Abstract
Deep brain stimulation (DBS) has been associated with increased apathy in patients with PD, yet studies lack longitudinal data and have not assessed differences between sites of implantation (i.e. STN versus GPi). We assessed apathy prior to surgery and 6 months post-surgery using a longitudinal designe–latent growth curve modeling. We hypothesized that apathy would increase post-surgery, and be related to subthalamic nucleus (versus globus pallidus interna) implantation. Forty-eight PD patients underwent unilateral surgery to either GPi or STN and completed the Apathy Scale prior to surgery and 2, 4, and 6 months post-surgery. Forty-eight matched PD controls completed the Apathy Scale at a 6-month interval. Results indicated apathy increased linearly from pre- to 6-months post-DBS by .66 points bi-monthly, while apathy in the control group did not change. There was no relationship between apathy and DBS site. Higher baseline depression was associated with higher baseline apathy, but not with change in apathy. Middle-aged adults (<65) had a steeper trajectory of apathy than older adults (≥65). Apathy trajectory was not related to motor severity, laterality of DBS, levodopa medication reduction, or motor changes after surgery.
Keywords: Parkinson’s disease, Apathy, Deep brain stimulation, Latent growth curve modeling
1. Introduction
Deep brain stimulation (DBS) is an important therapeutic advancement for the treatment of motor symptoms in Parkinson’s disease (PD) [1]. However, its effects on non-motor symptoms are not well understood. Early studies first reported the development of apathy following DBS surgery in adverse event listings [2,3]. Recently, studies have begun to investigate the predictors and mechanisms of apathy after DBS [4-6]. Apathy is defined as a loss of motivation [7]. Apathy includes symptoms such as loss of initiative, loss of interest, low energy, and flat emotions. Some studies suggest that DBS worsens apathy in some patients. Thirteen studies have quantified apathy after DBS. Ten of these studies found increased apathy after surgery, [4-6,8-14] and two studies found no change in apathy [15,16], and one looked at the acute effects of stimulation, but not the chronic effect of apathy [17].
A limitation of most studies is the lack of longitudinal data. The majority of studies compare apathy at only two time points, once before and once after surgery, and they employed statistical tests such as analyses of variance (ANOVAs). These methods cannot capture the longitudinal pattern of change in apathy symptoms. In contrast, we used latent growth curve modeling with multiple assessments of apathy to better characterize pre-surgery status and rate of change in apathy symptoms after DBS. Latent growth curve modeling is a type of longitudinal structural equation modeling (SEM) that emphasizes initial status and individual change. It can quantify “true” initial status as well as both group means (i.e. fixed effects) and individual differences (i.e. random effects) [18]. Capturing inter-individual differences in change allows for better identification and prediction of individuals who are more likely to suffer DBS-related side effects such as apathy.
This study investigated predictors of baseline apathy and rate of change in apathy after DBS. Some prior research reported a positive correlation between age and apathy after DBS [19]. Yet, other studies have not found a relationship between age and apathy [8,14]. Motor severity and depression severity have been linked to worsened apathy in non-surgical PD patients [20,21], but their relationship to apathy after DBS is understudied. Furthermore, some research suggests the location of DBS electrodes plays a role. For instance, apathetic patients in one study had bilateral STN DBS electrodes placed more ventrally and internally in STN than did patients without apathy [6]. Either the lesion produced by the placement of the stimulator, or the spread of stimulation may affect downstream limbic circuits thought to underlie motivation [6,22]. The GPi, the most common alternative target to the STN, is larger and easier to target the sensorimotor region rather than non-motor regions. The GPi versus STN may be less likely to affect non-motor circuits (i.e. and therefore apathy) than the small/compact STN.
Little is known about the relationship between laterality and apathy. One study involving 70 patients with a mixture of etiologies (i.e. TBI, hypoxia, CVA) reported more apathy in patients with right than left hemispheric lesions [23]. Another study reported a case of profound apathy after a penetrating brain injury of the right basal ganglia [24]. This is the first study to examine the relationship between laterality and apathy in PD. Regarding levodopa, there are mixed findings regarding whether apathy after DBS is related to reductions in levodopa dosage [4-6,8,12,14,15]. One study found 40% of bilateral STN DBS patients who completely withdrew from dopaminergic medications developed apathy [12]. However, complete withdrawal from levodopa represents a rare subgroup of patients. Several studies with substantial levodopa reduction, but not complete withdrawal, found no relationship between levodopa reduction and apathy [4-6,15].
