Abstract
Despite advances in reperfusion therapy, acute coronary syndromes can still result in myocardial injury and subsequent MI. Molecular, cellular, and interstitial events antecedent to the acute MI culminate in deleterious changes in the size, shape, and function of the left ventricle (LV), collectively termed LV remodeling. Three distinct anatomical and physiologic LV regions can be described post-MI: the infarct, borderzone, and remote regions. Given the complexity of post-MI remodeling, imaging modalities must be equally diverse to elucidate this process. The focus of this review will first be upon cardiovascular magnetic resonance imaging (MRI) of the anatomical and pathophysiological LV regions of greatest interest with regard to the natural history of the post-MI remodeling process. This review will examine imaging modalities which provide translational and molecular insight into burgeoning treatment fields for the attenuation of post-MI remodeling, such as cardiac restraint devices and stem cell therapy.
Introduction
Acute coronary syndrome, which can be defined as a constellation of clinical symptoms associated with acute myocardial ischemia, strikes nearly a million people in the United States each year (1) and is responsible for tens of billions of dollars in hospital charges. (2) Optimal treatment with timely reperfusion therapy and pharmacologic intervention has made acute coronary syndrome increasingly survivable, even in the face of rising incidence given the growing elderly population. (1) Despite advances in reperfusion therapy, acute coronary syndromes can still result in myocardial injury and subsequent myocardial infarction (MI), evoking cellular and extracellular processes in the reperfusion phase leading to cell death, inflammation, and scar formation. Molecular, cellular, and interstitial events antecedent to the acute MI culminate in changes in the size, shape, and function of the left ventricle (LV), collectively termed LV remodeling. Despite successful reperfusion, lifestyle modification, and pharmacotherapy, the LV remodeling process continues unabated, resulting in an accrual rate of almost 1 million new patients per year at risk for developing heart failure. (1) Accordingly, strategies to selectively and specifically monitor the molecular pathways which underlie the LV remodeling process hold great import to alleviate the socioeconomic as well as the healthcare resource burden of post-MI remodeling.
In order to identify appropriate imaging targets in the context of post-MI LV remodeling, the anatomical and biologic underpinnings of the process must be considered. For the purposes of the remodeling process, the post-MI LV can be divided into 3 distinct anatomic regions: the MI itself, the surrounding borderzone region, and the remaining remote myocardium. Regardless of reperfusion, degradation of normal extracellular matrix occurs within the MI region, accompanied by invasion of inflammatory cells and the induction of bioactive peptides and cytokines, leading to the necessary substructure for scar formation. (3,4) The fully perfused myocardium surrounding the MI, described as the borderzone region, is a metabolically active amalgam of inflammatory cells, fibroblasts, and viable myocytes. The borderzone region is also the site of infarct expansion, a dynamic process defined as the extension of changes in structure and function between the MI and remote myocardium. Infarct expansion is a process of great import, as it has been identified as an independent predictor of mortality for post-MI patients. (5) The remote region is normally perfused and can be millimeters to centimeters away from the MI and borderzone regions. Despite conventional belief, the remote region is not immune to the post-MI remodeling process, as changing wall strain patterns post-MI lead to myocyte hypertrophy and interstitial fibrosis. (6) Each of these 3 anatomical regions are distinctly different, and therefore the heterogeneity of the regions and cellular targets within the post-MI LV must be recognized in order to achieve appropriate imaging modalities.
While post-MI remodeling is a continually evolving process, a milestone can be described at the 30 day post-MI time point when the infarct itself is roughly healed. Several large animal studies have been conducted examining this period of the remodeling process and the unique events happening in each of the 3 anatomical regions. (7–16,18,19) Jugdutt et al. utilized a canine model to characterize the collagen dynamics, as well as the effects of angiotensin-converting enzyme inhibition (ACE-I) on collagen deposition, within the infarct zone during this time frame. (7,8) Subsequently, MI collagen and the myofibroblasts which produce it have become molecular imaging targets, capable of reflecting ACE-I therapeutic effects. (9) In the borderzone region vascular endothelial growth factor, a marker of angiogenesis during the post-MI remodeling process, has been studied in pigs, providing a molecular imaging target to assess borderzone myocardial perfusion status. (10,11,12) Integrins specific to angiogenic vessels have also been utilized as a novel target for imaging angiogenesis in post-MI large animal models. (13) Non-invasive imaging of angiogenesis in the borderzone region holds important implications for risk stratification of patients post-MI and the potential to evaluate the response to therapeutic measures aimed at myocardial angiogenesis. Unique portfolios of matrix metalloproteinases, a family of proteases which have been shown to be integral to the extracellular matrix remodeling process, have been characterized in all post-MI regions in sheep models (14,15) and provide non-invasive molecular imaging targets to interrogate the post-MI remodeling process. Apoptotic markers are clearly involved with pathways which can contribute to adverse remodeling post-MI. (16) At present the imaging of apoptotic pathways has been limited to small animal models (17) but clearly holds relevance for clinically relevant large animal models. A summary of domains of the biologic remodeling process (i.e. extracellular matrix, vasculature, and signaling pathways) are presented in Table 1 as well as potential imaging targets. Imaging of the post-MI remodeling process holds great import for both prognostic information and potential monitoring of therapeutic interventions.
