearance. The presence of subclinical myocardial necrosis was associated with elevations in acute phase proteins (CRP, ceruloplasmin, p<0.01 each) and reduction in systemic anti-oxidant enzyme activities (arylesterase, p<0.01), but showed no significant associations with multiple specific measures of oxidant stress, and borderline associations with myeloperoxidase, a marker of leukocyte activation.
Conclusions
In stable cardiology patients, prodromal subclinical myocardial necrosis is associated with substantially higher long-term risk for MACE. The underlying mechanisms contributing to this minimal troponin leak phenomenon warrants further investigation.
Keywords: Coronary artery disease, myocardium, ischemia, troponin, atherosclerosis
INTRODUCTION
Detection of circulating cardiac troponins is associated with the presence of ongoing myocardial necrosis, and fulfills the contemporary definition of myocardial infarction in the presence of ischemic signs and symptoms1. The prognostic role of cardiac troponin levels (either T or I subtypes) is well established across the spectrum of acute coronary syndromes including within patients with renal insufficiency and end-stage kidney disease, and serves as a reflection of myocardial necrosis or stress2.
The presence of “subclinical myocardial necrosis” as a prodrome to longer term adverse cardiac event risk has been debated (i.e. “troponin leak”). Some non-ischemic conditions such as renal insufficiency have also been associated with detection of circulating cardiac troponin levels2–4, particularly in asymptomatic patients with end-stage kidney diseases5, 6. In fact, several recent reports have even associated detectable (but non-diagnostic) cardiac troponin levels in stable non-cardiac subjects in the community with heightened risk of developing future cardiovascular events 7–9. At present, it is unclear whether microvascular ischemic insults, or various oxidative and inflammatory mediators, contribute to myocyte injury and/or apoptosis and progressive myocyte loss, leading to graded release of troponin fragments into the circulation. Herein we sought to examine the phenotype, prognostic significance, as well as underlying pathophysiologic mechanisms that may contribute to the development of subclinical myocardial necrosis in stable cardiac patients.
METHODS
Study population
The Cleveland Clinic GeneBank study is a large, prospective cohort study that established a well-characterized clinical repository with clinical data and longitudinal outcomes comprised from consenting subjects undergoing elective diagnostic coronary angiography from 2001–6. All GeneBank participants gave written informed consent approved by the Cleveland Clinic Institutional Review Board. All blood samples were collected at the time of cardiac catheterization procedure where arterial sheath access has been obtained and prior to diagnostic catheterization or treatment (including heparin). This analysis included a cohort of 3,828 consecutive consenting subjects without clinical evidence of acute coronary syndrome at the time of enrollment and confirmed by including only those with cardiac troponin I [cTnI] <0.03 ng/mL, no history of revascularization within 30 days before enrollment, and at least 3 years of adjudicated follow-up data.
We defined coronary artery disease (CAD) as any clinical history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass surgery, or angiographic evidence of significant stenosis (≥50%) in one or more major coronary arteries. Dyslipidemia was defined as low-density lipoprotein cholesterol >130 mg/dL, high-density lipoprotein cholesterol <50 mg/dL, or triglyceride >150 mg/dL. An estimate of creatinine clearance (CrCl) was calculated using the Cockcroft-Gault equation, since a large majority of subjects had relatively preserved renal function. Adjudicated outcomes were prospectively ascertained over the ensuing 3 years for all subjects following enrollment. Major adverse clinical event (MACE) was defined as death, non-fatal myocardial infarction, or non-fatal stroke following enrollment.
Subclinical Myocardial Necrosis
Plasma levels of cardiac troponin I were measured using the STAT Troponin I assay (Abbott Laboratories, Abbott Park IL) in a research-based immunoanalyzer that provides a 3-decimal point readout from venous blood samples collected by EDTA-tubes. This assay provides highly sensitive analytical measurement of cTnI with a reported limit of detection (LOD) reaching 0.009 ng/mL in the literature, and a diagnostic cut-off of 0.03 ng/mL for myocardial infarction defined by the upper limit of normal (99th percentile cut-off with 10% coefficient of variation)10, 11. We confirmed these results by running >10 replicates of the zero calibrator and 5 replicates of a low level sample (target value 0.035 ng/mL). Mean and standard deviations were determined for each, and LOD was determined at 0.008 ng/mL using the formula LOD = 2 × SD low × [measured levels ÷ (Mean low-Mean zero)]12. Based on the analytical characteristics of the cTnI assay, we defined subclinical myocardial necrosis as “definite” in the range of cTnI 0.009 – 0.029 ng/mL, “probable” in the range of cTnI 0.001 – 0.008 ng/mL, and “none” if cTnI <0.001 ng/mL.
Biomarkers of Inflammation, Leukocyte Activation, and Oxidative Stress
High sensitivity C-reactive protein (hsCRP), ceruloplasmin, myeloperoxidase (MPO), as well as serum creatinine and fasting lipid profiles, were all measured simultaneously with the cTnI assay using the same analysis platform. Total leukocyte counts were analyzed on the Advia 120 Hematology Analyzer (Siemens, New York, New York). Serum arylesterase activity level was determined using a modification of a spectrophotometry-based assay as previously described13. Briefly, initial hydrolysis rates at 25°C of phenylacetate substrate (3.6 mM) were determined at 270 nm in 1:50 diluted serum in reactions mixtures composed of 9 mM Tris hydrocholoride, pH 8.0, and 0.9 mM calcium chloride. An extinction coefficient (at 270 nm) of 1310 M−1cm−1 was used for calculating units of arylesterase activity, which are expressed as the amount of phenyl acetate hydrolyzed in µM/min/mL of serum. In a random subset of subjects (n=123), multiple distinct fatty acid oxidation products (hydroxyeicosatetraenoic acids [HETEs], hydroxyoctadecadienoic acids [HODES] and F2α-Isoprostanes) within plasma stored in the presence of anti-oxidant cocktail and under inert (argon) atmosphere were determined using high performance liquid chromatography with on-line stable isotope dilution electrospray ionization tandem mass spectrometry, as previously described14.
