Abstract
Purpose
To improve local control for inoperable non-small cell lung cancer (NSCLC), a phase I dose escalation study for locally advanced and medically inoperable patients was devised to escalate tumor dose while limiting the dose to organs at risk including the esophagus, spinal cord, and residual lung. Helical tomotherapy provided image-guided IMRT, delivered in a 5-week hypofractionated schedule to minimize the effect of accelerated repopulation.
Methods
Forty-six patients judged not to be surgical candidates with Stage I-IV NSCLC were treated. Concurrent chemotherapy was not allowed. Radiotherapy was delivered via helical tomotherapy and limited to the primary site and clinically proven or suspicious nodal regions without elective nodal irradiation. Patients were placed in 1 of 5 dose bins, all treated for 25 fractions, with dose per fraction ranging from 2.28 to 3.22 Gy. The bin doses of 57 to 80.5 Gy result in 2 Gy/fraction normalized tissue dose (NTD) equivalents of 60 to 100 Gy. In each bin, the starting dose was determined by the relative normalized tissue mean dose modeled to cause < 20% Grade 2 pneumonitis. Dose constraints included spinal cord maximum NTD of 50 Gy, esophageal maximum NTD < 64 Gy to ≤ 0.5 cc volume, and esophageal effective volume of 30%.
Results
No grade 3 RTOG acute pneumonitis (NCI-CTC v.3) or esophageal toxicities (CTCAE v.3.0 and RTOG) were observed at median follow-up of 8.1 months. Pneumonitis rates were 70% grade 1 and 13% grade 2. Multivariate analysis identified lung NTDmean (p=0.012) and administration of adjuvant chemotherapy following radiotherapy (p=0.015) to be independent risk factors for grade 2 pneumonitis. Only 7 patients (15%) required narcotic analgesics (RTOG grade 2 toxicity) for esophagitis, with only 2.3% average weight loss during treatment. Best in-field gross response rates were 17% complete response, 43% partial response, 26% stable disease, and 6.5% in-field thoracic progression. The out-of-field thoracic failure rate was 13%, and distal failure rate was 28%. The median survival was 18 months with 2-year overall survival of 46.8% ± 9.7% for this cohort, 50% of whom were stage IIIB and 30% stage IIIA.
Conclusions
Dose escalation can be safely achieved in NSCLC with lower than expected rates of pneumonitis and esophagitis using hypofractionated image-guided IMRT. The maximum tolerated dose has yet to be reached.
Keywords: Hypofractionation, Dose Escalation, Tomotherapy, Image Guided Radiotherapy, IMRT
Introduction
Although the optimal dose and fractionation scheme for inoperable NSCLC has yet to be established clearly, a dose-response effect for local control has been demonstrated (1-4). Improvement in survival for NSCLC is unlikely without improvement in local control. The maximum dose which may be delivered to tumor and involved lymph nodes is limited by normal tissue tolerances, including esophagus, spinal cord, and normal residual lung. Helical tomotherapy allows for delivery of image-guided, intensity modulated radiation therapy (IG-IMRT) to permit conformal delivery to disease while minimizing the dose delivered to normal tissues.
Radiation pneumonitis represents a major dose limiting toxicity for NSCLC, and treatment planning for locally advanced disease with bilateral hilar or mediastinal involvement can be challenging with 3-D conformal techniques. The M.D. Anderson Cancer Center retrospectively compared 68 patients treated with IMRT to 222 patients treated with 3-D conformal radiotherapy to median doses of 63 Gy. Despite the IMRT group's larger gross tumor volume (194 mL vs. 142 mL, p = 0.002), the rate of Grade ≥ 3 pneumonitis at 12 months was 8% compared with 32% for 3D-conformal (p = 0.002) (5). The Memorial Sloan Kettering Cancer Center also reported on their initial experience with IMRT for 55 NSCLC patients, with 11% grade 3 acute pulmonary toxicity and 4% late pulmonary toxicity (6). Image guidance coupled with IMRT offers further opportunity to decrease high doses delivered to normal tissues by decreasing the margin allowed for setup uncertainty. Prolongation of overall treatment time allows for accelerated repopulation of tumors while undergoing radiotherapy. Improvements in local control for head and neck cancer have been achieved with accelerated courses of radiation therapy using hyperfractionation (7, 8). The CHART trial randomized 563 locally advanced NSCLC patients to 36 fractions of 1.5 Gy 3 times per day to give 54 Gy in 12 consecutive days or conventional radiotherapy delivered in 30 fractions of 2 Gy to a total dose of 60 Gy in 6 weeks. Overall there was a 22% reduction in the relative risk of death, which translated to an absolute improvement in 2 year survival of 9% from 20 to 29% (p = 0.008) and a 21% reduction in the relative risk of local progression (p = 0.033). The CHART trail indirectly demonstrated the role of repopulation as a cause of failure, and provided further evidence that improvement in local control leads to improvement in long term survival in NSCLC (9). Hypofractionated radiotherapy represents an accelerated form of radiation delivery, and once daily treatments improve convenience for patients.