Table 1.
Molecular imaging targets in the post-MI remodeling process.
Given the complexity of post-MI remodeling, imaging modalities must be equally diverse to elucidate this process. Subsequently, it is not possible to integrate the entire body of imaging studies which are applicable to LV remodeling in this brief review. Rather, the focus of this review will first be upon cardiovascular magnetic resonance imaging (MRI) of the anatomical and pathophysiological LV regions of greatest interest with regard to the natural history of the post-MI remodeling process. Second, this review will examine imaging modalities which provide translational and molecular insight into burgeoning treatment fields for the attenuation of post-MI remodeling, such as cardiac restraint devices and stem cell therapy.
Post-MI LV Remodeling: Biophysical Characterization by MRI
Cardiac MRI is able to provide accurate, reproducible, high spatial and temporal resolution images in any plane, making it especially suited for longitudinal assessment of post-MI LV remodeling (Figure 1). (20,21) Several studies have utilized MRI techniques to gain molecular and cellular insight into the post-MI remodeling process. Specifically, Friedrich et al. have demonstrated the use of MRI to characterize reversibly and irreversibly injured myocardium in and around the MI region (Figure 2). (22) They hypothesize that mapping areas of post-MI myocardial edema, using T2-weighted and late enhancement MRI, can guide therapeutic modalities to the borderzone region in attempts to attenuate infarct expansion. Other reports have targeted the reduction of post-MI edema, as detected by MRI, as a novel method to attenuate LV remodeling at the molecular level. (23) Following MI, contrast-enhanced MRI techniques provide visualization of microvascular obstruction in the infarct region. (24) Regions of microvascular obstruction, which can persist despite reperfusion, appear as subendocardial dark areas surrounded by hyperenhanced injured myocardium, and have been reported as a marker for adverse LV remodeling. (25) In a landmark report by Wu, et al. contrast enhanced MRI was demonstrated to correlate microvascular obstruction and infarct size with long-term prognosis, including the development of heart failure. (26)
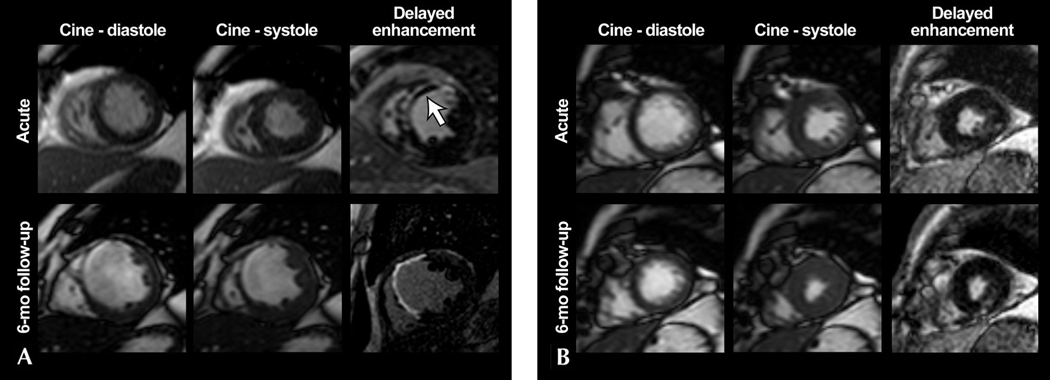
Figure 1.
Delayed-enhancement MRI examples of two different myocardial infarctions (MI) demonstrating the relationship between LV remodeling and functional recovery. A. A large anteroseptal transmural acute MI with areas of microvascular obstruction within the infarct region (white arrow) is shown in the acute phase. Images 6-months post-MI show substantial LV remodeling, with wall thinning and absence of regional functional recovery. B. A small inferolateral subendocardial MI with regional hypokinesis is shown in the acute phase. Subsequent 6-month post-MI images show a lack of LV remodeling and complete recovery of regional contractility, in contrast to the MI shown in A. Reproduced with permission from Reference #21.
Figure 2.