The present study examined the trajectory of apathy scores 6 months after unilateral DBS. We aimed to: 1) identify the temporal course of apathy after surgery and 2) determine the relationship between change in apathy and pre-surgical (i.e. age, motor severity, depression) and surgical variables (i.e. site, laterality, levodopa, motor scores). We hypothesized that patients would have increased apathy after DBS surgery and predicted a positive relationship between apathy and age, motor severity and depression. Based on the smaller size and ease of simulating non-motor pathways in STN, we hypothesized that apathy would be related to STN versus GPi implantation. Based on previous literature, we did not expect apathy to be related to changes in levodopa. Finally, we predicted that right-sided DBS would be related to post-surgical apathy.
2. Methods
Participants were 48 patients with PD who underwent unilateral DBS surgery to GPi, n = 15 (11 left, 4 right) or STN, n = 33 (20 left, 13 right). DBS targets were chosen in two ways for this study. For patients enrolled in the NIH COMPARE cohort [25] the target was randomized STN versus GPi. For the rest of the cohort, targets were chosen individually for patients by an interdisciplinary team including a neurologist, neurosurgeon, neuropsychologist and psychiatrist. The target choice of STN versus GPi in these cases was individualized to the patients’ needs. Additionally, there was one case in the cohort included with a DRS <130. This case was reviewed by the interdisciplinary team, and felt to be suitable for inclusion in the cohort despite not meeting one of the typical center standard requirements to receive DBS. Control participants were a convenience sample of 48 matched non-surgery candidate PD patients who attended the Movement Disorders Center for routine neurological appointments. Mood, motor, and demographic data were collected after informed consent was provided according to university and federal guidelines. All participants met diagnostic criteria for idiopathic PD in accordance with U.K. Brain Bank criteria [26]. Participants completed the Apathy Scale (AS) and Beck Depression Inventory-II (BDI-II) at baseline. The DBS group completed the AS 2, 4, and 6 months after surgery. The control group completed the AS at two sessions 6 months apart (mean = 5.9 months, range = 4.2–8.0 months). A paired t-test was used to examine change over this interval. The AS is a 14-item scale measuring cognitive, emotional, and behavioral symptoms of apathy. A cut-off score of 14 points indicates clinically significant apathy [27,28].
2.1. Predictors
Predictors of baseline apathy were: 1) age group (older adult ≥65 years, middle-aged adult <65), 2) pre-surgery levodopa equivalent dosage (LED), 3) pre-surgery motor severity measured with the Unified Parkinson’s Disease Rating Scale (UPDRS) motor score “off” levodopa medication, and 4) pre-surgery Beck Depression Inventory-II (BDI-II). Predictors of the change in rate of apathy were: 1) pre-surgery BDI-II score, 2) age group, 3) motor improvement after DBS measured by residual change in UPDRS motor score after surgery,1 4) change in LED after surgery, 5) site (GPi versus STN), and 6) laterality (left versus right).
2.2. Structural equation modeling
We constructed latent growth curve models using structural equation modeling. Using maximum likelihood estimation, we ran an unconditional linear growth model of apathy with the slope factor fixed at 0, 1, 2, and 3. A quadratic unconditional growth model was examined, but did not fit the data. The latent factors tested were the intercept (baseline apathy score) and the slope (rate of change in apathy after surgery). Next, we introduced the eight exogenous variables. We ran the model with exogenous variables included and allowed all variables to correlate with each other, but not to predict the latent intercept and slope factors. This provides the “worst fitting” model against which subsequent conditional models are compared. The conditional model allowed the exogenous variables to predict intercept and slope factors. A chi-squared difference test was used to test for significant improvements with the conditional model.
AMOS 16 was used to analyze the data. Model fit was evaluated with traditional goodness of fit indices, including the omnibus χ2 test. A nonsignificant χ2 test statistic suggests a good fit. The closer the following are to 1, the better the fit: Normed Fit Index (NFI), Relative Fit Index (RFI), Incremental Fit Index (IFI), Tucker–Lewis Index (TLI) Comparative Fit Index (CFI). Root Mean Square Error of Approximation (RMSEA) of less than .05 indicates a good fit.
3. Results
3.1. Patient characteristics
DBS participants and control PD participants tended to be male (i.e. DBS = 75% male, control = 70.8% male), well educated (i.e. DBS = 14.4 years, control = 15.3 years), and in the middle stages of the disease when assessed off levodopa (DBS UPDRS-III = 41.1, control UPDRS-III = 38.3). See Table 1 for patient characteristics. Groups were not different on any baseline characteristic, including baseline depression scores or apathy scores, (AS, t [47] = −.19, p = .85).
Table 1.