In this report we present the early analysis of a prospective, dose per fraction escalation trial for NSCLC with helical tomotherapy initiated at the University of Wisconsin in 2004, with a specific emphasis on discussing toxicities. An interim analysis has been conducted to assess toxicities and safety. The primary endpoint of the study is to determine the maximum tolerated dose for NSCLC patients, and enrollment continues.
Materials and Methods
Forty-six patients with Stage I-IV NSCLC judged not to be surgical candidates were enrolled. Enrollment was limited to patients with newly diagnosed or recurrent histologically confirmed NSCLC with no prior thoracic radiation therapy or malignant pleural effusion. Stage IV patients were included when multiple lobes were involved and when definitive doses were deemed appropriate for adequate local control. Written informed consent was obtained from each patient according to the University of Wisconsin Hospital Institutional Review Board.
Concurrent chemotherapy was not allowed, although patients could receive sequential chemotherapy either before the initiation or after completion of radiation therapy. Bleomycin and gemcitabine chemotherapy were not allowed prior to radiation. Laboratory evaluation included creatinine < 2.0 mg/dL, hemoglobin > 10 g/dL (allowing for transfusion prior to entry), white blood cell count > 1200/microliter, and SGOT < 3 times normal. Pulmonary function requirements were forced expiratory volume in 1 second (FEV1) ≥ 1 liter and corrected diffusing capacity for carbon monoxide (DLCOc) > 50% predicted. Supplemental oxygen requirements excluded patients from enrollment on the protocol. Patients could have no prior invasive malignancies within the past 3 years, with the exceptions of non-melanoma skin cancers or stage I carcinoma of the breast, prostate, or head and neck. Myocardial infarction within 6 months, unstable angina, uncontrolled congestive heart failure, and uncontrolled significant arrhythmia were also exclusion criteria.
Radiotherapy was delivered via helical tomotherapy and limited to the primary site and clinically proven or radiographically suspicious nodal regions. Elective nodal irradiation was prohibited. Planning imaging included a thin slice treatment planning CT and a 4D-CT. A custom-made double vacuum based immobilization system was utilized to reduce and normalize respiratory motion. The 4D-CT was acquired to identify the motion-defined envelope of the gross tumor volume (GTV). PET imaging was also utilized to define the extent of the GTV. The TomoTherapy Treatment Planning system (TomoTherapy, Inc., Middleton, Wisconsin) was used for treatment planning. A 6 mm margin was added to the motion envelope to account for microscopic extension and setup error, forming the clinical target volume (CTV). Histopathological studies have indicated that a margin of 6 mm covers 95% of the microscopic extension of tumor in squamous cell carcinoma (10), and prior phantom studies evaluating automatic registration of megavoltage to kilovoltage CT images in helical tomotherapy have demonstrated total uncertainty within approximately 1 mm (11). A 2 mm expansion was added to the CTV to form the planning target volume (PTV). Ninety-eight percent of the PTV received the prescription dose determined by bin assignment. The jaw width was allowed to vary based on tumor size, with the majority of patients treated with 2.5 cm jaw width. Pitch settings were typically 0.287 for most plans, and modulation factor varied based on degree of conformality required near normal tissues.
