Abstract
OBJECTIVE
Invasive candidiasis is a leading cause of infection-related morbidity and mortality in extremely low-birth-weight (<1000 g) infants. We quantify risk factors predicting infection in high-risk premature infants and compare clinical judgment with a prediction model of invasive candidiasis.
METHODS
The study involved a prospective observational cohort of infants <1000 g birth weight at 19 centers of the NICHD Neonatal Research Network. At each sepsis evaluation, clinical information was recorded, cultures obtained, and clinicians prospectively recorded their estimate of the probability of invasive candidiasis. Two models were generated with invasive candidiasis as their outcome: 1) potentially modifiable risk factors and 2) a clinical model at time of blood culture to predict candidiasis.
RESULTS
Invasive candidiasis occurred in 137/1515 (9.0%) infants and was documented by positive culture from ≥ 1 of these sources: blood (n=96), cerebrospinal fluid (n=9), urine obtained by catheterization (n=52), or other sterile body fluid (n=10). Mortality was not different from infants who had positive blood culture compared to those with isolated positive urine culture. Incidence varied from 2–28% at the 13 centers enrolling ≥ 50 infants. Potentially modifiable risk factors (model 1) included central catheter, broad-spectrum antibiotics (e.g., third-generation cephalosporins), intravenous lipid emulsion, endotracheal tube, and antenatal antibiotics. The clinical prediction model (model 2) had an area under the receiver operating characteristic curve of 0.79, and was superior to clinician judgment (0.70) in predicting subsequent invasive candidiasis. Performance of clinical judgment did not vary significantly with level of training.
CONCLUSION
Prior antibiotics, presence of a central catheter, endotracheal tube, and center were strongly associated with invasive candidiasis. Modeling was more accurate in predicting invasive candidiasis than clinical judgment.
Keywords: Candidiasis, premature infant, risk factors
In the extremely low-birth-weight (ELBW; <1000g) infant, invasive candidiasis is common, often fatal, and frequently leads to poor neurodevelopmental outcomes.1,2 Invasive candidiasis (Candida infections of the blood and other sterile body fluids) is the second most common cause of infectious disease-related death in the extremely premature infant. Despite antifungal treatment, 20% of infants who develop invasive candidiasis die, and neurodevelopmental impairment occurs in nearly 60% of survivors.1,2
Rates of invasive candidiasis vary 10-fold among similar academic tertiary care neonatal intensive care units (NICUs).3 This variation among nurseries is found throughout the world4–9 and has not been explained, but exposure to environmental risk factors (e.g., incubator humidity), third-generation cephalosporins, and foreign bodies such as catheters have all been associated with development of disease.3,10,11
The high morbidity related to invasive candidiasis leads to the consideration of empirical antifungal therapy and even prophylactic approaches in high-risk infants. Selection of older children and adults for empirical antifungal therapy for invasive candidiasis has long relied upon the presence of fever and neutropenia;12,13 however, fever and neutropenia are rarely present in the premature infant. The combination of extreme prematurity, thrombocytopenia, and use of broad-spectrum antibiotics has been suggested for guiding the initiation of empirical therapy.14
Four randomized trials for prophylaxis have been conducted: 2 small trials showed no benefit,15,16 and 2 trials conducted at high-incidence centers showed benefit.17,18 The Infectious Disease Society of America has suggested that prophylaxis be considered at high incidence centers.19 Widespread use of antifungal prophylaxis17 and overuse of empirical therapy14 may lead to antifungal drug resistance, a potential public health threat. We therefore enrolled a cohort of ELBW infants to identify risk factors for invasive candidiasis in order to better develop future prevention initiatives, to prospectively test prediction models for empirical therapy against clinical judgment, and to explore other risk stratification strategies for empirical therapy.
METHODS
The Cohort
Eligible study participants included neonates ≤ 1000 g birth weight, alive at 72 hours and <120 days, inborn or out-born, born between March 2004 and July 2007 at Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NRN) sites, whose parents gave informed consent for the study. The NRN is a consortium of tertiary academic neonatal centers; the study included 2 NRN funding cycles. A total of 19 centers contributed infants to this study.
Trained research personnel collected maternal demographic, perinatal, and delivery data as well as infant data until the first of the following end points: positive blood culture for candidiasis, discharge, day of life 120, transfer to another hospital, or death. Clinical data for these neonates were recorded at each sepsis evaluation. Thus, infants could contribute clinical data from multiple sepsis evaluations that were negative for Candida, but only 1 episode positive for Candida, and no sepsis episodes after development of invasive candidiasis. Candida organisms isolated by sterile body fluid were sent to the Duke University Mycology Research Unit for species identification confirmation.
Outcomes
Invasive candidiasis was defined as positive culture from normally sterile body fluid such as blood, urine (in/out catheterization, suprapubic aspiration), peritoneal fluid, or cerebrospinal fluid (CSF). Sepsis evaluations (n=6833) were conducted in accordance with local center standard practices; however, a recommendation was made regarding acquisition of specimens for culture—blood (0.5–1.0 ml), CSF, and urine from suprapubic aspiration or in/out catheterization. Cultures were processed locally. Those that were positive for Candida were sub-cultured locally and shipped to Duke University for independent confirmation by the Duke University Mycology Research Unit. All culture results from normally sterile body fluids were recorded until day of life 120, and cultures positive for Candida from any of these sites defined invasive candidiasis. Antifungal therapy was prescribed at the discretion of the attending neonatologist; amphotericin B deoxycholate, lipid complex amphotericin, and fluconazole were the antifungal agents prescribed most frequently. Because this study was focused on risk and diagnosis, treatment duration and dosing were not recorded.