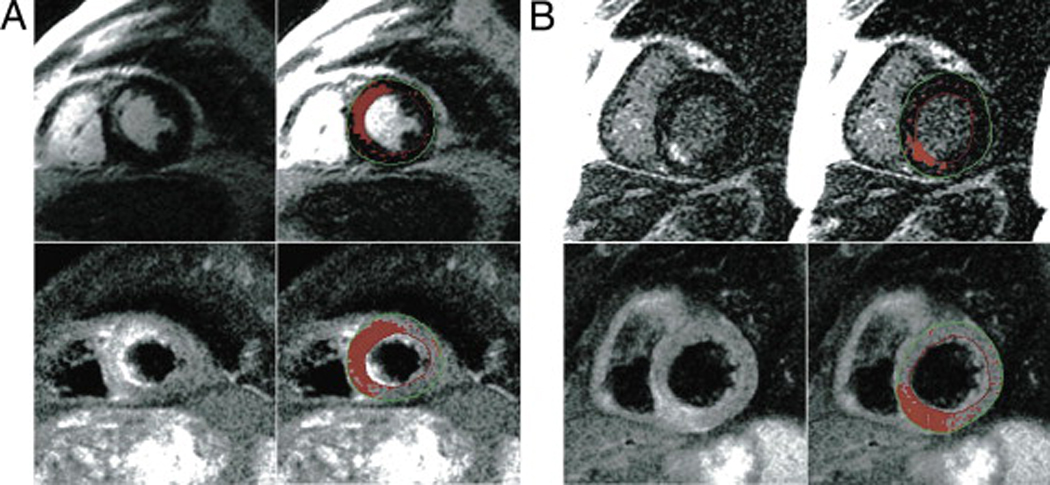
Irreversible and reversible injury in acute reperfused infarcts in two patients as visually and semiautomatically defined. A. Cardiac MRI 3 days after an acutely reperfused infarct with a subtotal (99%) occlusion of the left anterior descending artery in a 71-year-old patient. B. Cardiac MRI 1 day after reperfusion of an occluded right coronary artery in a 57-year-old patient. Red areas indicate the result of a semiautomatic delineation of pixels with abnormal signal as defined by a signal intensity of >2 SD above mean signal intensity of the remote myocardium. The spatial extent of the myocardial injury in the edema-sensitive T2 imaging is consistently larger than that of the necrosis-sensitive late enhancement. Reproduced with permission from Reference #22.
The topography of the LV following MI is a heterogeneous landscape, with great prognostic value held in the infarct characteristics and myocardial perfusion status. (27,28) This landscape is made traversable to researchers and clinicians with the help of a variety of imaging modalities. While computed tomography (CT) can provide high resolution static images to identify the region of MI, the current consensus is that MRI is able to provide better cardiac images than CT (29,30) with an additional feature of superior tissue characterization. For example, both clinical and pre-clinical studies (31,32) have utilized MR diffusion tensor imaging to study myocardial fiber architecture characteristics, describing distinct unique qualities among the MI, borderzone and remote regions. Specifically, the percentage of left-handed helical fibers was shown to have increased from the remote zone (13.3±5.8%) to the borderzone (19.2±9.7%) and infarct zone (25.8±18.4%) in patients a median of 26 days post-MI. (32) Myocardial fiber architecture correlated with infarct size and left ventricular function in these studies, linking structural and functional post-MI remodeling. (32) Furthermore, by identifying these distinct anatomic LV regions, this imaging technology can enable scientists and clinicians to intervene in the post-MI remodeling process.
Another technique which has been utilized to characterize the distinct myocardial regions involved in post-MI remodeling is magnetic resonance spectroscopy (MRS). A multitude of myocardial metabolites are able to be non-invasively detected using MRS, characterizing the regional metabolic changes which occur in post-MI remodeling. Hu et al reported a 50% reduction in the phosphocreatine to adenosine triphosphate ratio in the borderzone as compared to the remote region of a porcine MI model, indicating that borderzone energy defects likely contribute to local dysfunction post-MI. (33) Feygin et al utilized MRS to examine borderzone bioenergetics following mesenchymal stem cell injection, reporting significant improvement in the phosphocreatine to adenosine triphosphate ratio 4 weeks post-MI in a porcine model. (34) Hence, MRS allows interrogation of specific myocardial regions post-MI and provides an assessment of therapeutic response. At the present time, however, the spatial and temporal resolution limitations of MRS have not yet allowed high-resolution cardiac metabolic imaging to become a clinical reality.
Regional Imaging Post-MI: Prognostic and Therapeutic Potential
Molecular imaging of the unique regions involved in post-MI LV remodeling holds great prognostic importance. Non-invasive, longitudinal assessment of qualities such as the fiber architecture and metabolism of the borderzone region hold the potential to risk-stratify patients. By identifying those at risk for continued post-MI adverse remodeling, these patients could be directed to adjuvant therapies in an effort to reduce the morbidity of the LV remodeling process. Hence molecular imaging techniques hold promise for further individualizing the treatments patients receive post-MI.
Given the prevalence and dire consequences of the LV remodeling process, a multitude of devices and therapies have been developed in an effort to battle this clinical entity. The high quality images produced by cardiac MRI have become an excellent tool for guiding the use of such technologies. One specific group of devices aimed at attenuating LV remodeling post-MI are the ventricular restraint devices, which apply epicardial pressure to combat dilation of the LV and reduce LV wall stress. (35,36) Magovern et al. utilized cardiac MRI to evaluate the placement of a nitinol mesh device (Paracor Surgical Inc., Sunnyvale, CA) in an ovine MI model. MRI at 6 weeks post-MI demonstrated less increase in LV end-diastolic volume index and end systolic volumes in device treated animals as compared to controls, as shown in Figure 3. (35) This study set the stage for future work which would utilize MRI techniques to characterize the regional effects of a cardiac restraint device on post-MI remodeling. Specifically, Blom et al. utilized cardiac MRI and novel three-dimensional surface modeling techniques to measure the degree of infarct expansion and global LV remodeling in an ovine MI model with the CorCap (Acorn Cardiovascular, St.Paul, MN) ventricular restraint device. (36) The study found animals treated with the device at 3 days post-MI had decreased infarct area and improved borderzone contractile function as compared to control animals at 12 weeks post-MI. (36) Device treated animals demonstrated no expansion in either the infarcted or perfused regions following device placement, compared to control animals which displayed more than 300% expansion in the surface area of the infarct region. These results supported the conclusion that early ventricular restraint post-MI is effective to limit infarct expansion and LV remodeling. Cardiac MRI techniques have transitioned the examination of these specific devices from pre-clinical studies to clinical application, providing valuable non-invasive follow-up capabilities (Figure 4).