Statistical Analysis
The Student’s t-test or Wilcoxon-Rank sum test for continuous variables and chi-square test for categorical variables were used to examine the difference between the groups. Since a substantial proportion of patients had non-detectable cTnI levels (up to 37% of study cohort, corresponding to the first tertile), the study population was divided according to approximate tertiles based on cTnI levels rounded to the nearest 0.001 ng/mL. Unadjusted trends (adjusted for age and gender only) for all-cause mortality rates as well as non-fatal myocardial infarction/stroke rates with increasing tertiles of cTnI were evaluated with the Cochran-Armitage test using a time-to-event approach. Adjustments were made for individual traditional cardiac risk factor, Framingham risk factors (including age, gender, cigarette smoking, low-density and high-density lipoprotein cholesterol, systolic blood pressure) plus diabetes mellitus, log-transformed hsCRP, and CrCl to predict incident 3-year MACE risks. Logistic regression models were developed to calculate odds ratios (ORs) associated with the lowest tertile compared with the upper tertiles (i.e. non-detectable versus detectable cTnI). Kaplan–Meier analysis with Cox proportional hazards regression was used for time-to-event analysis to determine Hazard ratio (HR) and 95% confidence intervals (95%CI) for MACE. Levels of cTnI were then adjusted for traditional CAD risk factors in a multivariable model including Framingham risk factors, log-transformed hsCRP, and CrCl. We confirmed that both the proportionality hazards and linearity assumptions were met. All analyses were performed using SAS version 8.2 (Cary, NC) and R 2.8.0 (Vienna, Austria). P values <0.05 were considered statistically significant.
RESULTS
Study Population
The following is the breakdown of reasons for cardiac catheterization within the study cohort, noting that subjects can have more than one reason per person: history of positive or indeterminate stress test (50%), evaluation for possible ischemic causes of symptoms (66%), preoperative evaluation (12%), history of cardiomyopathy (3%). In our study cohort, 52% of subjects (1,993/3,828) had evidence of cTnI detectable below the upper limit of normal (<0.03 ng/mL), including 18% (702/3,828) with “definite” (cTnI 0.009–0.29 ng/mL) and 34% (1,291/3,828) with “probable” (cTnI 0.001–0.008 ng/mL) subclinical myocardial necrosis. Baseline characteristics of the study population are shown in Table 1, stratified according to presence or absence of subclinical myocardial necrosis.
Table 1.
Subject characteristics
| Subclinical Myocardial Necrosis | |||
|---|---|---|---|
| None (n=1,835) |
Probable (n=1,291) |
Definite (n=702) |
|
| cTnI range (ng/mL) | <0.001 | 0.001–0.008 | 0.009–0.029 |
| Demographics and Clinical Data | |||
| Age (years) | 63 ± 11 | 63 ± 11 | 66 ± 11 |
| Male (%) | 66 | 65 | 69 |
| History of diabetes mellitus (%) | 29 | 31 | 41* |
| History of hypertension (%) | 70 | 71 | 75 |
| Cigarette smoking (former / current %) | 65 / 11 | 64 / 9 | 66 / 10 |
| Prior coronary artery disease (%) | 68 | 66 | 76* |
| History of heart failure (%) | 11 | 15 | 33* |
| LV ejection fraction <50% (%) | 13 | 23 | 42* |
| Maximal stenosis ≥50% (%) | 65 | 69 | 76* |
| Framingham ATP III Risk Score | 13 (11, 15) | 14 (11, 15) | 14 (12, 16) |
| Laboratory Data | |||
| LDL cholesterol (mg/dL) | 95 (78, 117) | 97 (80, 118) | 92 (76, 112) |
| HDL cholesterol (mg/dL) | 34 (28, 41) | 34 (28, 41) | 32 (27, 40) |
| Triglycerides (mg/dL) | 114 (84, 162) | 115 (84, 166) | 115 (83, 156) |
| C-reactive protein (mg/L) | 1.99 (0.91, 4.51) | 2.11 (0.86, 4.58) | 2.68 (1.19, 6.24)* |
| Creatinine clearance (ml/min/1.73m2) | 100 (77, 126) | 101 (76, 126) | 85 (62, 113)* |
| Medications | |||
| Aspirin (%) | 74 | 73 | 70 |
| Statin (%) | 60 | 58 | 54 |
| ACE inhibitors (%) | 44 | 51 | 60* |
| Beta-blockers (%) | 59 | 63 | 63# |
| Nitrates (%) | 29 | 28 | 32 |
Values expressed in mean ± standard deviation or median (interquartile range). Abbreviations: cTnI = cardiac troponin I; LDL = low-density lipoprotein; HDL = high-density lipoprotein; hsCRP = high-sensitivity C-reactive protein; ATP III = Adult Treatment Panel III guidelines.
p<0.01,
p<0.05
Overall, 68% of subjects had ≥50% stenosis at any coronary artery, 32% of subjects had prior revascularization, and 84% had no subsequent revascularization within 30 days following catheterization. There were no significant differences in the prevalence of none, probable, or definite subclinical myocardial necrosis between these subgroups. However, we observed a trend towards higher rates of any (probable or definite) subclinical myocardial dysfunction in those who underwent subsequent revascularization within 30 days of cardiac catheterization compared to those who did not (61% versus 51%), as well as those with ≥50% stenosis at any coronary artery compared to those without (55% versus 47%). Only 17% had a history of heart failure and 22% had LV ejection fraction <50%. Patients with evidence of subclinical myocardial necrosis were more likely to be older, with more cardiovascular risk factors and underlying heart failure/impaired LV ejection fraction, and with slightly lower renal function at baseline, though overall still within the normal range (Table 1).