Risk Factors
Study nurses recorded the presence of the following risk factors in the previous 24 hours each time an infant had a blood culture obtained: use of endotracheal tube, use of central catheters, Candida-like dermatitis on physical examination, use of skin emollients, receipt of intravenous lipid emulsion, use of humidity in the incubator, systemic steroid use, highest and lowest glucose, insulin use, enteral feeding, ingested breast milk, heparin flushes, and heparin in intravenous fluid. Lowest platelet count in the 24 hours surrounding the blood culture was recorded. Study nurses also recorded all systemic antifungal and antibiotic use for all days in the nursery. Broad-spectrum antibiotics were defined as the use of third-generation cephalosporins, carbapenems, or beta-lactam/beta lactamase inhibitor products. Because necrotizing enterocolitis and spontaneous perforation can be a result of invasive candidiasis, these data were not includedas part of the study.
Choice and use of antimicrobial therapy were left to the discretion of the attending neonatologist; however, the use of Gram-positive (ampicillin or nafcillin) and limited Gram-negative (aminoglycoside) therapy was encouraged based on studies conducted within the network.1,3 Two centers routinely used antifungal prophylaxis: 1 used fluconazole (n=50), and 1 used nystatin (n=117). None of the centers routinely employed empirical antifungal therapy.
Clinical Judgment
At the time blood cultures were obtained, the bedside clinicians were asked to estimate the probability of invasive candidiasis, and identified themselves by professional background (nurse practitioner or physician) and level of training (resident, fellow, attending). Antifungal use was also recorded. Antifungal therapy (yes/no) on the date of blood culture was used as the standard to determine if the clinician believed that the neonate had invasive candidiasis.
Analyses
For analyses in which the infant was the unit of observation (n=1515), proportions were calculated and P values were determined using chi-square tests. For analyses in which the unit of observation was the blood culture (n=6833), and infants could therefore contribute multiple observations, reported odds ratios, confidence intervals, and P values were based on generalized linear mixed models that adjust for correlated outcomes obtained from the same infant and correlation between infants at the same center.
Two models were generated, and the primary outcome for each model was invasive candidiasis:
The risk factor model was constructed using backward selection of factors related to candidiasis from hospitalization of the mother for labor, through birth of the infant, until the time of invasive disease, day of life 120, or discharge. Variables with a significance of P<0.1 were retained in the final model. The goal of this model is to help delineate components of supportive care that vary considerably among units and may explain the large differences in rates of candidiasis between nurseries.
-
The clinical predictive model included components of the history and clinical presentation at the time of blood culture that can be used to estimate the probability of candidiasis. The goal of this model is to determine if modeling is more accurate than clinical judgment for the diagnosis of invasive candidiasis. From the clinical prediction model and clinical judgment model, 2 sets of receiver operating characteristic (ROC) curves and confidence intervals were generated based on the accuracy (sensitivity and 1–specificity) of predicting invasive candidiasis.20
The first pair of ROC curves compared the clinical predictive model with clinician judgment—whether the infant was receiving antifungal therapy on the day of culture.
The second set of ROC curves compared the clinical judgment of attending neonatologists with other health care providers—pediatric residents, fellows, and nurse practitioners.
Sample Size
We estimated the cumulative incidence of invasive candidiasis to be approximately 10% in ELBW infants. We prespecified that an absolute difference in the upper and lower bound of the confidence interval of 15% would provide sufficient precision for subsequent risk factor modification. This goal would be met with a sample size of at least 100 cases of culture-proven invasive candidiasis. Because the initiating trigger for data collection was the acquisition of the blood culture, and the use of urine to document disease is somewhat controversial, it was decided to target 100 cases of bloodstream infection. It was also prespecified that no more than 1750 infants would be enrolled and that enrollment would cease with either 100 cases of bloodstream infection or 1750 ELBW infants enrolled. The day that the 100th positive blood culture was reported, enrollment stopped. Following monitoring of the data and confirmation of cultures at the central laboratory, it was discovered that 4 of the blood cultures thought to be positive had been mistakenly reported and that only 96 infants had positive blood cultures.
The Institutional Review Boards at each of the participating centers approved this study, and informed consent was obtained from each infant’s parent or legal guardian.
Role of the Funding Source
The funding sources for this manuscript did not play a role in the study design; the collection, analysis, and interpretation of the data; the writing of the report; or the decision to submit the paper for publication.
RESULTS
Cohort
From March 2004 to July 2007, 6493 infants ≤ 1000 g birth weight were cared for in the Neonatal Research Network, and 5252 were alive at 72 hours. Nineteen NICUs from the network enrolled 1515 ELBW infants (Table 1) during this time period. Of the infants enrolled, 137/1515 (9.0%) developed invasive candidiasis documented by positive culture from 1 or more of the following sources: blood (n=96), CSF (n=9), urine obtained by catheterization or suprapubic aspiration (n=52), or other sterile body fluid (n=10). Of the 1515 infants enrolled, 1051 (69%) were born via C-section; 941 (63%) were exposed to antenatal antibiotics; 841 (56%) were white; 384 (25%) were <25 weeks gestational age; and 680 (45%) were <750 g birth weight. Gestational age <25 weeks, lower birth weight, vaginal delivery, and receipt of antenatal antibiotics were strongly associated with subsequent invasive candidiasis in bivariate analysis.