During the pilot phase of the study, 5 patients were treated to the lowest dose allowed on the protocol, 2.28 Gy per fraction for 25 fractions over 5 weeks. The starting dose of 2.28 Gy per fraction to 57 Gy was selected to give the same biologic effective dose (BED) for normal tissue late effects as 60 Gy delivered in 30 fractions of 2 Gy per fraction. Subsequent patients were placed in 1 of 5 dose bins, all treated for 25 fractions, with dose per fraction ranging from 2.28 to 3.22 Gy. These doses, ranging from 57 to 80.5 Gy yielded normalized tissue dose (NTD) equivalents (in 2 Gy fractions) of 60-100 Gy. The relative normalized tissue mean dose (RNTDmean) is the ratio of the mean NTD of lung to the normalized prescription PTV dose. Higher volume of normal lung irradiated and higher dose per fraction result in higher RNTDmean, allowing stratification of patients on the basis of pneumonitis risk as detailed in the appendix at the end of this manuscript. In each dose bin, the starting dose was determined by the RNTDmean predicted for < 20% Grade 2 pneumonitis based on previously reported multi-institutional data incorporated into a normal tissue complication probability model (12). The schema for bin assignments and starting doses for each bin are shown in Table I. Other dose constraints were spinal cord maximum NTD of 50 Gy, esophageal maximum NTD < 64 Gy to ≤ 0.5 cc volume, and esophageal effective volume of 30%.
Table I.
Schema for bin assignments based on relative mean normalized total dose delivered to normal lung. Starting doses for each bin are indicated by an asterisk.
| Dose level (Gy per fraction) | Total Dose (Gy) | Equivalent Dose in 2Gy fractions | Bin 1 0 – 0.119 |
Bin 2 0.12 – 0.179 |
Bin 3 0.18 – 0.239 |
Bin 4 0.24 – 0.309 |
Bin 5 0.31 – 0.41 |
|---|---|---|---|---|---|---|---|
| 2.28 | 57 | 60 | 0-11 | 11-12 | 12-15 | 15-22* | 22-40* |
| 2.53 | 63.25 | 70 | 0-11 | 11-13 | 13-16* | 16-26 | 40-50 |
| 2.77 | 69.25 | 80 | 0-12 | 12-15 | 15-22 | 22-40 | 40-65 |
| 3.00 | 75 | 90 | 0-12 | 12-16* | 16-26 | 26-50 | 50-80 |
| 3.22 | 80.5 | 100 | 0-13* | 13-21 | 21-38 | 38-61 | 61-80+ |
| 3.42 | 85.5 | 110 | 10-21 | 15-23 | 26-50 | High | High |
| 3.62 | 90.5 | 120 | 15-23 | 18-26 | 38-61 | High | High |
| Number of Patients | 4 | 3 | 8 | 24 | 2 | ||
NTD = Normalized Total Dose in 2 Gy fractions
rNTDmean = Volume Relative NTDmean of both lungs
Shaded area ∼ 20% pneumonitis (Grade 2 or greater) risk level 5 Pilot Phase Patients
The primary endpoint of the study was to determine the maximum tolerated dose (MTD). The NCI-CTC v.3 grading criteria for pulmonary toxicities were utilized. Grade 1 pneumonitis was defined as asymptomatic radiographic findings. Grade 2 pneumonitis entailed clinical symptoms which did not interfere with activities of daily living, and patients were usually treated with steroids. Grade 3 pneumonitis was defined as interfering with activities of daily living such that oxygen was required (and for the purposes of DLT definition, at least 7 days of usage was required). Grade 4 pneumonitis was defined as life threatening with the need for ventilatory support. Esophageal toxicities were scored using the Radiation Therapy Oncology Group (RTOG) toxicity scale: grade 1 esophagitis required topical anesthetic or non-narcotic analgesics for mild dysphagia or odynophagia; grade 2 esophageal toxicity consisted of moderate dysphagia or odynophagia, usually requiring narcotic analgesics; grade 3 esophageal toxicity consisted of severe dysphagia or odynophagia with >15% weight loss; and grade 4 esophageal toxicity consisted of complete obstruction, ulceration, fistula, or perforation.
Results
Forty-seven patients with NSCLC were enrolled. One patient elected to discontinue treatment after 10 fractions (22.8 Gy) with no evidence of toxicity, and hospice care was initiated. The remaining 46 patients completed treatment as scheduled. As detailed in Table II, 15% of patients had stage I or II disease, 30% were stage IIIA, 50% were stage IIIB, and 5% were Stage IV. As shown in Table I, the majority of patients were placed into bin 4 (52%) and bin 5 (4%), reflecting the extent of locally advanced disease. Median age was 67 years (range 43-85). Forty-three percent of patients were treated with radiation therapy without chemotherapy, 24% of patients received induction chemotherapy prior to radiation therapy, and 33% of patients received adjuvant chemotherapy after completion of radiation therapy.