Subclinical Myocardial Necrosis and Prognosis
Overall, patients with evidence of subclinical myocardial necrosis (either probable or definite) were associated with a 2.2-fold increased risk of 3-year incidence of MACE (Hazard ratio [HR] 2.18; 95% confidence interval [95%CI] 1.77–2.68, p<0.0001) when compared to those with no evidence of subclinical myocardial necrosis. When analyzed at the LOD cut-off (0.008 ng/mL), those with evidence of subclinical myocardial necrosis still conferred significantly higher risk of death (HR 2.23, 95%CI 1.81–2.74, p<0.01), non-fatal MI and stroke (HR 2.23, 95%CI 1.81–2.74, p<0.01), and MACE (HR 2.27, 95%CI 1.86–2.76, p<0.01) at 3-year follow-up. After adjusting for traditional risk factors including Framingham risk factors, hsCRP, and CrCl, evidence of subclinical myocardial necrosis (probable or definite) within stable cardiac patients remained a significant increased risk of incident MACE at 3 years (HR 1.77; 95%CI 1.43–2.18, p<0.01). Such prognostic value remained significant in various subgroups stratified by age, gender, presence of diabetes mellitus, or in those with CrCl ≥60 ml/min (but not <60 ml/min). Also, the results were similar when analyzed only within the cohort of patients without any subsequent revascularization over the ensuing three years of follow-up (68% of cohort, Table 2). The results also extended to those with no history of heart failure (adjusted HR for MACE 2.09; 95%CI 1.51–2.90, p<0.01), those with preserved (i.e. ≥50%) LV ejection fraction (adjusted HR for MACE 2.83; 95%CI 1.80–4.45, p<0.01), as well as those with statin therapy at baseline (adjusted HR for MACE 2.04; 95%CI 1.43–2.92, p<0.01).
Table 2.
Adjusted hazard ratio (HR) for major adverse cardiac events at 3-year follow-up.
| All Subjects | Medically-Managed Coronary Disease | |||||
|---|---|---|---|---|---|---|
| Subclinical Myocardial Necrosis (n) |
None (n=1,835) |
Probable (n=1,291) |
Definite (n=702) |
None (n=894) |
Probable (n=627) |
Definite (n=374) |
| cTnI Range (ng/mL) | <0.001 | 0.001–0.008 | 0.009–0.029 | <0.001 | 0.001–0.008 | 0.009–0.029 |
| All-cause death | ||||||
| Number of events | 66 | 96 | 101 | 43 | 69 | 72 |
| Unadjusted HR | 1.0 | 2.06 (1.51, 2.82) * | 4.09 (3.00, 5.58) * | 1.0 | 2.32 (1.58, 3.39) * | 4.28 (2.93, 6.23) * |
| Age-/Gender-adjusted HR | 1.0 | 1.86 (1.36, 2.55) * | 3.36 (2.45, 4.61) * | 1.0 | 2.06 (1.41, 3.03) * | 3.56 (2.42, 5.24) * |
| Risk-factor adjusted HR | 1.0 | 1.70 (1.24, 2.34) * | 2.43 (1.73, 3.41) * | 1.0 | 1.96 (1.33, 2.87)* | 2.90 (1.94, 4.35) * |
| Non-fatal MI/stroke | ||||||
| Number of events | 62 | 60 | 41 | 40 | 34 | 21 |
| Unadjusted HR | 1.0 | 1.76 (1.38, 2.25) * | 2.92 (2.27, 3.76) * | 1.0 | 1.78 (1.32, 2.41) * | 2.79 (2.06, 3.80) * |
| Age-/Gender-adjusted HR | 1.0 | 1.64 (1.28, 2.10) * | 2.54 (1.96, 3.28) * | 1.0 | 1.62 (1.20, 2.19) * | 2.40 (1.75, 3.28) * |
| Risk-factor adjusted HR | 1.0 | 1.54 (1.20, 1.98) * | 1.98 (1.51, 2.61) * | 1.0 | 1.56 (1.15, 2.12) * | 2.04 (1.47, 2.84) * |
| Death/non-fatal MI/stroke | ||||||
| Number of events | 128 | 156 | 142 | 83 | 103 | 93 |
| Unadjusted HR | 1.0 | 1.74 (1.38, 2.20) * | 3.00 (2.36, 3.81) * | 1.0 | 1.80 (1.35, 2.40) * | 2.88 (2.14, 3.87) * |
| Age-/Gender-adjusted HR | 1.0 | 1.62 (1.28, 2.05) * | 2.60 (2.04, 3.32) * | 1.0 | 1.63 (1.22, 2.19) * | 2.48 (1.84, 3.36) * |
| Risk-factor Adjusted HR | 1.0 | 1.54 (1.21, 1.95) * | 2.09 (1.61, 2.71) * | 1.0 | 1.58 (1.18, 2.12) * | 2.12 (1.54, 2.93) * |
Values presented as Hazard Ratio (HR) and 95% confidence interval in brackets “Medically-managed coronary disease” is defined as those with significantly obstructive (>50% stenosis at any vessel) coronary disease but without revascularization within 30 days following catheterization.
p<0.01 and
p<0.05 (comparing with “none” category): 1) unadjusted; 2) adjusted for age and sex; and 3) adjusted for individual Framingham risk factors, including age, gender, low-density lipoprotein (LDL), high-density lipoprotein (HDL) cholesterol, systolic blood pressure, smoking, diabetes, creatinine clearance, high-sensitivity C-reactive protein;
Abbreviation: cTnI = cardiac troponin I; MI = myocardial infarction; HR = Hazard ratio.