Patient characteristics
| Characteristic | DBS patients |
PD control patients |
|---|---|---|
| N = 48 | N = 48 | |
| Age | 60.3 (9.0), range 41–79 | 60.4 (9.0), range 39–79 |
| Men: women | 36:12, (75% male) | 34:14 (70.8% male) |
| Years of education | 14.4 (2.8), range 7–20 | 15.3 (3.3), range 9–20 |
| Motor score (UPDRS, off levodopa) | 41.3 (11.7), range 23–81 | 38.3 (13.7), range 10–76 |
| Baseline levodopa equivalent dosage | 1028.9 (527.7), range 150–2500* | 792.07 (618.31), range 0–2834.5 |
| 6 month levodopa equivalent dosage | 1017.4 (632.4), range 0–3500 | 877.27 (632.47), range 0–3000 |
| Years of symptoms | 10.7 (4.8), range 3–27 | 8.8 (5.0), range 1–24 |
| Baseline apathy score (n = 48) | 10.19 (5.6), range 1–25 | 10.2 (6.0), range 1–25 |
| 2 month apathy score (n = 38) | 10.8 (6.6), range 1–33 | n/a |
| 4 month apathy score (n = 40) | 11.8 (7.8), range 1–33 | n/a |
| 6 month apathy score (n = 29) | 12.6 (6.0), range 1–24 | 10.3 (6.5), range 0–26 |
Note: = p < .05 for baseline LED. All other variables not significantly different (all p > .05).
3.2. Aim 1: unconditional growth model
An unconditional linear growth model was run with slope factor loadings fixed to 0, 1, 2, and 3. Time points for the slope factor indicate a baseline pre-surgery time point of 0, and each unit increase represents a 2-month difference. Means for both intercept and slope factors were positive and significant (intercept p < .001; slope p = .014), indicating that apathy scores were significantly different from 0 at baseline and there was a significant linear increase in apathy over time. Thus, preconditions for further modeling were met.
The mean pre-surgery apathy score was 10.19 points (p < .001), and scores rose .660 points every 2 months (p = .014). See Fig. 1. There was significant variance (i.e. individual differences) in both the intercept and slope factors. The intercept and slope were moderately and negatively correlated (r = −.427). Patients higher in initial apathy tended to have slower rates of gain in apathy over time. The fit of the unconditional model was: χ2(38) = 61.2, p = .01, NFI = .689, RFI = .361, IFI = .854, TLI = .598, CFI = .804, RMSEA = .114, p = .04, AIC = 165.2. The unconditional model represents the “worst fitting model” with no predictors.
Fig. 1.
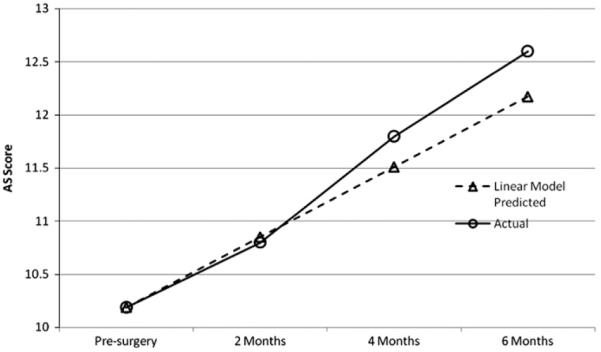
Graph of means of apathy scores comparing means predicted by linear growth model and actual means.
3.3. Aim 2: conditional growth model
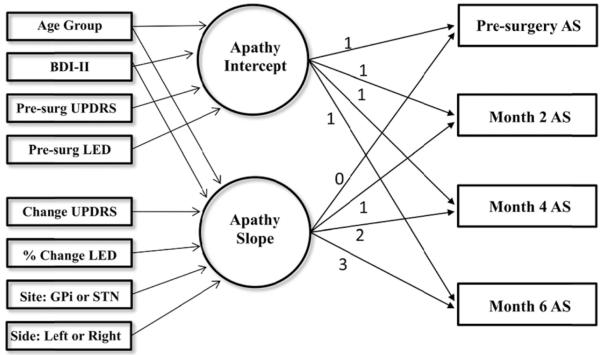
Predictors were introduced into the model (see Fig. 2). The conditional model fit the data well, χ2(28) = 21.1, p = .82, NFI = .893, RFI = .701, IFI = 1.04, TLI = 1.16, CFI = 1.0, RMSEA = .00, p = .90, AIC = 145.1. Fit statistics between the unconditional model and the conditional model are compared in Table 2. A chi-square difference test indicated predictors significantly improved the fit of the model (, p < .001). Predictors accounted for 50.6% of the variance in baseline apathy and 21.7% of the variance in the rate of change in apathy.
Fig. 2.