Table II.
Stage Grouping and Bin Assignments.
| Stage | # Pts. | Pilot | Bin 1 | Bin 2 | Bin 3 | Bin 4 | Bin 5 |
|---|---|---|---|---|---|---|---|
| IB | 2 | 1 | 1 | ||||
| IIA | 1 | 1 | |||||
| IIB | 4 | 2 | 1 | 1 | |||
| IIIA | 14 | 1 | 1 | 1 | 1 | 9 | 1 |
| IIIB | 23 | 2 | 1 | 5 | 14 | 1 | |
| IV | 2 | 1 | 1 | ||||
| Total | 46 |
At median follow-up of 8.1 months, no patient developed grade 3 or greater pneumonitis or esophagitis. The incidence of grade 2 pneumonitis was only 13%, and patients were treated with tapered steroids as needed. Asymptomatic grade 1 pneumonitis was noted in 70%. Grade 2 esophagitis was recorded in 15% of patients, treated typically with narcotic analgesics. Grade 1 esophagitis was recorded in 24% of patients, and patients responded to non-narcotic analgesics. The average weight loss was only 2.3% for both grade 1 and grade 2 esophagitis while under treatment. For the entire cohort of patients, average weight loss was 1.6% while under treatment.
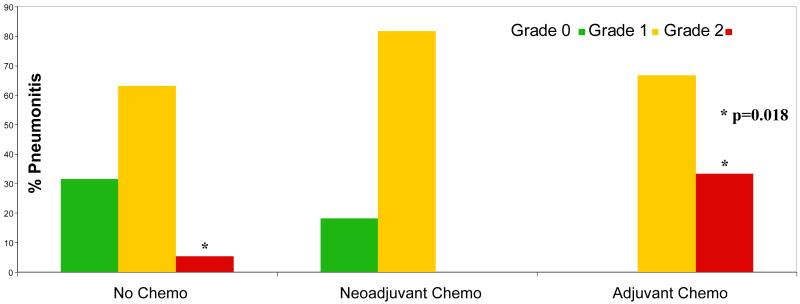
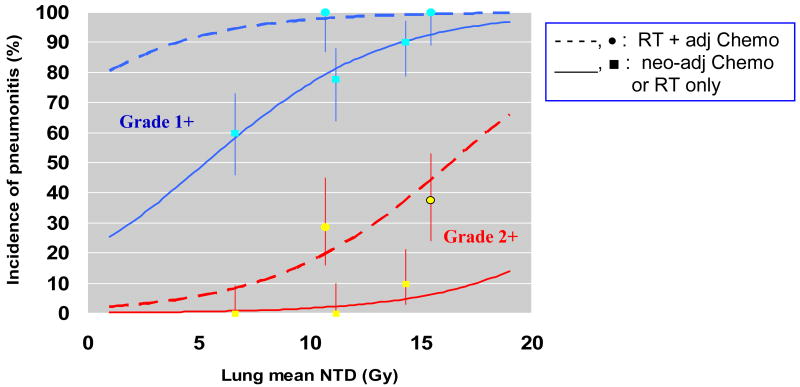
As shown in Figure 1, the administration of adjuvant chemotherapy after completion of radiation therapy conferred a statistically significant (p=0.018) higher incidence of pneumonitis compared to induction chemotherapy prior to radiotherapy or radiotherapy alone. Multivariate analysis identified that lung mean normalized tissue dose and administration of adjuvant chemotherapy following completion of radiation therapy were the only significant independent risk factors for development of grade 2 pneumonitis. Figure 2 demonstrates the relationship of these parameters with regard to the incidence of pneumonitis from ordinal regression analysis.
Figure 1.
Administration of adjuvant chemotherapy after radiation therapy conferred a statistically significant higher risk for grade 2 pneumonitis compared with induction (neoadjuvant) chemotherapy or no chemotherapy.
Figure 2.
Graded response analysis illustrates that mean lung normalized total dose and administration of adjuvant chemotherapy are independent risk factors for pneumonitis. The top curves represent grade 1 or higher pneumonitis and the lower curves represent grade 2 or higher pneumonitis.