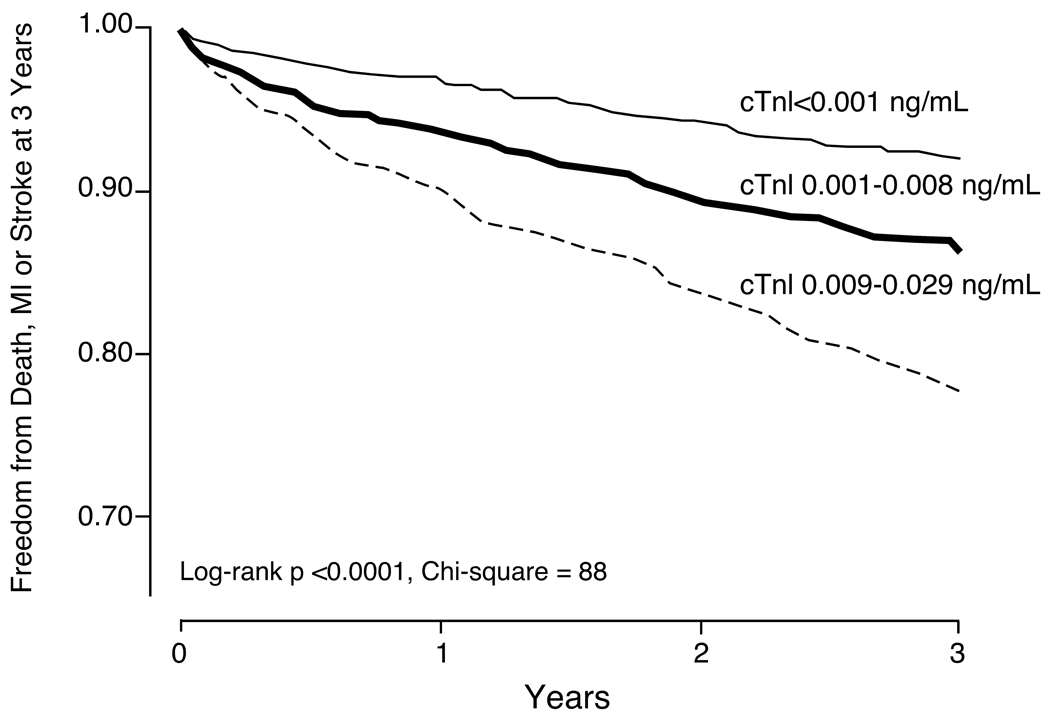
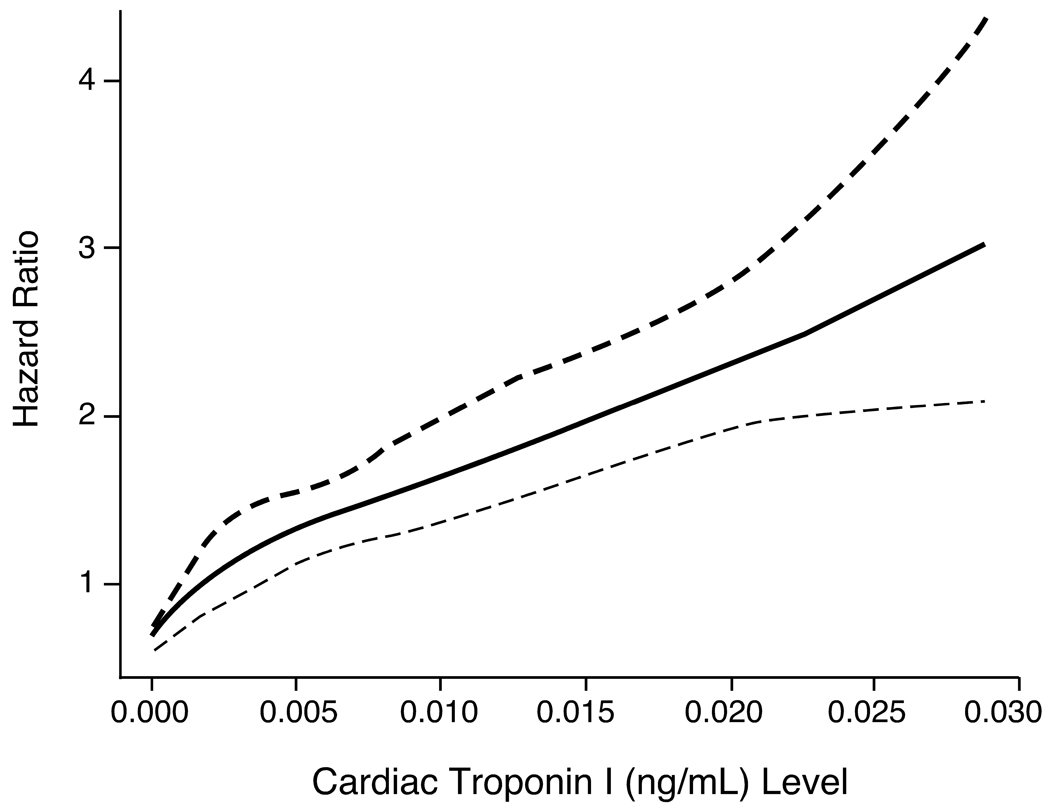
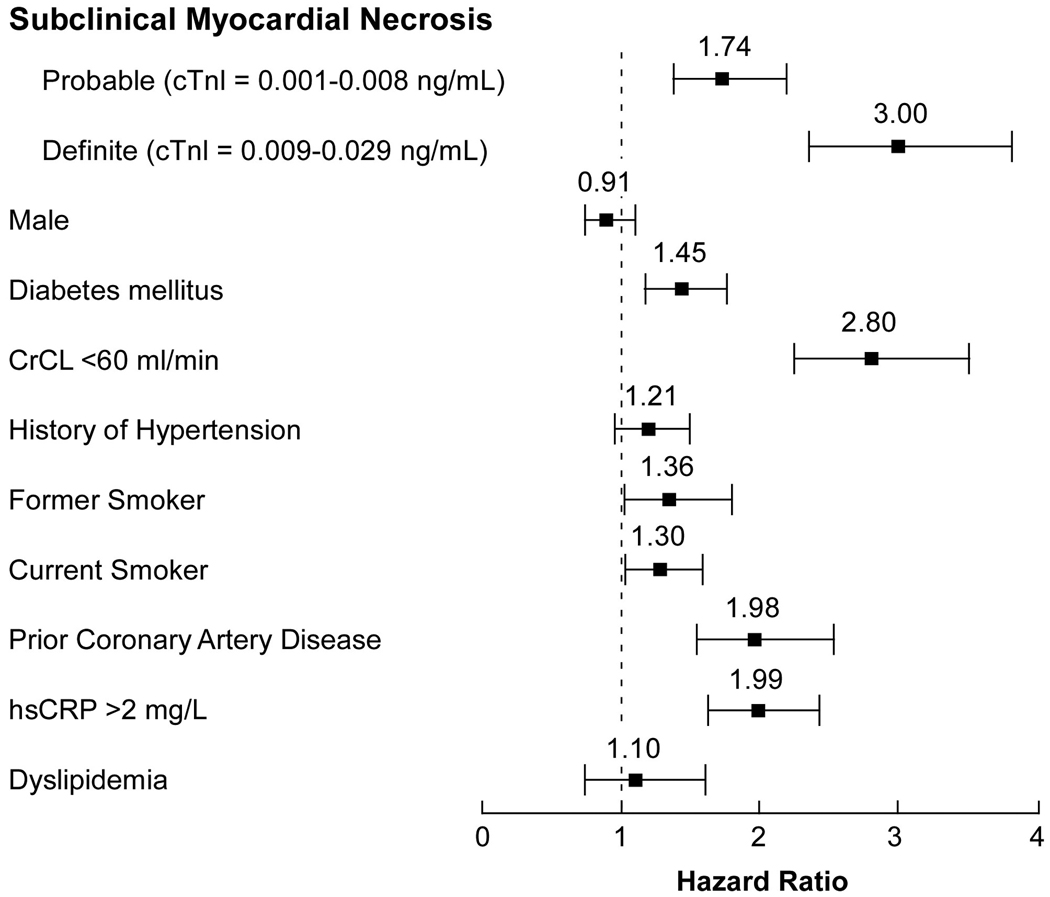
There was a noticeable risk increase in those with probable subclinical myocardial necrosis (cTnI between 0.001–0.008 ng/mL) versus those without any detectable cTnI (<0.001 ng/mL). This graded risk increase can be clearly illustrated in the Kaplan-Meier analysis shown in Figure 1. The Hazard ratios for adverse cardiac events appear to rise in a nearly linear fashion above cTnI levels of 0.004 ng/mL (Figure 2). Table 2 demonstrates the relationship between cTnI ranges with 3-year unadjusted and adjusted risks for incidence of death, myocardial infarction and stroke, as well as MACE at 3 years, stratified by definite or probable subclinical myocardial necrosis. Remarkably, the presence of subclinical myocardial necrosis provided superior prognostic value for adverse long term outcomes compared to other established cardiac risk factors (Figure 3).
Figure 1.
Kaplan-Meier analysis for 3-year major adverse clinical events, stratified according to cTnI tertiles (rounded to the nearest 0.001 ng/mL).
Figure 2.
Cubic spline curve of Hazard Ratios for major adverse clinical events (death, non-fatal MI, and stroke) at 3 years with cTnI levels.
Figure 3.
Forest plot of risk prediction for major adverse clinical events at 3 years according to cTnI cohorts in comparison with standard cardiovascular risk factors in multivariable Cox-proportional hazard analysis. Major adverse clinical events defined as death, non-fatal myocardial infarction (MI), and stroke over the ensuing 3 years of follow-up.