Path diagram. Factors for intercept and slope are represented by circles, observed variables by boxes on the right, and predictors by boxes on the left. Disturbance terms for the factors, and all the exogenous variables were allowed to correlate, but this is not shown to reduce visual clutter. Age Group = older adult or younger adult, BDI-II = Beck Depression Inventory, UPDRS = Uni ed Parkinson’s Disease Rating Scale, LED = Levodopa equivalent dosage, GPi = Globus pallidus interna, STN = Subthalamic nucleus.
Table 2.
Comparison of fit statistics for the unconditional and conditional models
| Fit statistic | Unconditional model | Conditional model |
|---|---|---|
| χ2 (df) | 61.2 (38) p = .01 | 21.1 (28), p = .82 |
| Variance in initial status | 30.49 | 15.00 |
| Variance in rate of change | 2.28 | 1.42 |
| Pseudo R2 (Proportional reduction in variance) |
n/a | Intercept = .507 Slope = .292 |
| AIC | 165.2 | 145.1 |
| RMSEA | .114, p = .04 | .000, p = .90 |
3.4. Predictors of pre-surgical apathy
Higher pre-surgical depression was significantly related to higher pre-surgical apathy (b = .542, SE = .085, p < .001). There was no relationship between pre-surgical apathy and age (p = .17), LED (p = .69), or motor severity (p = .57).
3.5. Predictors of apathy post-surgery
There was no relationship between pre-surgery depression score and rate of change in apathy (p = .23). There was a significant relationship between age group and rate of change in apathy, indicating that middle-aged adults (<65 years) had a faster rise of apathy after DBS than older adults (b = −1.0, SE = .234, p = .04). Change in UPDRS motor score and percent change in LED were not related to change in apathy (p = .095, p = 52, respectively). Site of implantation (p = .20) and laterality of implantation (p = .92) were not related to change in apathy.
3.6. Additional analyses: baseline variables, surgery variables and levodopa equivalent dosage (LED)
We ran additional analyses to further investigate the relationship between apathy and duration of disease (years of symptoms), baseline Dementia Rating Scale (DRS) scores, surgery factors (i.e. number of microelectrode passes during surgery) and stimulation parameters (i.e. frequency, pulse width, and voltage at 6 months). None were significantly related to apathy. We calculated the change in LED after unilateral DBS. Prior to surgery, patients were taking an average of 1028.9 units of levodopa (SD = 527.7, range 150–2500). After DBS, patients were taking 1017.4 units (SD = 632.4, range 0–3500). The average change in LED was an increase of approximately 5% (mean increase = 4.8%, range = 177% reduction to 100% increase). Specific to site of implantation, STN patients reduced their LED by a mean of .4% (range = 250% reduction to 100% increase) while GPi patients increased their LED by a mean of 15.7% (range = 177% reduction to 40% increase). As noted earlier, this increase in LED was not related to change in apathy, p = .52.
4. Discussion
This study used latent growth curve modeling to examine longitudinal data from 48 PD patients who underwent unilateral STN or GPi DBS surgery. Further, we also examined a matched control group of 48 non-surgical PD patients and hypothesized that apathy would increase in the DBS group, but not in the control group. This hypothesis was supported. Apathy scores in the DBS group increased by .66 points every 2 months while scores in the non-surgical group did not change. Next, we hypothesized a positive relationship between apathy and age, motor severity, and depression. This was only partially supported. Higher pre-surgical depression was related to higher pre-surgical apathy, but not to change in apathy. Contrary to our prediction, middle-aged adults had a steeper rise in apathy after DBS than older adults. Motor severity was not related to baseline apathy or change in apathy after DBS. STN (versus GPi) implantation, laterality, and change in levodopa equivalent dose were not related to change in apathy.
Our finding of increased apathy is consistent with the current literature. Thirteen prior studies have investigated apathy after DBS. Ten studies report an increase in chronic apathy after DBS [4-6,8-12,14], two report no change in apathy after DBS[15,16], and one reports a reduction in acute apathy when stimulators were turned from OFF to ON, but did not examine chronic apathy [17]. See Table 3 for a review. No studies found improvement in chronic apathy following DBS. This is the first study to investigate unilateral STN and GPi found DBS whereas other studies have all been bilateral STN DBS. Importantly, we found a longitudinal trajectory of increased apathy as a result of unilateral surgery.
Table 3.