TABLE 1.
Demographic and Center Differences for Incidence of Candidiasis
| Variable | Category | Percent with positive sterile culture for Candida | Odds ratio (95% CI) vs. reference category | Unadjusted P value |
|---|---|---|---|---|
| Mode of delivery | Vaginal | 14% (64/464) | 2.14 (1.5, 3.06) | <0.0001 |
| C-section (reference) | 7% (73/1051) | |||
| Antenatal antibiotics | 1 = Yes | 10% (96/941) | 1.53 (1.04, 2.25) | 0.0308 |
| 2 = No (reference) | 7% (39/564) | |||
| Race | Black | 10% (62/606) | 1.24 (0.86, 1.77) | 0.2531 |
| Other | 5% (3/62) | 0.55 (0.17, 1.8) | ||
| White (reference) | 8% (71/841) | |||
| Gestational age (weeks) | <25 | 19% (74/384) | 11.7 (4.66, 29.38) | <0.0001 |
| 25–27 | 7% (58/881) | 3.45 (1.37, 8.71) | ||
| 28+ (reference) | 2% (5/250) | |||
| Gestational age (weeks) | 22 | 25% (1/4) | 38.67 (1.93, 776.23) | <0.0001 |
| 23 | 20% (17/85) | 29 (3.77, 222.79) | ||
| 24 | 19% (56/295) | 27.18 (3.72, 198.8) | ||
| 25 | 9% (31/334) | 11.87 (1.6, 87.94) | ||
| 26 | 5% (16/312) | 6.27 (0.82, 47.82) | ||
| 27 | 5% (11/235) | 5.7 (0.73, 44.67) | ||
| 28 | 3% (4/133) | 3.6 (0.4, 32.65) | ||
| 29+ (reference) | 1% (1/117) | |||
| Birth weight (g) | <750 | 13% (88/680) | 2.38 (1.65, 3.44) | <0.0001 |
| 750–1000 (reference) | 6% (49/835) | |||
| Birth weight (g) | ≤500 | 7% (4/54) | 1.4 (0.45, 4.34) | <0.0001 |
| 501–600 | 12% (21/182) | 2.29 (1.17, 4.46) | ||
| 601–700 | 17% (51/296) | 3.65 (2.05, 6.48) | ||
| 701–800 | 7% (23/324) | 1.34 (0.7, 2.56) | ||
| 801–900 | 6% (21/344) | 1.14 (0.59, 2.2) | ||
| 901–1000 (reference) | 5% (17/315) | |||
| Positive sterile culture for Candida | 1 = Yes | 34% (47/137) | 3.13 (2.13, 4.59) | <0.0001 |
| 2 = No (reference) | 14% (197/1378) |
CI = confidence interval.
Risk Factors
In centers that enrolled at least 50 infants, the incidence of invasive candidiasis varied from 2% to 28%. One hundred and thirty-seven infants developed invasive candidiasis, while 6697 sepsis evaluations resulted in negative cultures for invasive candidiasis. In multivariable analysis, potentially modifiable risk factors at the time of blood culture acquisition associated with candidiasis included presence of an endotracheal tube, presence of central catheter, receipt of intravenous lipid emulsion, administration of broad-spectrum antibiotics in the week prior to culture, and intrapartum antibiotics (Table 2). Due to missing data, 6777 cultures were included in this model. Of the infants exposed to broadly acting antibiotics, 492 received third-generation cephalosporins, 59 received carbapenems, and 141 received beta-lactam/beta-lactamases. Of the 137 infants, 86 grew C. albicans, 41 C. parapsilosis, (3 grew both C. albicans and C. parapsilosis) 5 C. glabrata, 4 were not speciated, 1 C. lusitaniae, 1 C. tropicalis, and 1 C. guilliermondi.
TABLE 2.
Potentially Modifiable Risk Factors for Invasive Candidiasis at the Time of Culturea
| Effect | Adjusted odds ratio (95% CI) | P value |
|---|---|---|
| Broadly acting | 0.0003 | |
| antibiotics | 1.98 (1.37, 2.86) | |
| Central catheter | 1.94 (1.17, 3.21) | 0.0098 |
| IV lipid emulsion | 1.66 (0.98, 2.81) | 0.0596 |
| Endotracheal tube | 1.58 (1.07, 2.35) | 0.0226 |
| Antenatal antibiotics | 1.40 (0.97, 2.03) | 0.0747 |
Presence of central catheter, use of broadly acting antibiotics in the week prior to culture, use of intralipids, presence of endotracheal tube, and receipt of intrapartum antibiotics.
CI = confidence interval.
Clinician Judgment and Clinical Predictive Model
On the day of blood culture/sepsis evaluation, 40 infants (29% of those who developed candidiasis) received empirical antifungal therapy. Of the sepsis episodes that resulted in candidiasis for which clinicians provided a priori estimate of disease, 25% (32/128) were thought probably or highly likely to be infected with Candida by the bedside clinician (Table 3). In center-adjusted analysis, administration of antifungal therapy as an indication that the clinician thought the infant had candidiasis had an area under the ROC curve of 0.70 (95% CI 0.66–0.75). Centers with high incidence of candidiasis were no more accurate in predicting infection than centers with low incidence.