In an effort to better understand biologic processes associated with development of subclinical myocardial necrosis, we examined the relationship between detectable cTnI levels and markers of inflammation, leukocyte activation, and systemic oxidative stress (Table 3). In subjects with subclinical myocardial necrosis, we observed a graded and significantly higher levels in acute phase reactants such as hsCRP and ceruloplasmin, consistent with heightened systemic inflammation. In contrast, there did not appear to be an association between levels of cTnI and leukocyte count or plasma MPO levels, an index of leukocyte activation. Nevertheless, patients with definite subclinical myocardial necrosis had significantly higher plasma MPO levels compared to those with no subclinical myocardial necrosis (p=0.04) or those with probable subclinical myocardial necrosis (p=0.03). Interestingly, quantification of multiple distinct fatty acid oxidation products within plasma failed to show any evidence of enhanced oxidant stress among subjects with versus without evidence of subclinical myocardial necrosis (Table 3). However, serum levels of arylesterase activity, a cardioprotective anti-oxidant activity catalyzed by the HDL associated protein paraoxonase-1 (PON1)7, were significantly (p<0.01) lower in those with evidence of subclinical myocardial necrosis (Table 3). When tested for interaction with baseline statin therapy, only hsCRP demonstrated significant interaction (p=0.014) and arylesterase activity with borderline significance (p=0.05). Cardiac troponin I levels and all other inflammatory and oxidative biomarkers demonstrated no significant interaction with statin use.
Table 3.