Apathy outcome following chronic deep brain stimulation reported by study, surgical treatment, sample size (n), evaluation period, apathy measures, and outcome. Note: Abbreviations used: AS = Apathy Scale, FRSBE = Frontal Systems of Behavior Scale UPDRS = Unified Parkinson’s Disease Rating Scale, SD = standard deviation.
| Study | N | Control Group | Apathy Scale | (Pre-DBS) | Post 1 | Post 2 | Mean % reduction of LED after surgery |
Relationship: reduction of LED to apathy |
Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Saint-Cyr et al. [9] |
11 | None | Frontal Lobe Personality Scale (FLOPS) apathy subscale |
Testing period not specified |
3–6 months | 9–12 months | 47–54% | Not examined | FLOPS Apathy subscale significantly increased at 9–12 months for caregiver rated version. No significant difference in patient version |
| Funkiewiez et al. [10] |
77 | None | UPDRS item 4 | Testing period not specified |
1 year | 3 years | 67% | Not examined | Apathy mean score on UPDRS item 4 significantly increased at 3 years post-DBS, trend increase at 1 year post-DBS versus pre-DBS. |
| Czernecki et al. [17] |
18 | 23 demographically matched assessed “on” and “off” L-dopa |
AS measured acutely with STN stimulator turned “on” then “off” (Off L-dopa for both conditions) |
N/A | 10 months | N/A | 84% | Not examined | Apathy mean score significantly decreased (improved) when STN stimulation was on versus off. Controls apathy score significantly decreased when L-dopa was given versus withdrawn |
| Funkiewiez et al. [11] |
22 | None | AS | Testing period not specified |
3 months | N/A | 77.5% | Not examined | Apathy increased at 3 month post-DBS (Individual patient data: 1 patient ≥14 pre-DBS and 5 patients ≥14 post-DBS) |
| Drapier et al. [6] |
15 | 15 demographically matched PD patients after 9 mo. |
AS & Marin Apathy Evaluation Scale |
3 months | 3 months | 6 months | 18.8% from pre to 3 months, 22.2% from pre to 6 months |
No difference in LED between apathetic and non-apathetic, no correlation of apathy and LED |
Apathy increased at 3 and 6 months post-DBS. No difference in apathy for controls |
| Castelli et al. [16] |
62 | None | UPDRS item 4 | 2 wks | 12–20 months | N/A | 55.8% | Not examined | No significant difference was detected in apathy on the UPDRS item 4 post-DBS versus pre-DBS. |
| Castelli et al. [15] |
19 | None | AS | 2 weeks | 13–23 months | N/A | 52.1% | No correlation between apathy and LED |
No significant difference detected in mean apathy scores post-DBS. Individual patient data showed: 1 SD (4 point) increase-31% (6/19), decrease-16% (3/19), no 4 change-53% (10/19). |
| Drapier et al. [13] |
17 | None | Marin Apathy Evaluation Scale |
3 months | 3 months | N/A | 18.8% from pre to 3 months |
Not examined | Apathy mean scores significantly increased at 3 month post-DBS |
| Czernecki et al. [12] |
8 | None | AS | Testing period not specified |
Testing period not specified |
N/A | 100% | Complete withdrawal of dopamine was related to apathy |
Apathy significantly improved with ropinirole in a sample of completely dopaminergic medication withdrawn patients. Pre- to post-DBS apathy data not provided |
| Le Jeune et al. [4] |
12 | None | AES-Clinician version | 3 months | 3 months | N/A | % not reported, but there was a significant reduction in levodopa |
Not related | Apathy worsened at 3 months post-bilateral STN DBS surgery. Worsening apathy was related to hypometabolism of glucose in bilateral anterior cingulate and prefrontal lobe. |
| Porat et al. [8] |
22 | None | Neuropsychiatric Inventory—single item |
3 months | 24 months | N/A | % not reported. | No differences between LED reduction in patients developing apathy versus those that did not |
Apathy increased significantly after surgery. It was not related to motor symptom improvement. |
| Denheyer et al. [14] |
16 | None | Frontal Systems of Behavior Scale |
None | Average of 40 months post-DBS, range 13–78 months |
46% | Increased FRSBE apathy scores associated with less reduction in levodopa |
Apathy assessed retrospectively with the FRSBE by both patients and caregivers significantly increased post-surgery. |
|
| Thobois et al. [5] | 63 | None | AS | Testing period not specified |
Testing conducted monthly over the course of a year via phone |
82% | No relationship in overall levodopa reduction and change in apathy |
Apathy significantly increased over the course of a year in 54% of patients by a mean of 5 months. It was reversible by dopaminergic agonist administration in half of these patients by the 12 month follow-up. Authors suggest individual variation in mesolimbic denervation plus dopamine reduction leads to apathy. |
In terms of predictors of apathy, older age and motor severity did not predict baseline apathy score. Higher baseline depression scores predicted higher baseline apathy scores, which is consistent with studies in PD and other neurological disease groups that report a positive correlation between apathy and depression [14,20,29]. Higher baseline depression score was not related to change in apathy after surgery. In our study, the only significant predictor of apathy was being a middle-aged (versus older) adult. To further investigate this, we performed a post-hoc t-test examining apathy scores at baseline between older and younger adults. While older adult status was not a significant predictor of baseline apathy in our SEM due perhaps to power and/or multi-collinearity with baseline BDI scores, we found a trend for apathy scores at baseline to be lower in middle aged adults (M = 9.03, SD = 5.6), than in older adults (M = 11.95, SD = 5.14), t (46) = −1.81, p = .077. Apathy scores may have increased faster because they started out lower (i.e. return to the mean). Future studies should confirm the relationship between change in apathy and age.