TABLE 3.
Clinician Judgment of Invasive Candidiasis
| Variable | Candidiasis (n = 137) | No candidiasis (n = 6697) | P value |
|---|---|---|---|
| Empirical antifungal therapy | |||
| Yes | 40 (29%) | 478 (7%) | <0.001 |
| No | 96 (71%) | 6219 (93%) | |
| Probability of candidemia | |||
| Very low | 13 (10%) | 1806 (29%) | <0.001 |
| Low | 42 (33%) | 2765 (45%) | |
| Possible | 41 (32%) | 1416 (23%) | |
| Probable | 21 (16%) | 148 (2%) | |
| High | 11 (9%) | 35 (1%) | |
Components of the history, physical exam, and initial laboratory evaluation that predicted candidiasis included vaginal delivery, week of gestational age, Candida-like dermatitis on physical exam, central catheter, lack of enteral feeding, hyperglycemia, days of antibiotic exposure in week prior to culture, and platelet count (Table 4). These elements comprised the clinical prediction model. Due to missing data, primarily for platelet count (missing 1062) and lowest glucose (missing 1100), 4862 cultures were included in this model. Day of life did not predict invasive candidiasis in the adjusted model.
TABLE 4.
Predictive Model of Invasive Candidiasisa
| Effect | Adjusted odds ratio (95% CI) | P value |
|---|---|---|
| Candida-like dermatitis | 3.22 (1.68, 6.20) | 0.0005 |
| Central catheter | 1.85 (1.08, 3.16) | 0.0242 |
| Vaginal vs. C-section | 1.84 (1.25, 2.70) | 0.0021 |
| Enteral feeding | 1.52 (1.01, 2.28) | 0.0429 |
| Lower gestational age (wk) | 1.29 (1.12, 1.49) | 0.0005 |
| Lowest glucose (50 mg/dl)b | 1.22 (0.99, 1.49) | 0.0603 |
| Lower platelet count (50,000)c | 1.17 (1.06, 1.28) | 0.0012 |
| Antibiotic days | 1.13 (1.05, 1.22) | 0.0013 |
Presence of Candida-like dermatitis on exam, mode of delivery, presence of central catheter, enteral feeding, lowest glucose in preceding 24 hours in increments of 50 mg/dl, antibiotic days in week prior to culture, platelet count in increments of 50,000, and gestational age in increments of weeks.
Odds of invasive candidiasis increased with increasing blood glucose.
Odds of invasive candidiasis increased with decreasing platelet count.
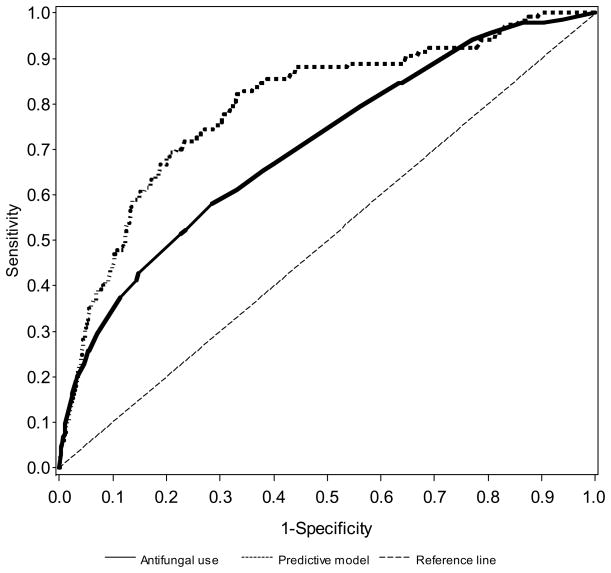
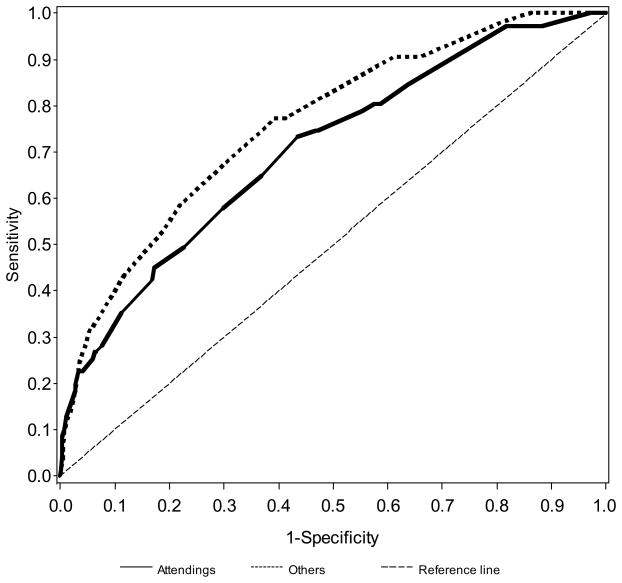
The clinical prediction model was superior to clinical judgment (P=0.0022). The area under the ROC curve was 0.79 (95% CI 0.75–0.84; Figure 1). Accuracy of clinician judgment in predicting candidiasis did not vary significantly with level of expertise. Judgment as to whether the infant did or did not have invasive candidiasis was exercised by: attending alone (13%), fellow alone (16%), nurse practitioner alone (15%), resident alone (19%), and physician or nurse with attending input (37%). The area under the ROC curve was similar whether or not an attending physician was involved in the decision to start empirical antifungal therapy (Figure 2). The area under the curve (AUC) without attending input was 0.76 (95% CI 0.69–0.82), and the AUC with attending input incorporated into the decision to start antifungal therapy was 0.70 (95% CI 0.64–0.77). The models with and without attending input were based on n=3037 and n=2928 cultures, respectively.