Inflammatory and Oxidative Biomarkers in Subgroup Stratified by Presence of Subclinical Myocardial Necrosis
| Biomarkers | Subclinical Myocardial Necrosis | |||
|---|---|---|---|---|
| None (n=1,835) |
Probable (n=1,291) |
Definite (n=702) |
p-value | |
| Acute Phase Reactants | ||||
| C-reactive protein | 1.71 (0.84–3.81) | 2.11 (0.86–4.59) | 2.68 (1.19–6.25) | <0.01 |
| Ceruloplasmin | 22 (19.0–25.6) | 23.2 (20.3–27.4) | 24.2 (20.8–28.1) | <0.01 |
| Leukocyte Activation | ||||
| Total leukocyte count | 6.1 (5.0–7.4) | 6.0 (5.0–7.2) | 6.2 (5.0–7.5) | 0.78 |
| Myeloperoxidase | 98 (68–193) | 102 (71–186) | 113 (78–221) | 0.07 |
| Anti-oxidative Pathways | ||||
| Arylesterase activity | 105.2 (89.6–123.4) | 103(87.8–120.5) | 95.5 (79.7–113.8) | <0.01 |
| Oxidative Fatty Acids | (n=33) | (n=61) | (n=29) | |
| 5-HETE | 14.7 (9.6–20.3) | 12.8 (8.8–20.1) | 15.3 (12.0–21.0) | 0.91 |
| 8-HETE | 2.2 (1.6–3.0) | 2.1 (1.3–3.1) | 2.5 (1.7–3.4) | 0.53 |
| 9-HETE | 29.2 (18–42.9) | 24.6 (16.5–42.6) | 33.0 (22.0–43.9) | 0.28 |
| 11-HETE | 5.05 (4.05–5.94) | 4.57 (3.47–6.29) | 4.80 (3.38–6.20) | 0.37 |
| 12-HETE | 6.62 (5.07–8.50) | 5.77 (3.94–9.05) | 6.18 (4.97–8.20) | 0.79 |
| 15-HETE | 22.2 (14.5–27.4) | 18.1 (11.6–26.7) | 19.9 (10.8–25.5) | 0.36 |
| 9-HODE | 29.3 (22.9–35.1) | 27.3 (16.9–38.6) | 28.6 (16.8–37.7) | 0.74 |
| 13-HODE | 31.5 (23–38.8) | 28.2 (18.7–43.8) | 28.5 (17.8–40.5) | 0.48 |
| 8-isoPGF2α | 7.9 (2.0–18.5) | 6.1 (1.7–15.6) | 7.4 (2.7–15.8) | 0.46 |
Abbreviations: HETE, hydroxyeicosatetraenoic acid; HODE, hydroxyloctadecadienoic acid; 8-iso PGF2α, 8-isoprostane prostaglandin F2α, all expressed in pM/mL.
All levels expressed in median (interquartile range), p value by ANOVA
DISCUSSION
Myocardial necrosis is considered the primary consequence of ischemia during acute coronary syndromes, and provides not only robust prognostic value but clinical indication for therapeutic interventions. Over the years, improved recognition of assay limitations, as well as detection of a subset of patients with a “troponin leak” below the standard diagnostic range, has emerged. Discussions regarding the clinical utility of cardiac troponins in plasma have focused on analytical precision and accuracy, and revised definitions of 99th percentile cutoff values among apparently healthy subjects has been proposed as the upper limit of normal for diagnostic purposes 15. The prognostic utility of detecting a “troponin leak” below this cutoff has been debated, and correspondingly, whether pathophysiologic mechanisms are at play leading to detection of a “subclinical cardiac troponin leak” has remained unclear. Moreover, the clinical necessity of improved sensitivity cardiac troponin assays has also been intensively debated because few studies have examined the clinical prognostic value, particularly among stable patients, or with use of a central reference laboratory and a high sensitivity assay capable of monitoring levels below the classic clinical threshold for diagnosis of an ischemic event. Our observations provide strong support for the concept that any detectable cTnI levels heralds enhanced long-term adverse cardiovascular event risk. We also demonstrated that within the cohort with detectable subclinical myocardial necrosis, there were heightened inflammatory and reduced anti-oxidative processes, even though there was only borderline evidence of significant leukocyte activation and no evidence of enhanced systemic indices of oxidant stress. The findings of heightened cardiovascular risk at levels equal or greater to traditional cardiovascular risk factors are consistent with recent report using a high-sensitivity cardiac troponin T assay 16, and supports the notion that detection of any cTnI within plasma warrants more globally aggressive risk reduction efforts and closer long-term monitoring since this represents a high-risk group of patients. It also suggests that therapeutic efforts at reducing cTnI leak, and incident development of future adverse cardiovascular events in this group should also be considered.
Our data significantly extend the clinical information available about detection of subclinical myocardial necrosis in multiple aspects. Recent registry data have suggested the prognostic value of detectable levels of cardiac troponin (thus far determined qualitatively as above the “upper limit of normal”) in patients, such as those presenting with acute decompensated heart failure for detecting in-hospital mortality 17, or in those undergoing percutaneous coronary intervention for detecting up to one-year mortality risk 18. Instead, our study focused entirely on individuals with cTnI levels below the upper limit of normal, and a large number of subjects without any subsequent coronary revascularization. The large, single-center cohort experience of stable subjects undergoing cardiac evaluation allows careful description of a study cohort regarding their selection, stability, and degree of atherosclerotic burden. The advantage of analyzing cTnI levels with the same assay in a core reference laboratory using a research-based immunoanalyzer allows the opportunity to quantify long-term risk beyond a dichotomous categorization of “positive” versus “negative” level (i.e. – above versus below the limit of detection) grossly utilized in published literature on this topic. Indeed, a clear dose response relationship for increased risk and relatively tight confidence intervals was observed within our cohort (Figure 2). The availability of traditional clinical and biochemical risk markers for multivariable adjustments also strengthen the support of the independent added clinical significance of detecting the presence of subclinical myocardial necrosis.