Per site of electrode implantation, we found no relationship between change in apathy and laterality or STN versus GPi implantation. Studies in other disorders have broadly suggested right hemispheric lateralization for apathy, but we found no difference between right and left lateralization in the development of apathy after DBS. No other study has examined this in PD. Per STN versus GPi, the STN has a smaller and more difficult to target sensorimotor region than the GPi. Thus, we hypothesized that either the lesion itself or stimulation effects of STN would be more likely to affect non-motor pathways potentially involved in apathy than would the GPi. This was not supported and both targets were equally likely to lead to apathy. Consistent with this, some authors have argued against the idea that apathy is a specific side effect of stimulation of the limbic part of the STN [5]. They base this on the psycho-stimulating effects of acute bilateral STN stimulation [30]. Further, acute bilateral STN has been shown to reduce acute apathy scores [17]. Also, apathetic versus non-apathetic groups often have similar improvements in motor symptoms (i.e. also found in the present study), suggesting they were accurately targeting the sensorimotor versus non-motor regions of the STN.
Turning to apathy and levodopa, we found no relationship between apathy and change in levodopa equivalent dose (LED) after surgery. Several studies did not find a relationship between apathy and reduction in levodopa, despite substantial reductions in overall LED (see Table 3) [4-6,8,10,15]. Furthermore, one study found increased apathy scores were associated with less reduction (i.e. rather than more reduction) in levodopa [14]. In our study, overall LED change was a slight increase (i.e. 5%), yet apathy still increased after surgery. Taken together, results from previous studies and our own study suggest levodopa reduction alone is not sufficient for the development of apathy.
Instead, Thobois and coworkers [5] recently suggested a more complex interaction between medication changes and underlying neuropathology. They examined 63 bilateral STN patients with self-report inventories, motor testing, and PET imaging (i.e. subgroup of 25 for PET) and found that baseline non-motor fluctuations were predictive of change in apathy after DBS. Post-operative reduction in overall dopaminergic treatment per se did not predict the occurrence of apathy. However, they suggest that underlying individual variations in denervation of the mesolimbic system are the substrate for “unmasking” hypodopaminergic behaviors such as apathy when medications are reduced after surgery. PET imaging suggested a relationship between apathy and the left orbitofrontal cortex, dorsolateral prefrontal cortex, thalamus, internal globus pallidus, and bilaterally in anterior and posterior cingulate cortices. Authors hypothesized that based on this, certain individuals with PD have a lower dopaminergic tone at baseline within the mesocorticolimbic pathways, and these patients are the ones at risk for developing post-operative apathy.
5. Limitations
A limitation of the present study is that 19 of the 48 DBS patients (39.5%) did not complete the AS at the 6-month assessment. However, to address this we used Full Information Maximum Likelihood (FIML), which calculates parameter estimates based on all available data, unlike list-wise deletion. Patients with and without 6-month data did not differ in terms of age, gender, disease variables, surgery variables, or stimulation parameters, or baseline apathy. Groups did differ on education, with those without apathy data at 6 months being more highly educated than those with apathy data. To determine the potential effect of this, we investigated whether increases in apathy were maintained with covariate correction (i.e. education) for missingness using propensity score weighting. This method weights available cases based on the probability of undergoing attrition. We conducted a logistic regression in which education was allowed to predict attrition status and found an acceptable C-statistic (.65). Thus, post-hoc analyses suggest that even if the highly educated patients were included in the final time point, the overall results remain the same.2 Another limitation of the present study was the inability to examine dopamine agonist versus levodopa separately in relation to apathy. Some studies suggest a relationship between dopamine agonists and apathy [12], whereas others do not suggest a specific role of dopamine agonists [5]. Further limitations include short follow-up (6 months), relatively small sample size, and lack of a full set of neuropsychological testing data. While apathy was not related to DRS score, other studies have shown a relationship of apathy and executive functioning. This relationship should be examined in future studies.