FIGURE 1.
Receiver operating characteristic curves for predictive model vs. clinical judgment (administration of antifungal therapy on the day of culture).
FIGURE 2.
Receiver operating characteristic curves for attending vs. other clinician judgment for the administration of antifungal therapy on the day of culture.
Mortality
Invasive candidiasis increased risk of death: 47/137 (34%) infants with candidiasis died compared with 197/1378 (14%) without candidiasis. Mortality was highest in the infants from whom Candida was isolated from multiple sources (e.g., urine and blood or urine and CSF): 16/28 (57%) of these infants died (Table 5).
TABLE 5.
Culture Location and Mortality
| Source of positive culture for Candida | Percent of infants who died |
|---|---|
| Blood only | 28% (19/69) |
| Urine only | 26% (9/34) |
| CSF only | 50% (1/2) |
| Other sterile source only | 50% (2/4) |
| Multiple sources | 57% (16/28) |
CSF = cerebrospinal fluid.
Mortality was similar in patients who had Candida isolated only from blood (19/69; 28%) and those with Candida isolated only from urine (9/34: 26%). Too few infants received systemic antifungal prophylaxis to conduct analysis for the influence of this intervention on incidence of, or mortality related to, candidiasis. Of the 39 infants who received empirical therapy, 13 (33%) died, and of the 97 who did not receive empirical therapy 34 (35%) died. In a center-adjusted model to predict mortality, only gestational age predicted death.
DISCUSSION
Risk Factors
We identified components of the history, physical exam, and clinical presentation that suggest subsequent development of invasive candidiasis: vaginal delivery, lower gestational age at delivery, dermatitis, central catheter, enteral feeding, elevated glucose, increased number of antibiotic days, and lower platelet count. Several of the risk factors that we have outlined (use of central catheters and endotracheal tube, broadly acting antibiotics, intravenous lipid emulsion; Table 2) are components of clinical care that may be potentially modified by centers with high rates of invasive candidiasis. Some risk factors (e.g., antenatal antibiotic use) require a multi-disciplinary approach to modify. Several of the components of the presentation (e.g., gestational age or platelet count) cannot be modified by the practice of the neonatologist but can be incorporated into the assessment of the probability of invasive disease.
Center, gestational age, and empirical therapy with third-generation cephalosporins, carbapenems, and beta-lactam/beta-lactamase products were strongly associated with subsequent development of invasive candidiasis. The incidence of invasive candidiasis varied from 2–28% in similar academic NICUs.1,3 We have previously reported that physician choice in empirical antibiotic therapy influences rates of candidiasis in retrospective individual patient and center-based analyses.3 This prospective cohort study confirms the association between use of third-generation cephalosporin and other broadly acting antimicrobial agents in the nursery and subsequent development of Candida infection.
Marked center variation has been observed in the frequency with which clinicians caring for neonates use third-generation cephalosporins—rather than an aminoglycoside—as empirical therapy for possible Gram-negative infections.21 The choice of cephalosporins (which eliminate much of the gut flora including bifidobacteria), other broadly acting antimicrobial agents, or aminoglycoside has marked center variation. These data support the use of aminoglycosides, which provide more focused therapy, as empirical coverage for Gram-negative organisms.
Although 9 of the centers had an incidence ≥ 9%, of the centers that enrolled more than 50 infants, only 4 had an incidence of candidiasis greater than 10%. Wide variation between in the incidence of invasive candidiasis between neonatal intensive care units has been shown in multiple publications.1,22 Four randomized trials of fluconazole prophylaxis with sample size ≥ 100 have been completed. In 1 low incidence study, fluconazole reduced colonization but not disease. Three high-incidence studies (13–26%)15,17,18 have been completed. In 1 high-incidence study, fluconazole failed to reduce invasive disease.16 In 2 high-incidence studies, prophylaxis reduced the incidence of candidemia to approximately 3%. Several sites in the network have a similar incidence without prophylaxis. This study identifies several interventions that may be targeted to reduce the risk of candidiasis.
Mortality
Of infants with invasive candidiasis, one third died; nearly 60% of infants from whom Candida was isolated from more than 1 sterile body fluid died (Table 5).
Mortality was similar in those from whom Candida was isolated from only the blood or urine (Table 5). These data suggest that Candida isolated from any normally sterile body fluid (including urine by suprapubic aspiration or in/out catheterization) should be treated as definitive evidence of systemic disease—just as if the organism were isolated from the blood. These clinical data are consistent with animal model data23 in which Candida injected into the blood of rodents were first isolated from the urine; when small amounts of Candida were injected, blood cultures were often negative while urine cultures were more frequently positive.