Seventeen percent of patients achieved a complete response as the overall best in-field response. Partial response defined as 50% or greater decrease in the sum of the products of diameters of all measured lesions occurred in 43% of patients. Stable disease was the best response for 26% of patients, and 6.5% developed immediate progressive disease after completion of radiotherapy. Ultimately, six patients (13%) developed out-of-field intra-thoracic failures, four of whom also had in-field progression. Only 2 patients failed in mediastinal lymph node regions without in-field failure. Thirteen patients (28%) developed distant metastases, five of whom also manifested in-field thoracic failure and one of whom manifested an out-of-field thoracic failure. Half of the distant failures manifested brain metastases as a component of failure.
The actuarial 2-year overall survival is calculated to be 46.8% ± 9.7%, with a median survival of 18 months. Kaplan Meier curves for overall survival and progression free survival are shown in Figure 3. When patients on the protocol were compared stage for stage to patients reported by Mountain et al. (13), the expected 2-year survival was 21.5%, indicating that the survival of our patients does not appear to be inferior to historic controls, although follow-up is early.
Figure 3.
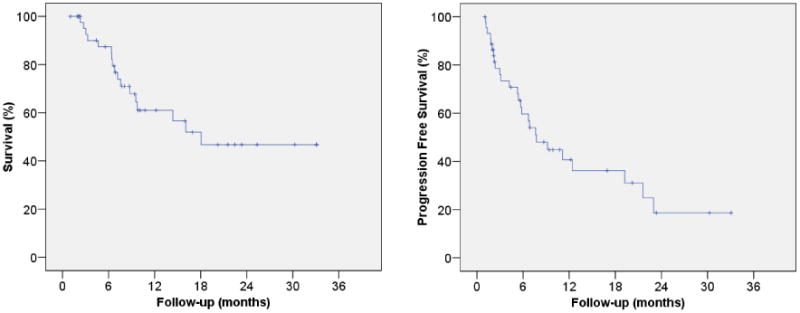
Kaplan Meier curves for overall survival and progression free survival.
Discussion
Although much investigation has focused on optimization of systemic agents for NSCLC, the standard radiation dose of ∼60 Gy has been accepted since publication of results from RTOG 7301 (1). Patients were treated with elective nodal irradiation on the RTOG 7301 protocol with 2-dimensional treatment planning. Since the advent of CT based treatment planning, elective nodal irradiation has been abandoned in many centers and protocols (2, 4, 14, 15), with a focus on involved field treatment to gross tumor and gross lymph node involvement only. A Chinese prospective randomized trial from Shandong Cancer Center treated stage IIIA or IIIB patients with one cycle of induction cisplatin and etoposide followed by concurrent chemoradiation with cisplatin and etoposide. Patients were randomized to either elective nodal irradiation to a total dose of 60-64 Gy or involved field radiation to a dose of 68-74 Gy. Rates of all grades of radiation pneumonitis were significantly lower in the involved field arm compared to the arm receiving elective nodal irradiation (17% vs. 29%, p=0.044). Five-year local control (51% vs. 36%, p=0.032) and 2-year overall survival (39.4% vs. 25.6%, p=0.048) were also improved in the involved field arm which received higher doses. The rate of isolated failure within elective nodal failure regions within 5 years was only 7% for the involved field arm and 4% for the arm receiving elective nodal irradiation (4). The Memorial Sloan Kettering Cancer Center similarly reported elective nodal failure rate of 6.1%, with a median time to elective nodal failure of 6 months after involved field radiation therapy. The 2-year elective nodal control rate was 91% in patients who maintained local control (15).
Prospective dose escalation trials using involved field radiation therapy have demonstrated improved outcomes for patients treated to higher radiation doses. The University of Michigan conducted a phase I dose escalation study using 3-D conformal radiation therapy for stage I-IIIB patients treated at 2.1 Gy per fraction to doses from 63-103 Gy using a risk-stratified normal tissue complication model based on the effective volume (Veff), defined as the volume of normal lung that would have to be irradiated uniformly to a given reference dose to result in a similar risk of pneumonitis. Multivariate analysis revealed weight loss (p = 0.011) and radiation dose (p = 0.0006) were the only significant predictors for overall survival. The 5-year overall survival was 4%, 22%, and 28% for patients receiving 63-69, 74-84, and 92-103 Gy, respectively. Radiation dose was the only significant independent predictor (p=0.015) for locoregional progression-free survival. The 5-year control rate was 12%, 35%, and 49% for 63-69, 74-84, and 92-103 Gy, respectively (2). The Memorial Sloan Kettering Cancer Center also conducted a phase I dose escalation study with 3-D conformal radiation therapy which initially included elective lymph node areas but subsequently converted to an involved field approach with a normal tissue complication probability model, treating patients to 70.2 to 84 Gy in 1.8 Gy fractions. For patients with stage I-II disease, local control rate for patients who received < 80 Gy was 14% at 2 years compared to 88% for those who received ≥ 80 Gy (p<0.001). Overall survival was also improved significantly in patients who received ≥ 80 Gy for both Stage I-II and Stage IIA-IIIB patients (16).