6. Conclusions
Our results suggest increased apathy after DBS is robust even after unilateral DBS. Future research may wish to combine latent growth curve modeling, which excels at characterizing longitudinal data and individual differences, with imaging results such that individual differences in underlying pathology (i.e. binding potentials representing dopaminergic cell loss in the mesocorticolimbic system) can be entered into growth curve modeling and this hypothesized predictor of apathy can be tested directly.
Acknowledgment
Funding support: This work was supported by the National Institutes of Health (F31-NS591422 to LKD, K-23 NS50633 to MSO, R-01NS044997 to DB) and the National Parkinson Foundation Center of Excellence.
Footnotes
The review of this paper was entirely handled by the Co-Editor-in-Chief, R.L. Rodnitzky.
Residual change scores were created by taking the average motor improvement from before to after surgery, and calculating how much each individual differed from that average.
As a further check, patients were grouped into quintiles using the resultant predicted probabilities representing groups who were least likely to most likely to fail to complete the 6 month assessment. Using this method, individuals who were more likely to have missing data based on education were forced to contribute more to subsequent analyses and those less likely contributed less. Results indicated an increase in apathy scores from baseline to 6 months post-surgery, with a significant mean of 1.77 points (p = .048).
References
- [1].Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N Engl J Med. 2003 Nov 13;349(20):1925–34. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- [2].Volkmann J, Allert N, Voges J, Weiss PH, Freund HJ, Sturm V. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced PD. Neurology. 2001 Feb 27;56(4):548–51. doi: 10.1212/wnl.56.4.548. [DOI] [PubMed] [Google Scholar]
- [3].Trepanier LL, Kumar R, Lozano AM, Lang AE, Saint-Cyr JA. Neuropsychological outcome of GPi pallidotomy and GPi or STN deep brain stimulation in Parkinson’s disease. Brain Cogn. 2000 Apr;42(3):324–47. doi: 10.1006/brcg.1999.1108. [DOI] [PubMed] [Google Scholar]
- [4].Le Jeune F, Drapier D, Bourguignon A, Peron J, Mesbah H, Drapier S, et al. Subthalamic nucleus stimulation in Parkinson disease induces apathy: a PET study. Neurology. 2009 Nov 24;73(21):1746–51. doi: 10.1212/WNL.0b013e3181c34b34. [DOI] [PubMed] [Google Scholar]
- [5].Thobois S, Ardouin C, Lhommee E, Klinger H, Lagrange C, Xie J, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain. 2010 Apr;133(Pt 4):1111–27. doi: 10.1093/brain/awq032. [DOI] [PubMed] [Google Scholar]
- [6].Drapier D, Drapier S, Sauleau P, Haegelen C, Raoul S, Biseul I, et al. Does subthalamic nucleus stimulation induce apathy in Parkinson’s disease? J Neurol. 2006 Aug;253(8):1083–91. doi: 10.1007/s00415-006-0177-0. [DOI] [PubMed] [Google Scholar]
- [7].Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243–54. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- [8].Porat O, Cohen OS, Schwartz R, Hassin-Baer S. Association of preoperative symptom profile with psychiatric symptoms following subthalamic nucleus stimulation in patients with Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2009;21(4):398–405. doi: 10.1176/jnp.2009.21.4.398. [DOI] [PubMed] [Google Scholar]
- [9].Saint-Cyr JA, Trepanier LL, Kumar R, Lozano AM, Lang AE. Neuropsychological consequences of chronic bilateral stimulation of the subthalamic nucleus in Parkinson’s disease. Brain. 2000 Oct;123(Pt 10):2091–108. doi: 10.1093/brain/123.10.2091. [DOI] [PubMed] [Google Scholar]
- [10].Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, et al. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J Neurol Neurosurg Psychiatr. 2004 Jun;75(6):834–9. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Funkiewiez A, Ardouin C, Cools R, Krack P, Fraix V, Batir A, et al. Effects of levodopa and subthalamic nucleus stimulation on cognitive and affective functioning in Parkinson’s disease. Mov Disord. 2006 Oct;21(10):1656–62. doi: 10.1002/mds.21029. [DOI] [PubMed] [Google Scholar]
- [12].Czernecki V, Schupbach M, Yaici S, Levy R, Bardinet E, Yelnik J, et al. Apathy following subthalamic stimulation in Parkinson disease: a dopamine responsive symptom. Mov Disord. 2008 May 15;23(7):964–9. doi: 10.1002/mds.21949. [DOI] [PubMed] [Google Scholar]