One of the more remarkable observations in the present study is how pervasive “troponin leak” is within a stable cardiology patient population. Almost half of the cohort had detectable cTnI levels, among which 65% were within the “probable” range (0.001–0.008 ng/mL). Of interest, only approximately a third (37 %) of patients with measurable plasma cTnI levels also demonstrated significant (≥50% stenosis in one or more major vessels) obstructive coronary artery disease on coronary angiography, suggesting the other two thirds of subjects are at increased risk but this would not be observed using traditional risk stratification methods, or invasive testing that relies upon significant luminal narrowing (i.e. most imaging methods). While evidence of macrovascular ischemia as a contributor to the observed troponin leak seems remote, the presence of vulnerable plaque with microvascular ischemic insults or microembolisms may be more prevalent than previously appreciated, and serve as a contributory mechanism to the development of subclinical myocardial necrosis. The concomitant presence of heightened inflammatory and reduced anti-oxidative processes among subjects with subclinical myocardial necrosis suggests the potential for some common underlying pathophysiologic processes at play. Interestingly, our results did not identify differences in systemic levels of leukocytes or elevations in multiple distinct oxidized fatty acid species, and only borderline associations with MPO levels, a marker of leukocyte activation. These results may suggest that pathophysiologic process leading to subclinical myocardial necrosis may be regional or partially independent of leukocyte-mediated progression of disrupting vulnerable plaques, as seen in macrovascular ischemia.
The prognostic value in the “borderline” range of cTnI may vary widely depending on which assays is being used 19. There are several potential interferences that may influence circulating levels of cTnI, including the presence of heterophile antibodies or other non-cardiac confounders such as renal insufficiency 10, 20, 21. The prevalence of 18% with definite subclinical myocardial necrosis (0.009–0.29 ng/mL range) in our study population is comparable with some but not all prior reports in the literature (up to 22% in an elderly population with >0.01 µg/L by Beckman Coulter Access AccuTnI assay in a European cohort 22). In particular, detectable cardiac troponin T levels in otherwise healthy elderly subjects was also associated with poorer long-term prognosis (even though only 4% of that specific population had detectable levels) 7. Whether the detection of subclinical myocardial necrosis can allow therapeutic targeting of potential modifiable risk factors for reversal or delay of the progression of disease processes remains to be determined. Further investigations are warranted regarding the potential for aggressive preventive strategies based on identification of an at-risk population defined by the presence of subclinical myocardial necrosis.
Study Limitations
A strength of the present study is that the study cohort is an aggressively treated cardiology population in the modern era of high potency statin therapy. However, this also leads to a clear selection bias in the study population since they were deemed to have enough clinical suspicion (either by positive or equivocal stress test results or clinical presentation) to warrant an elective diagnostic invasive coronary angiographic evaluation. The diagnostic range of cTnI levels used in this study overlaps with a large majority of otherwise “healthy controls 10,” which argues to the broader potential generality of the present findings beyond the relatively higher risk population examined in the present clinical setting. Such low ranges are not routinely reported in standard laboratory evaluations, and their accuracy and reproducibility have not been extensively evaluated even though their sensitivities have been comparable to other sensitive cardiac troponin assays 23.
CONCLUSION
In stable patients undergoing elective cardiac evaluation, detectable cTnI levels by high sensitivity assay are commonly observed despite being well below the consensus defined diagnostic range. The present studies indicate that subclinical myocardial necrosis is a relatively common pathophysiologic phenomenon in the cardiology patient setting, and that detection of any cTnI within plasma may provide independent risk prediction of both near- and long-term adverse clinical events.
Acknowledgments
FINANCIAL SUPPORT
This research was supported by National Institutes of Health grants P01HL087018-020001, P01 HL076491-055328, P50 HL077107-050004, and the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (1UL1RR024989). Supplies and funding for performance of clinical laboratory studies were provided for by Abbott Laboratories.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
Dr. Tang received research grant support from Abbott Laboratories, Inc. Dr. Wu, Dr. Nicholls, Ms. Brennan, Mr. Pepoy, Ms. Mann, Mr. Pratt, and Dr. Van Lente have no potential conflicts to disclose with regard to this study. Dr. Hazen reports being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Dr. Hazen reports having been paid as a consultant or speaker for the following companies: AstraZeneca Pharmaceuticals LP, BG Medicine, Inc., Merck & Co., Inc., Pfizer Inc., PrognostiX, Inc. and Takeda. Dr. Hazen reports receiving research funds from Abbott, and PrognostiX Inc. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics and the companies shown below: PrognostiX Inc., Abbott Laboratories, Inc., Biosite Incorporated, Frantz Biomarkers, LLC, and Siemens.
AUTHOR CONTRIBUTIONS
Drs Tang and Hazen had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Tang, Hazen
Acquisition of data: Tang, Hazen
Analysis and interpretation of data: Tang, Wu, Nicholls, Brennan, Pepoy, Mann, Pratt, Van Lente, Hazen
Drafting of the manuscript: Tang
Critical revision of the manuscript for important intellectual content: Tang, Wu, Nicholls, Van Lente, Hazen
Statistical analysis: Wu, Brennan
Obtained funding: Hazen
Study supervision: Tang, Hazen
There are no medical writers or editors involved in the preparation of the manuscript.