RTOG 9311 was a multi-institutional phase I/II prospective trial which treated patients with involved field 3-D conformal radiation therapy to doses from 70.9 to 90.3 Gy using 2.15 Gy fractions. Patients were stratified based on V20 < 25% vs. V20 of 25-36%, and patients with V20 of 25-36% were only treated to 77.4 Gy. The observed locoregional control and overall survival rates were similar among the various dose levels. The duration of treatment for patients treated to the highest dose level was in excess of 8 weeks, and accelerated repopulation may have played a role in the absence of improved response to higher doses (14). The hypofractionated regimen used in our protocol was specifically designed for this issue.
The observed toxicities on our protocol were lower than expected, with no grade 3 toxicity. Grade 2 pneumonitis occurred in only 13% of patients. The mean relative normalized total dose delivered to normal lung was significantly associated with grade 2 pneumonitis, validating the concept of binning patients based on the dose of radiation delivered to normal lung. In addition, the administration of adjuvant chemotherapy following radiation therapy was also a significant predictor of pneumonitis on multivariate analysis. In the University of Michigan study, no patient developed grade 4 or 5 pulmonary toxicity, 11% of patients developed grade 2 pneumonitis, and 4.6% developed grade 3 pneumonitis with 3-D conformal radiation therapy. The rates of grade 2 and 3 pulmonary fibrosis were 10.1% and 3.7%, respectively, with median follow-up of 9.2 years. The incidence of pulmonary toxicity was not associated with the dose prescribed to the tumor but was significantly associated with mean lung dose, V20, and the normal-tissue complication probability of the lung (17). Similarly, RTOG 9311 found that mean lung dose and V20 were prognostic factors for late pneumonitis (14). The University of Michigan established the maximum tolerated dose of 65.1 Gy for the bin with the largest effective volume, Veff > 0.31 (17). RTOG 9311 safely escalated patients with V20 <25% to 83.8 Gy, but the 90.3 Gy dose level was deemed to be too toxic. Memorial Sloan Kettering's phase I study found that the maximum tolerated dose using a normal tissue complication probability constraint of 25% was 84 Gy. With the use of more conformal techniques such as helical tomotherapy and IMRT, the maximum tolerated dose may be increased relative to 3-D conformal techniques, but this observation will require further validation.
The esophageal constraints used in our protocol were established to allow for minimal esophagitis. Esophageal maximum normalized total dose < 64 Gy to ≤ 0.5 cc volume limited dose escalation in many cases, but the esophageal effective volume of 30% was met readily in most cases. Grade 1 and 2 esophagitis occurred in 24% and 15% of patients, respectively. By way of comparison, the University of Michigan esophagitis rates were 39.5% for grade 1, 16.5% for grade 2, and 2.7% for grade 3 (17). RTOG 9311 estimated the rate of late Grade 3 or worse esophageal toxicity at 18 months of 8%, 0%, 4%, and 6%, for Group 1 patients receiving 70.9, 77.4, 83.8, and 90.3 Gy, respectively. For Group 2, the rates were 0% and 5% for patients receiving 70.9 and 77.4 Gy, respectively (14). The esophageal constraints in our protocol have now been relaxed, given the low incidence of significant esophagitis.
Conclusion
Our study demonstrates that higher doses of radiation therapy than are conventionally administered (∼60 Gy) may be delivered safely in a hypofractionated schedule with helical tomotherapy with lower than expected toxicities. Although median follow-up is relatively short, no grade 3 esophageal or pulmonary toxicities have been observed. Treatment is well tolerated with <3% weight loss in patients manifesting grade 1 or 2 esophagitis. The maximum tolerated dose has not been reached, and the protocol continues to accrue patients.
Table III.