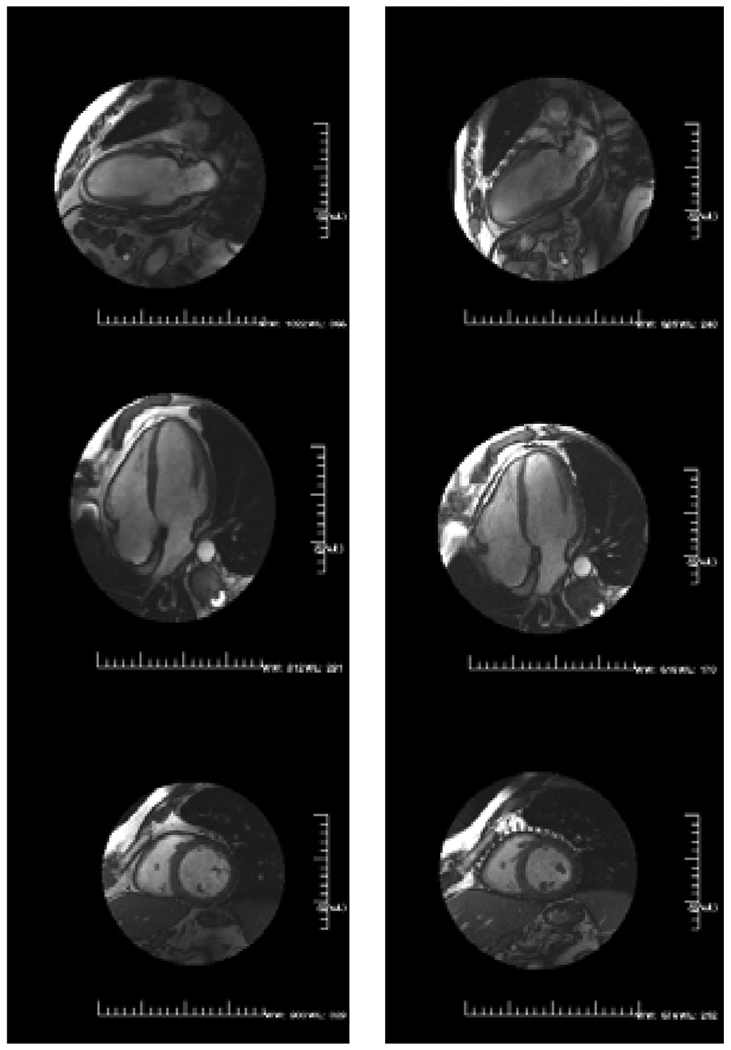
Figure 3.
Long-axis MRI images of sheep hearts obtained 6 weeks after infarction in device (left) and control (right) animals. Hearts are shown in diastole on the top panels and systole on the bottom. Mesh appears as dark area around ventricles. Reproduced with permission from Reference #35.
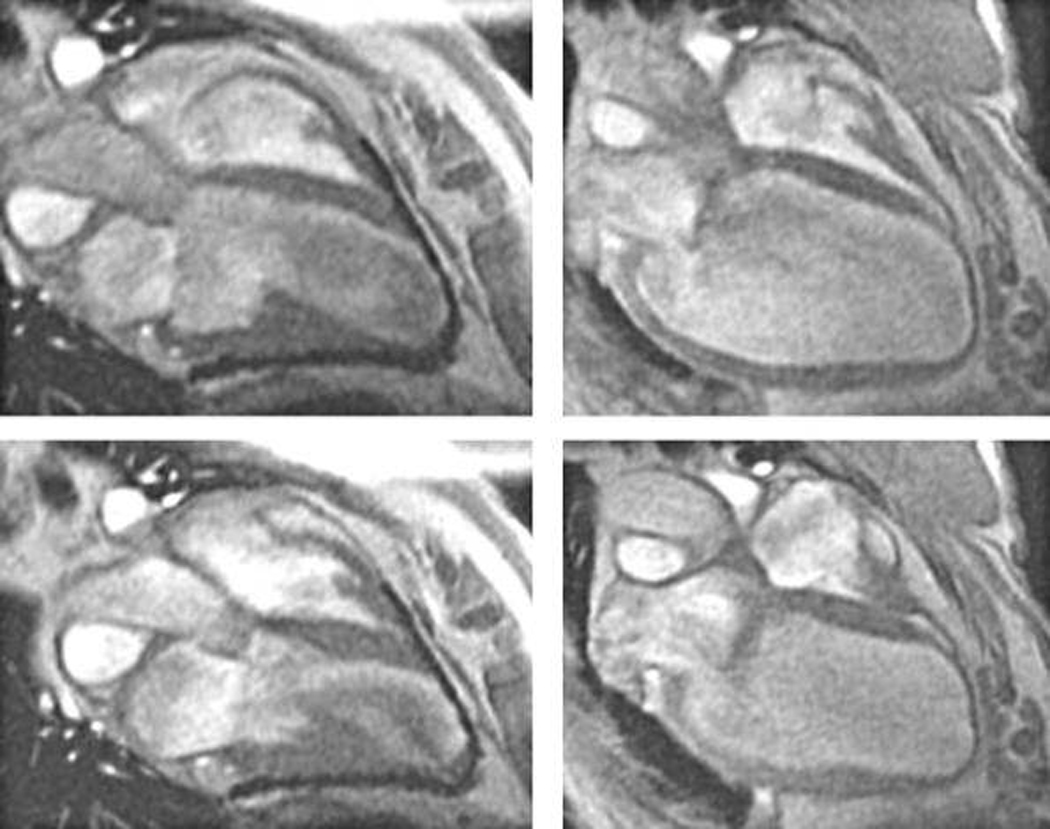
Figure 4.