REFERENCES
- 1.Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, Wu AH, Christenson RH. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115:e356–e375. doi: 10.1161/CIRCULATIONAHA.107.182882. [DOI] [PubMed] [Google Scholar]
- 2.Freda BJ, Tang WH, Van Lente F, Peacock WF, Francis GS. Cardiac troponins in renal insufficiency: review and clinical implications. J Am Coll Cardiol. 2002;40:2065–2071. doi: 10.1016/s0735-1097(02)02608-6. [DOI] [PubMed] [Google Scholar]
- 3.Francis GS, Tang WH. Cardiac troponins in renal insufficiency and other non-ischemic cardiac conditions. Prog Cardiovasc Dis. 2004;47:196–206. doi: 10.1016/j.pcad.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Van Lente F, McErlean ES, DeLuca SA, Peacock WF, Rao JS, Nissen SE. Ability of troponins to predict adverse outcomes in patients with renal insufficiency and suspected acute coronary syndromes: a case-matched study. J Am Coll Cardiol. 1999;33:471–478. doi: 10.1016/s0735-1097(98)00592-0. [DOI] [PubMed] [Google Scholar]
- 5.Khan NA, Hemmelgarn BR, Tonelli M, Thompson CR, Levin A. Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: a meta-analysis. Circulation. 2005;112:3088–3096. doi: 10.1161/CIRCULATIONAHA.105.560128. [DOI] [PubMed] [Google Scholar]
- 6.deFilippi C, Wasserman S, Rosanio S, Tiblier E, Sperger H, Tocchi M, Christenson R, Uretsky B, Smiley M, Gold J, Muniz H, Badalamenti J, Herzog C, Henrich W. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA. 2003;290:353–359. doi: 10.1001/jama.290.3.353. [DOI] [PubMed] [Google Scholar]
- 7.Daniels LB, Laughlin GA, Clopton P, Maisel AS, Barrett-Connor E. Minimally elevated cardiac troponin T and elevated N-terminal pro-B-type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008;52:450–459. doi: 10.1016/j.jacc.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zethelius B, Johnston N, Venge P. Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men: a community-based cohort study. Circulation. 2006;113:1071–1078. doi: 10.1161/CIRCULATIONAHA.105.570762. [DOI] [PubMed] [Google Scholar]
- 9.Schulz O, Paul-Walter C, Lehmann M, Abraham K, Berghofer G, Schimke I, Jaffe AS. Usefulness of detectable levels of troponin, below the 99th percentile of the normal range, as a clue to the presence of underlying coronary artery disease. Am J Cardiol. 2007;100:764–769. doi: 10.1016/j.amjcard.2007.03.096. [DOI] [PubMed] [Google Scholar]
- 10.Lam Q, Black M, Youdell O, Spilsbury H, Schneider HG. Performance evaluation and subsequent clinical experience with the Abbott Automated ARCHITECT® STAT Troponin-I assay. Clin Chem. 2006;52:298–300. doi: 10.1373/clinchem.2005.057216. [DOI] [PubMed] [Google Scholar]
- 11.Package Insert for Abbott Achitect i-STAT assay. Abbott Park IL: Abbott Laboratories; 2007. [Google Scholar]
- 12.Westgard JO. Basic Method Validation. Madison, WI: Westgard QC, INC; 1999. [Google Scholar]
- 13.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shishehbor MH, Zhang R, Medina H, Brennan ML, Brennan DM, Ellis SG, Topol EJ, Hazen SL. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Radic Biol Med. 2006;41:1678–1683. doi: 10.1016/j.freeradbiomed.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: Analytical issues for biochemical markers of acute coronary syndromes. Circulation. 2007;115:e352–e355. doi: 10.1161/CIRCULATIONAHA.107.182881. [DOI] [PubMed] [Google Scholar]
- 16.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E. A Sensitive Cardiac Troponin T Assay in Stable Coronary Artery Disease. N Engl J Med. doi: 10.1056/NEJMoa0805299. Published online at November 25, 2009 at DOI: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peacock WFt, De Marco T, Fonarow GC, Diercks D, Wynne J, Apple FS, Wu AH. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–2126. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 18.Jeremias A, Kleiman NS, Nassif D, Hsieh WH, Pencina M, Maresh K, Parikh M, Cutlip DE, Waksman R, Goldberg S, Berger PB, Cohen DJ. Prevalence and prognostic significance of preprocedural cardiac troponin elevation among patients with stable coronary artery disease undergoing percutaneous coronary intervention: results from the evaluation of drug eluting stents and ischemic events registry. Circulation. 2008;118:632–638. doi: 10.1161/CIRCULATIONAHA.107.752428. [DOI] [PubMed] [Google Scholar]
- 19.Zahid M, Good CB, Singla I, Sonel AF. Clinical significance of borderline elevated troponin I levels across different assays in patients with suspected acute coronary syndrome. Am J Cardiol. 2009;104:164–168. doi: 10.1016/j.amjcard.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Kenny PR, Finger DR. Falsely elevated cardiac troponin-I in patients with seropositive rheumatoid arthritis. J Rheumatol. 2005;32:1258–1261. [PubMed] [Google Scholar]
- 21.Kazmierczak SC, Sekhon H, Richards C. False-positive troponin I measured with the Abbott AxSYM attributed to fibrin interference. Int J Cardiol. 2005;101:27–31. doi: 10.1016/j.ijcard.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Eggers KM, Lind L, Ahlstrom H, Bjerner T, Ebeling Barbier C, Larsson A, Venge P, Lindahl B. Prevalence and pathophysiological mechanisms of elevated cardiac troponin I levels in a population-based sample of elderly subjects. Eur Heart J. 2008;29:2252–2258. doi: 10.1093/eurheartj/ehn327. [DOI] [PubMed] [Google Scholar]
- 23.Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–867. doi: 10.1056/NEJMoa0900428. [DOI] [PubMed] [Google Scholar]