Clinical Judgment, Empirical Therapy, and Risk Factor Model
We provide ROC curves (Figures 1 and 2) to show that the use of a clinical prediction model outperformed the judgment of the bedside clinician (Figure 1); and that attending input into the estimation of candidiasis did not improve accuracy compared with nurse practitioners or physicians in training (Figure 2). The ROC compares sensitivity on the y-axis and 1–specificity on the x-axis. Thus, a “perfect” test reaches the upper left-hand corner, and a worthless test is represented by a diagonal dashed line across that bisects the graph from the lower left-hand corner to the upper right-hand corner. For most tests that use continuous value (e.g., creatinine), sensitivity can be made to look outstanding (nearly 100%). However, for virtually all tests, as sensitivity is improved, specificity worsens. The ROC curve is a graphic method to simultaneously provide test performance sensitivity and specificity.
The benefits of empirical therapy have not been proven in premature infants.19 These data do not support the widespread use of empirical antifungal in premature neonates. They do suggest, however, that if empirical therapy is to be administered, the decision should be based on systematic evaluation of risk factors rather than bedside judgment. We were surprised to see that enteral feeding on which the culture was obtained was associated with subsequent candidiasis in the predictive model. We do not interpret these data to suggest that clinicians should avoid enteral feeding; within these data, it may simply be that in the infants at highest risk of disease, enteral feeding is an additional factor to be considered when assessing risk of invasive candidiasis —— although one study has reported increased with repeated evaluation for feeding residuals.24
Conclusions
Our analyses have identified risk factors that may be targeted to reduce the incidence of invasive candidiasis in ELBW premature infants. If an infant has a positive urine culture obtained by catheterization or suprapubic aspiration, treatment with definitive antifungal therapy should be provided because the mortality is similar to blood culture-positive candidiasis. In addition, we found that a systematic risk factor assessment is more accurate in determining the risk of invasive candidiasis in premature infants when compared with bedside judgment. If empirical therapy is to be administered (or studied in the context of a randomized trial), systematic risk factor modeling can be used for patient selection.
Acknowledgments
Dr. Benjamin received support from the Thrasher Research Fund and the National Institute of Child Health and Human Development (NICHD), HD-044799-04. The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development provided grant support for the Neonatal Research Network’s Candidiasis Study (recruitment 2004–2007). The remaining authors have no conflicts to disclose.
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed, and analyzed the data for this study. On behalf of the NRN, Drs. Abhik Das (DCC Principal Investigator) and Marie Gantz (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Dr. Benjamin also had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chairs: Alan Jobe, MD, PhD, University of Cincinnati (2001–2006); Michael S. Caplan, MD, Northwestern University (2006–2011).
Brown University Women & Infants Hospital of Rhode Island (U10 HD27904): Abbot R. Laptook, MD; William Oh, MD; Angelita Hensman, BSN, RNC.
Case Western Reserve University Rainbow Babies & Children’s Hospital (CCTS UL1 RR24989, GCRC M01 RR80, U10 HD21364): Michele C. Walsh, MD, MS; Nancy S. Newman, BA, RN.
Duke University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (CCTS UL1 RR24128, GCRC M01 RR30, U10 HD40492): Ronald N. Goldberg, MD; C. Michael Cotten, MD; Kathy Auten, BS; Katherine A. Foy, RN.
Emory University Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (CCTS UL1 RR25008, GCRC M01 RR39, U10 HD27851): Barbara J. Stoll, MD; Ellen Hale, RN, BS; Ann Blackwelder, RNC, MS; Michelle Tidwell, RN, BSN.
Eunice Kennedy Shriver National Institute of Child Health and Human Development: Rosemary D. Higgins, MD; Stephanie Archer, MS.
Floating Hospital for Children at Tufts Medical Center (GCRC M01 RR54, U10 HD53119): Ivan D. Frantz III, MD; Brenda L. MacKinnon, RNC; Ellen Nylen, RN; Anne Furey, MPH.
Indiana University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (GCRC M01 RR750, U10 HD27856): Brenda B. Poindexter, MD, MS; James A. Lemons, MD; Dianne Herron, RN; Lucy Miller, RN, BSN, CCRC; Leslie D. Wilson, RN, BSN.
RTI International (U10 HD36790): Abhik Das, PhD; W. Kenneth Poole, PhD; Betty Hastings; Carolyn Petrie Huitema, MS; Kristin Zaterka-Baxter, RN; Scott E. Schaefer, MS; Jeanette O’Donnell Auman, BS.
Stanford University Lucile Packard Children’s Hospital (GCRC M01 RR70, U10 HD27880): Krisa P. Van Meurs, MD; David K. Stevenson, MD; M. Bethany Ball, BS, CCRC; Melinda S. Proud, RCP.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (GCRC M01 RR32, U10 HD34216): Waldemar A. Carlo, MD; Monica V. Collins, RN, BSN, MaEd; Shirley S. Cosby, RN, BSN.
University of California–San Diego Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461): Neil N. Finer, MD; Maynard R. Rasmussen, MD; David Kaegi, MD; Kathy Arnell, RN; Clarence Demetrio, RN; Chris Henderson, RCP, CRTT; Wade Rich, BS, RRT, CCRC.