Epicardial elastic support device in dilated cardiomyopathy. The left panel shows preoperative cardiac MRI images from a patient with dilated cardiomyopathy. The right panel shows the same patient 6 months after placement of the HeartNet (Paracor Medical, Inc., Sunnyvale, CA). Image reproduced with permission from Dr. Robert W.W. Biederman, Allegheny General Hospital, Pittsburgh, PA).
Another field which has drawn great attention in the treatment of LV remodeling is the use of cellular therapies. Specifically, the utilization of various types of stem cells in the post-MI context has been extensively studied. (37–39) MRI techniques utilizing magnetically labeled stem cells have demonstrated the ability to track these cells following delivery to the myocardium. In a pre-clinical study, Kim et al. utilized MRI techniques to track magnetically labeled mesenchymal stem cells for up to 3 months after injection into a prior MI site and to assess the functional response to the cell injection. (37) The study found the cells were able to be seen on MRI up to 10 weeks post-injection (Figure 5) and LV ejection fraction was improved as compared to control animals. Using similar MRI techniques, Hill et al. demonstrated the ability to track iron fluorophore particle labeled mesenchymal stem cells in a porcine model up to 3 weeks post-MI (Figure 6). The study also demonstrated that the iron labeled mesenchymal stem cells retained in vitro viability, proliferation, and differentiation capability as well as in vivo viability after allogeneic transplantation. (38) A quantity of 105 cells/injection was identified as the minimum detectable amount of cells on serial MRI. These findings make MRI detection of iron labeled cells an attractive alternative for the non-invasive evaluation of cellular therapies. This technique holds the potential to answer questions regarding cell engraftment, migration, and honing, in order to elucidate disparate results from early clinical trials involving cellular therapies in the post-MI context. (38,39)
Figure 5.
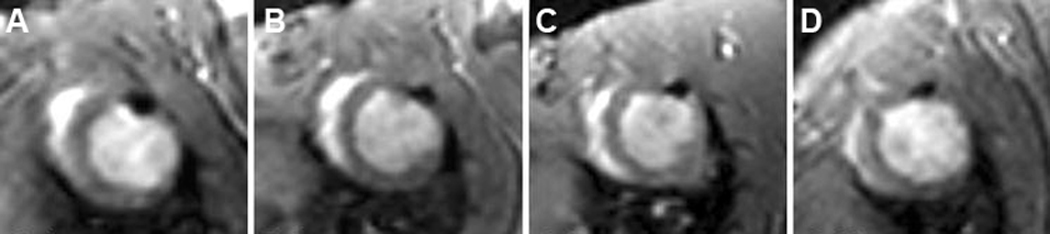
In vivo serial MRI. Serial short-axis views obtained at 1 (A), 2 (B), 4 (C), and 10 (D) weeks show a persistent signal-void after injection of Feridex-labeled mesenchymal stem cells. Reproduced with permission from Reference #37.
Figure 6.