- [13].Drapier D, Peron J, Leray E, Sauleau P, Biseul I, Drapier S, et al. Emotion recognition impairment and apathy after subthalamic nucleus stimulation in Parkinson’s disease have separate neural substrates. Neuropsychologia. 2008 Sep;46(11):2796–801. doi: 10.1016/j.neuropsychologia.2008.05.006. [DOI] [PubMed] [Google Scholar]
- [14].Denheyer M, Kiss ZH, Haffenden AM. Behavioral effects of subthalamic deep brain stimulation in Parkinson’s disease. Neuropsychologia. 2009 Dec;47(14):3203–9. doi: 10.1016/j.neuropsychologia.2009.07.022. [DOI] [PubMed] [Google Scholar]
- [15].Castelli L, Lanotte M, Zibetti M, Caglio M, Rizzi L, Ducati A, et al. Apathy and verbal fluency in STN-stimulated PD patients. An observational follow-up study. J Neurol. 2007 Sep;254(9):1238–43. doi: 10.1007/s00415-006-0510-7. [DOI] [PubMed] [Google Scholar]
- [16].Castelli L, Perozzo P, Zibetti M, Crivelli B, Morabito U, Lanotte M, et al. Chronic deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: effects on cognition, mood, anxiety and personality traits. Eur Neurol. 2006;55(3):136–44. doi: 10.1159/000093213. [DOI] [PubMed] [Google Scholar]
- [17].Czernecki V, Pillon B, Houeto JL, Welter ML, Mesnage V, Agid Y, et al. Does bilateral stimulation of the subthalamic nucleus aggravate apathy in Parkinson’s disease? J Neurol Neurosurg Psychiatr. 2005 Jun;76(6):775–9. doi: 10.1136/jnnp.2003.033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kristjansson SD, Kircher JC, Webb AK. Multilevel models for repeated measures research designs in psychophysiology: an introduction to growth curve modeling. Psychophysiology. 2007 Sep;44(5):728–36. doi: 10.1111/j.1469-8986.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- [19].Ory-Magne F, Brefel-Courbon C, Simonetta-Moreau M, Fabre N, Lotterie JA, Chaynes P, et al. Does ageing influence deep brain stimulation outcomes in Parkinson’s disease? Mov Disord. 2007 Jul 30;22(10):1457–63. doi: 10.1002/mds.21547. [DOI] [PubMed] [Google Scholar]
- [20].Levy ML, Cummings JL, Fairbanks LA, Masterman D, Miller BL, Craig AH, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10(3):314–9. doi: 10.1176/jnp.10.3.314. [DOI] [PubMed] [Google Scholar]
- [21].Zgaljardic DJ, Borod JC, Foldi NS, Rocco M, Mattis PJ, Gordon MF, et al. Relationship between self-reported apathy and executive dysfunction in non-demented patients with Parkinson disease. Cogn Behav Neurol. 2007 Sep;20(3):184–92. doi: 10.1097/WNN.0b013e318145a6f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fields JA, Troster AI. Cognitive outcomes after deep brain stimulation for Parkinson’s disease: a review of initial studies and recommendations for future research. Brain Cogn. 2000 Mar;42(2):268–93. doi: 10.1006/brcg.1999.1104. [DOI] [PubMed] [Google Scholar]
- [23].Finset A, Andersson S. Coping strategies in patients with acquired brain injury: relationships between coping, apathy, depression and lesion location. Brain Inj. 2000 Oct;14(10):887–905. doi: 10.1080/026990500445718. [DOI] [PubMed] [Google Scholar]
- [24].Grunsfeld AA, Login IS. Abulia following penetrating brain injury during endoscopic sinus surgery with disruption of the anterior cingulate circuit: case report. BMC Neurol. 2006;6:4. doi: 10.1186/1471-2377-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Okun MS, Fernandez HH, Wu SS, Kirsch-Darrow L, Bowers D, Bova F, et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: the COMPARE Trial. Ann Neurol. 2009 Mar 13; doi: 10.1002/ana.21596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatr. 1992 Mar;55(3):181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res. 1991 Aug;38(2):143–62. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- [28].Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1992;4(2):134–9. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- [29].Andersson S, Krogstad JM, Finset A. Apathy and depressed mood in acquired brain damage: relationship to lesion localization and psychophysiological reactivity. Psychol Med. 1999 Mar;29(2):447–56. doi: 10.1017/s0033291798008046. [DOI] [PubMed] [Google Scholar]
- [30].Funkiewiez A, Ardouin C, Krack P, Fraix V, Van Blercom N, Xie J, et al. Acute psychotropic effects of bilateral subthalamic nucleus stimulation and levodopa in Parkinson’s disease. Mov Disord. 2003 May;18(5):524–30. doi: 10.1002/mds.10441. [DOI] [PubMed] [Google Scholar]