Cincinnati Children’s Hospital Medical Center, University of Cincinnati Hospital, and Good Samaritan Hospital (GCRC M01 RR8084, U10 HD27853): Kurt Schibler, MD; Edward F. Donovan, MD; Kathleen Bridges, MD; Barb Alexander, RN; Cathy Grisby, BSN, CCRC; Holly Mincey, RN; Jody Shively, RN.
University of Iowa Children’s Hospital (CTSA UL1 RR24979, GCRC M01 RR59, U10 HD53109): Edward F. Bell, MD; Karen J. Johnson, RN, BSN.
University of Miami Holtz Children’s Hospital (GCRC M01 RR16587, U10 HD21397): Shahnaz Duara, MD; Ruth Everett-Thomas, RN, MSN.
University of New Mexico Health Sciences Center (GCRC M01 RR997, U10 HD53089): Kristi L. Watterberg, MD; Conra Backstrom Lacy, RN.
University of Rochester Golisano Children’s Hospital at Strong (GCRC M01 RR44, U10 HD40521): Dale L. Phelps, MD; Linda J. Reubens, RN, CCRC; Erica Burnell, RN; Cassandra A. Horihan, MS; Rosemary L. Jensen.
University of Texas Southwestern Medical Center at Dallas Parkland Health & Hospital System and Children’s Medical Center Dallas (CCTS UL1 RR24982, GCRC M01 RR633, U10 HD40689): Pablo J. Sánchez, MD; Walid A. Salhab, MD; Charles R. Rosenfeld, MD; Gaynelle Hensley, RN; Nancy A. Miller, RN; Melissa H. Leps, RN; Alicia Guzman.
University of Texas Health Science Center at Houston Medical School and Children’s Memorial Hermann Hospital (CCTS KL2 RR24149, CCTS UL1 RR24148, U10 HD21373): Kathleen A. Kennedy, MD, MPH; Jon E. Tyson, MD, MPH; Esther G. Akpa, RN, BSN; Beverly Harris, RN, BSN; Anna E. Lis, RN, BSN; Georgia E. McDavid, RN; Sarah Martin, RN, BSN; Patti L. Tate, RCP.
University of Utah University Hospital, LDS Hospital, and Primary Children’s Medical Center (CTSA UL1 RR25764, GCRC M01 RR64, U10 HD53124): Roger G. Faix, MD; Bradley A. Yoder, MD; Karen A. Osborne, RN, BSN; Jennifer J. Jensen, RN, BSN; Cynthia Spencer, RNC; R. Edison Steele, RN; Karena Strong, RN, BSN; Kimberlee Weaver-Lewis, RN, BSN.
Wake Forest University Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital (GCRC M01 RR7122, U10 HD40498): T. Michael O’Shea, MD, MPH; Nancy J. Peters, RN, CCRP.
Wayne State University Hutzel Women’s Hospital and Children’s Hospital of Michigan (U10 HD21385): Seetha Shankaran, MD; Rebecca Bara, RN, BSN.
Yale University Yale-New Haven Children’s Hospital (CCTS UL1 RR24139, GCRC M01 RR6022, U10 HD27871): Richard A. Ehrenkranz, MD; Rachel L. Chapman, MD; Patricia Gettner, RN; Monica Konstantino, RN, BSN.
ABBREVIATIONS
- AUC
area under the curve
- CSF
cerebrospinal fluid
- ELBW
extremely low-birth-weight
- NICU
neonatal intensive care unit
- NRN
Neonatal Research Network
- ROC
receiver operating characteristic
Footnotes
This study was registered on the United States government’s clinical trials web site: http://www.clinicaltrials.gov/ (clinicaltrials.gov identifier: NCT00109525).
AUTHOR CONTRIBUTIONS
Conception and design: Danny Benjamin
Analysis and interpretation of data: Danny Benjamin, Abhik Das, Marie Gantz
Drafting of the manuscript: Danny Benjamin
Revising manuscript critically for important intellectual content: Danny Benjamin, Barbara Stoll, Marie Gantz, Michele Walsh, Pablo Sanchez, Abhik Das, Seetha Shankaran, Rosemary Higgins, Kathy Auten, Nancy Miller, Thomas Walsh, Abbot Laptook, Waldemar Carlo, Kathleen Kennedy, Neil Finer, Shahnaz Duara, Kurt Schibler, Rachel Chapman, Krisa Van Meurs, Ivan Frantz III, Dale Phelps, Brenda Poindexter, Edward Bell, T. Michael O’Shea, Kristi Watterberg, Ronald Goldberg
Final approval of the manuscript submitted: Danny Benjamin, Barbara Stoll, Marie Gantz, Michele Walsh, Pablo Sanchez, Abhik Das, Seetha Shankaran, Rosemary Higgins, Kathy Auten, Nancy Miller, Thomas Walsh, Abbot Laptook, Waldemar Carlo, Kathleen Kennedy, Neil Finer, Shahnaz Duara, Kurt Schibler, Rachel Chapman, Krisa Van Meurs, Ivan Frantz III, Dale Phelps, Brenda Poindexter, Edward Bell, T. Michael O’Shea, Kristi Watterberg, Ronald Goldberg
References
- 1.Benjamin DK, Jr, Stoll BJ, Fanaroff AA, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality, and neurodevelopmental outcomes at 18–22 months. Pediatrics. 2006;117:84–92. doi: 10.1542/peds.2004-2292. [DOI] [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen NI, Adams-Chapman I, et al. National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 3.Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK., Jr The association of third generation cephalosporin use and invasive candidiasis in extremely low birth weight infants. Pediatrics. 2006;118:717–722. doi: 10.1542/peds.2005-2677. [DOI] [PubMed] [Google Scholar]