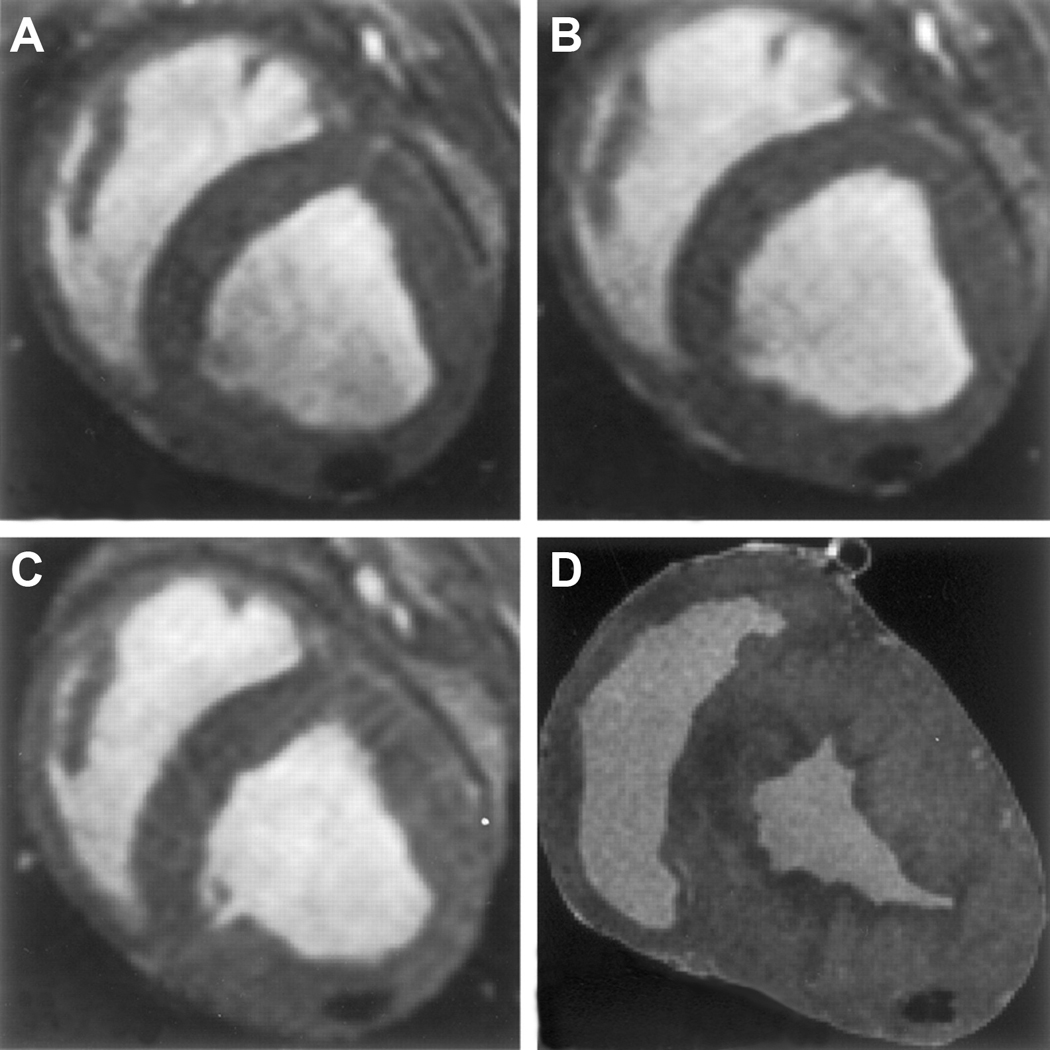
Serial in vivo and ex vivo MRI. Serial short-axis views of diastolic frames show a persistent signal void after injection of 105 iron fluorophore particle labeled mesenchymal stem cells, imaged on days 1 (A), 4 (B), and 21 (C). D, Corresponding view of explanted heart on high-resolution 3D MRI showing signal void. Reproduced with permission from Reference #38.
Conclusion
The post-MI remodeling process involves a heterogeneous anatomic and physiologic regional LV landscape. While this complex landscape is rife for therapeutic intervention, its intricacies must first be elucidated. Each unique anatomical region holds potential targets to attenuate the progression toward heart failure. With the assistance of advancing cardiac MRI techniques, these potential targets will progress toward becoming valuable clinical therapies and current treatment modalities will continue to be refined.
REFERENCES
- 1.Krumholz HM, Anderson JL, Bachelder BL, Fesmire FM, Fihn SD, Foody JM, Ho PM, Kosiborod MN, Masoudi FA, Nallamothu BK. ACC/AHA 2008 performance measures for adults with ST-elevation and non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Performance Measures for ST-Elevation and Non-ST-Elevation Myocardial Infarction) J Am Coll Cardiol. 2008;52:2046–2099. doi: 10.1016/j.jacc.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Russo CA, Andrews RM. The National Hospital Bill: The Most Expensive Conditions, by Payer, 2004. Rockville, Md: Agency for Healthcare Research and Quality; 2006. HCUP statistical brief No. 13. [PubMed] [Google Scholar]
- 3.Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995;27:1281–1292. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 4.Judd JT, Wexler BC. Prolyl hydroxylase and collagen metabolism after experimental mycardial infarction. Am J Physiol. 1975;228:212–216. doi: 10.1152/ajplegacy.1975.228.1.212. [DOI] [PubMed] [Google Scholar]
- 5.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 6.McCormick RJ, Musch TI, Bergman BC, Thomas DP. Regional differences in LV collagen accumulation and mature cross-linking after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 1994;266:H354–H359. doi: 10.1152/ajpheart.1994.266.1.H354. [DOI] [PubMed] [Google Scholar]
- 7.Jugdutt BI, Lucas A, Khan MI. Effect of angiotensin-converting enzyme inhibition on infarct collagen deposition and remodelling during healing after transmural canine myocardial infarction. Can J Cardiol. 1997;13:657–668. [PubMed] [Google Scholar]
- 8.Jugdutt BI, Joljart MJ, Khan MI. Rate of collagen deposition during healing and ventricular remodeling after myocardial infarction in rat and dog models. Circulation. 1996;94:94–101. doi: 10.1161/01.cir.94.1.94. [DOI] [PubMed] [Google Scholar]
- 9.van den Borne SW, Isobe S, Verjans JW, Petrov A, Lovhaug D, Li P, Zandbergen HR, Ni Y, Frederik P, Zhou J, Arbo B, Rogstad A, Cuthbertson A, Chettibi S, Reutelingsperger C, Blankesteijn WM, Smits JF, Daemen MJ, Zannad F, Vannan MA, Narula N, Pitt B, Hofstra L, Narula J. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J Am Coll Cardiol. 2008;52:2017–2028. doi: 10.1016/j.jacc.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 10.Harada K, Friedman M, Lopez JJ, Wang SY, Li J, Prasad PV, Pearlman JD, Edelman ER, Sellke FW, Simons M. Vascular endothelial growth factor administration in chronic myocardial ischemia. Am J Physiol. 1996;270(5 Pt 2):H1791–H1802. doi: 10.1152/ajpheart.1996.270.5.H1791. [DOI] [PubMed] [Google Scholar]