- 4.Blyth CC, Chen SC, Slavin MA, et al. Australian Candidemia Study. Not just little adults: candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics. 2009;123:1360–1368. doi: 10.1542/peds.2008-2055. [DOI] [PubMed] [Google Scholar]
- 5.Asmundsdóttir LR, Erlendsdóttir H, Haraldsson G, Guo H, Xu J, Gottfredsson M. Molecular epidemiology of candidemia: evidence of clusters of smoldering nosocomial infections. Clin Infect Dis. 2008;47:e17–e24. doi: 10.1086/589298. [DOI] [PubMed] [Google Scholar]
- 6.Sandven P, Bevanger L, Digranes A, Haukland HH, Mannsåker T, Gaustad P Norwegian Yeast Study Group. Candidemia in Norway (1991 to 2003): results from a nationwide study. J Clin Microbiol. 2006;44:1977–1981. doi: 10.1128/JCM.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez D, Almirante B, Park BJ, et al. Barcelona Candidemia Project Study Group. Candidemia in neonatal intensive care units: Barcelona, Spain. Pediatr Infect Dis J. 2006;25:224–229. doi: 10.1097/01.inf.0000202127.43695.06. [DOI] [PubMed] [Google Scholar]
- 8.Roilides E, Farmaki E, Evdoridou J, et al. Neonatal candidiasis: analysis of epidemiology, drug susceptibility, and molecular typing of causative isolates. Eur J Clin Microbiol Infect Dis. 2004;23:745–750. doi: 10.1007/s10096-004-1210-9. [DOI] [PubMed] [Google Scholar]
- 9.López Sastre JB, Coto Cotallo GD, Fernandez Colomer B. Neonatal invasive candidiasis: a prospective multicenter study of 118 cases. Am J Perinatol. 2003;20:153–163. doi: 10.1055/s-2003-40008. [DOI] [PubMed] [Google Scholar]
- 10.Saiman L, Ludington E, Pfaller M. Risk factors for candidemia in neonatal intensive care unit patient. The National Epidemiology of Mycosis Survey Study Group. Pediatr Infect Dis J. 2000;19:319–324. doi: 10.1097/00006454-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Benjamin DK, Jr, Ross K, Benjamin DK, McKinney RE, Jr, Auten R, Fisher RG. When to suspect fungal infections in neonates: a clinical comparison of Candida albicans and Candida parapsilosis fungemia with coagulase-negative staphylococcal bacteremia. Pediatrics. 2000;106:712–718. doi: 10.1542/peds.106.4.712. [DOI] [PubMed] [Google Scholar]
- 12.Pizzo PA, Robichaud KJ, Gill FA, Witebsky FG. Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia. Am J Med. 1982;72:101–111. doi: 10.1016/0002-9343(82)90594-0. [DOI] [PubMed] [Google Scholar]
- 13.EORTC International Antimicrobial Therapy Cooperative Group. Empiric antifungal therapy in febrile granulocytopenic patients. Am J Med. 1989;86(6 Pt 1):668–672. doi: 10.1016/0002-9343(89)90441-5. [DOI] [PubMed] [Google Scholar]
- 14.Benjamin DK, Jr, DeLong ER, Steinbach WJ, Cotten CM, Walsh TJ, Clark R. Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics. 2003;112(3 Pt 1):543–547. doi: 10.1542/peds.112.3.543. [DOI] [PubMed] [Google Scholar]
- 15.Kicklighter SD, Springer SC, Cox T, Hulsey TC, Turner RB. Fluconazole for prophylaxis against rectal colonization in the very low birth weight infant. Pediatrics. 2001;107:293–298. doi: 10.1542/peds.107.2.293. [DOI] [PubMed] [Google Scholar]
- 16.Parikh TB, Nanavati RN, Patankar CV, et al. Fluconazole prophylaxis against fungal colonization and invasive fungal infection in very low birth weight infants. Indian Pediatr. 2007;44:830–837. [PubMed] [Google Scholar]
- 17.Manzoni P, Stolfi I, Pugni L, et al. Italian Task Force for the Study and Prevention of Neonatal Fungal Infections; Italian Society of Neonatology. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med. 2007;356:2483–2495. doi: 10.1056/NEJMoa065733. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345:1660–1666. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 19.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 21.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117:67–74. doi: 10.1542/peds.2005-0179. [DOI] [PubMed] [Google Scholar]
- 22.Fridkin SK, Kaufman D, Edwards JR, Shetty S, Horan T. Changing incidence of Candida bloodstream infections among NICU patients in the United States: 1995–2004. Pediatrics. 2006;117:1680–1687. doi: 10.1542/peds.2005-1996. [DOI] [PubMed] [Google Scholar]
- 23.Hurley R, Winner HI. Experimental moniliasis in the mouse. J Pathol Bacteriol. 1963;86:75–82. doi: 10.1002/path.1700860109. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman D, Blackman A, Aurisy L, et al. Is this the next place for an infection control and prevention bundle? Society for Pediatric Research; Vancouver: 2010. Feeding tube colonization in NICU patients. Abstract 4411.405. [Google Scholar]