- 11.Voisine P, Bianchi C, Ruel M, Malik T, Rosinberg A, Feng J, Khan TA, Xu SH, Sandmeyer J, Laham RJ, Sellke FW. Inhibition of the cardiac angiogenic response to exogenous vascular endothelial growth factor. Surgery. 2004;136:407–415. doi: 10.1016/j.surg.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Boodhwani M, Sodha NR, Mieno S, Xu SH, Feng J, Ramlawi B, Clements RT, Sellke FW. Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation. 2007;116(11 Suppl):I31–I37. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meoli DF, Sadeghi MM, Krassilnikova S, Bourke BN, Giordano FJ, Dione DP, Su H, Edwards DS, Liu S, Harris TD, Madri JA, Zaret BL, Sinusas AJ. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J Clin Invest. 2004;113:1684–1691. doi: 10.1172/JCI20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH, 3rd, Edmunds LH, Jr, Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation. 2003;107:2857–2863. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 15.Dixon JA, Gorman RC, Stroud RE, Bouges S, Hirotsugu H, Gorman JH, 3rd, Martens TP, Itescu S, Schuster MD, Plappert T, St John-Sutton MG, Spinale FG. Mesenchymal cell transplantation and myocardial remodeling after myocardial infarction. Circulation. 2009;120(11 Suppl):S220–S229. doi: 10.1161/CIRCULATIONAHA.108.842302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekhterae D, Hinmon R, Matsuzaki K, Noma M, Zhu W, Xiao RP, Gorman RC, Gorman JH., 3rd Infarction Induced Myocardial Apoptosis and ARC Activation. J Surg Res. 2009 Jun 6; doi: 10.1016/j.jss.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sosnovik DE, Nahrendorf M, Panizzi P, Matsui T, Aikawa E, Dai G, Li L, Reynolds F, Dorn GW, 2nd, Weissleder R, Josephson L, Rosenzweig A. Molecular MRI detects low levels of cardiomyocyte apoptosis in a transgenic model of chronic heart failure. Circ Cardiovasc Imaging. 2009;2:468–475. doi: 10.1161/CIRCIMAGING.109.863779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamamoto H, Gorman JH, 3rd, Ryan LP, Hinmon R, Martens TP, Schuster MD, Plappert T, Kiupel M, St John-Sutton MG, Itescu S, Gorman RC. Allogeneic mesenchymal precursor cell therapy to limit remodeling after myocardial infarction: the effect of cell dosage. Ann Thorac Surg. 2009;87:794–801. doi: 10.1016/j.athoracsur.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jugdutt BI, Menon V, Kumar D, Idikio H. Vascular remodeling during healing after myocardial infarction in the dog model: effects of reperfusion, amlodipine and enalapril. J Am Coll Cardiol. 2002;39:1538–1545. doi: 10.1016/s0735-1097(02)01805-3. [DOI] [PubMed] [Google Scholar]
- 20.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 21.Azevedo CF, Cheng S, Lima JA. Cardiac imaging to identify patients at risk for developing heart failure after myocardial infarction. Curr Heart Fail Rep. 2005;2:183–188. doi: 10.1007/BF02696648. [DOI] [PubMed] [Google Scholar]
- 22.Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1581–1587. doi: 10.1016/j.jacc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Weis S, Shintani S, Weber A, Kirchmair R, Wood M, Cravens A, McSharry H, Iwakura A, Yoon YS, Himes N, Burstein D, Doukas J, Soll R, Losordo D, Cheresh D. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J Clin Invest. 2004;113:885–894. doi: 10.1172/JCI20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima JA, Judd RM, Bazille A, Schulman SP, Atalar E, Zerhouni EA. Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced MRI. Potential mechanisms. Circulation. 1995;92:1117–1125. doi: 10.1161/01.cir.92.5.1117. [DOI] [PubMed] [Google Scholar]
- 25.Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, Higashino Y, Fujii K, Minamino T. Clinical implications of the 'no reflow' phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223–228. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- 26.Wu KC, Zerhouni EA, Judd RM, Lugo-Olivieri CH, Barouch LA, Schulman SP, Blumenthal RS, Lima JA. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 27.Beek AM, Kühl HP, Bondarenko O, Twisk JW, Hofman MB, van Dockum WG, Visser CA, van Rossum AC. Delayed contrast-enhanced magnetic resonance imaging for the prediction of regional functional improvement after acute myocardial infarction. J Am Coll Cardiol. 2003;42:895–901. doi: 10.1016/s0735-1097(03)00835-0. [DOI] [PubMed] [Google Scholar]
- 28.Maes A, Van de Werf F, Nuyts J, Bormans G, Desmet W, Mortelmans L. Impaired myocardial tissue perfusion early after successful thrombolysis. Impact on myocardial flow, metabolism, and function at late follow-up. Circulation. 1995;92:2072–2078. doi: 10.1161/01.cir.92.8.2072. [DOI] [PubMed] [Google Scholar]
- 29.Gerber BL, Belge B, Legros GJ, Lim P, Poncelet A, Pasquet A, Gisellu G, Coche E, Vanoverschelde JL. Characterization of acute and chronic myocardial infarcts by multidetector computed tomography: comparison with contrast-enhanced magnetic resonance. Circulation. 2006;113:823–833. doi: 10.1161/CIRCULATIONAHA.104.529511. [DOI] [PubMed] [Google Scholar]
- 30.Nieman K, Shapiro MD, Ferencik M, Nomura CH, Abbara S, Hoffmann U, Gold HK, Jang IK, Brady TJ, Cury RC. Reperfused myocardial infarction: contrast-enhanced 64-Section CT in comparison to MR imaging. Radiology. 2008;247:49–56. doi: 10.1148/radiol.2471070332. [DOI] [PubMed] [Google Scholar]
- 31.Wu EX, Wu Y, Nicholls JM, Wang J, Liao S, Zhu S, Lau CP, Tse HF. MR diffusion tensor imaging study of postinfarct myocardium structural remodeling in a porcine model. Magn Reson Med. 2007;58:687–695. doi: 10.1002/mrm.21350. [DOI] [PubMed] [Google Scholar]
- 32.Wu MT, Tseng WY, Su MY, Liu CP, Chiou KR, Wedeen VJ, Reese TG, Yang CF. Diffusion tensor magnetic resonance imaging mapping the fiber architecture remodeling in human myocardium after infarction: correlation with viability and wall motion. Circulation. 2006;114:1036–1045. doi: 10.1161/CIRCULATIONAHA.105.545863. [DOI] [PubMed] [Google Scholar]
- 33.Hu Q, Wang X, Lee J, Mansoor A, Liu J, Zeng L, Swingen C, Zhang G, Feygin J, Ochiai K, Bransford TL, From AH, Bache RJ, Zhang J. Profound bioenergetic abnormalities in peri-infarct myocardial regions. Am J Physiol Heart Circ Physiol. 2006;291:H648–H657. doi: 10.1152/ajpheart.01387.2005. [DOI] [PubMed] [Google Scholar]
- 34.Feygin J, Mansoor A, Eckman P, Swingen C, Zhang J. Functional and bioenergetic modulations in the infarct border zone following autologous mesenchymal stem cell transplantation. Am J Physiol Heart Circ Physiol. 2007;293:H1772–H1780. doi: 10.1152/ajpheart.00242.2007. [DOI] [PubMed] [Google Scholar]
- 35.Magovern JA, Teekell-Taylor L, Mankad S, Dasika U, McGregor W, Biederman RW, Yamrozik J, Trumble DR. Effect of a flexible ventricular restraint device on cardiac remodeling after acute myocardial infarction. ASAIO J. 2006;52:196–200. doi: 10.1097/01.mat.0000199751.51424.78. [DOI] [PubMed] [Google Scholar]
- 36.Blom AS, Pilla JJ, Arkles J, Dougherty L, Ryan LP, Gorman JH, 3rd, Acker MA, Gorman RC. Ventricular restraint prevents infarct expansion and improves borderzone function after myocardial infarction: a study using magnetic resonance imaging, three-dimensional surface modeling, and myocardial tagging. Ann Thorac Surg. 2007;84:2004–2010. doi: 10.1016/j.athoracsur.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 37.Kim YJ, Huh YM, Choe KO, Choi BW, Choi EJ, Jang Y, Lee JM, Suh JS. In vivo magnetic resonance imaging of injected mesenchymal stem cells in rat myocardial infarction; simultaneous cell tracking and left ventricular function measurement. Int J Cardiovasc Imaging. 2009;25 Suppl 1:99–109. doi: 10.1007/s10554-008-9407-0. [DOI] [PubMed] [Google Scholar]
- 38.Hill JM, Dick AJ, Raman VK, Thompson RB, Yu ZX, Hinds KA, Pessanha BS, Guttman MA, Varney TR, Martin BJ, Dunbar CE, McVeigh ER, Lederman RJ. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003;108:1009–1014. doi: 10.1161/01.CIR.0000084537.66419.7A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, Hare JM, Bulte JW. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]