Patient Characteristics.
| Female | 19 |
| Male | 27 |
| Median Age | 67 |
| Induction Chemo | 11 |
| Adjuvant Chemo After RT | 15 |
| RT alone | 20 |
Acknowledgments
This work was funded by NIH NCI P01 CA88960-01-05: Improving Cancer Outcome with Adaptive Helical Tomotherapy.
Appendix
Our bin definitions are based on the University of Michigan Dose escalation trail (18) as to contribute to common data analysis. In the University of Michigan dose escalation trial patients where binned according to Veff. The general expression for Veff is given by:
where n is the Lyman volume effect parameter. For lung, this parameter turns out be n=1 which means that the mean dose to lung determines the incidence of complications. For n=1, the equation above becomes:
The University of Michigan dose escalation trail was conducted at a constant 2.1 Gy per fraction, and dose escalation was accomplished by increasing the number of fractions. However, our dose escalation trail held the number of fractions constant and instead increased the dose per fraction. Therefore, Veff must be calculated using normalized tissue dose (NTD) equivalents to keep the connection to the University of Michigan bin definitions which apply to a NTD schedule. Hence Veff becomes:
Since the residual healthy lung is defined in our dose escalation trail as lung–GTV, the part of the lung receiving the high dose will lie within the PTV, which receives the prescription dose. To be conservative it was decided to use the NTDmean for the tumor, calculated using Gy, as the reference NTD, NTDref. Hence, Veff then becomes:
Therefore, replacing Veff by relative-NTDmean in the University of Michigan bin definitions one arrives at the bins for the estimated % NTCP risk of Grade 2 pneumonitis by dose level and lung volume irradiated used in our protocol.
Footnotes
Conflict of Interest Statement: There are no conflicts of interest to disclose.
References
- 1.Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980;45:2744–2753. doi: 10.1002/1097-0142(19800601)45:11<2744::aid-cncr2820451108>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:324–333. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Belderbos JS, Heemsbergen WD, De Jaeger K, et al. Final results of a Phase I/II dose escalation trial in non-small-cell lung cancer using three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:126–134. doi: 10.1016/j.ijrobp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Yuan S, Sun X, Li M, et al. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol. 2007;30:239–244. doi: 10.1097/01.coc.0000256691.27796.24. [DOI] [PubMed] [Google Scholar]
- 5.Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:94–102. doi: 10.1016/j.ijrobp.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Sura S, Gupta V, Yorke E, et al. Intensity-modulated radiation therapy (IMRT) for inoperable non-small cell lung cancer: the Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Radiother Oncol. 2008;87:17–23. doi: 10.1016/j.radonc.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horiot JC, Bontemps P, van den Bogaert W, et al. Accelerated fractionation (AF) compared to conventional fractionation (CF) improves loco-regional control in the radiotherapy of advanced head and neck cancers: results of the EORTC 22851 randomized trial. Radiother Oncol. 1997;44:111–121. doi: 10.1016/s0167-8140(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 8.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 9.Saunders M, Dische S, Barrett A, et al. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: mature data from the randomised multicentre trial. CHART Steering committee. Radiother Oncol. 1999;52:137–148. doi: 10.1016/s0167-8140(99)00087-0. [DOI] [PubMed] [Google Scholar]
- 10.Giraud P, Antoine M, Larrouy A, et al. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys. 2000;48:1015–1024. doi: 10.1016/s0360-3016(00)00750-1. [DOI] [PubMed] [Google Scholar]
- 11.Boswell S, Tome W, Jeraj R, et al. Automatic registration of megavoltage to kilovoltage CT images in helical tomotherapy: an evaluation of the setup verification process for the special case of a rigid head phantom. Med Phys. 2006;33:4395–4404. doi: 10.1118/1.2349698. [DOI] [PubMed] [Google Scholar]
- 12.Kwa SL, Lebesque JV, Theuws JC, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998;42:1–9. doi: 10.1016/s0360-3016(98)00196-5. [DOI] [PubMed] [Google Scholar]
- 13.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 14.Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 15.Rosenzweig KE, Sura S, Jackson A, et al. Involved-field radiation therapy for inoperable non small-cell lung cancer. J Clin Oncol. 2007;25:5557–5561. doi: 10.1200/JCO.2007.13.2191. [DOI] [PubMed] [Google Scholar]
- 16.Rosenzweig KE, Fox JL, Yorke E, et al. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer. 2005;103:2118–2127. doi: 10.1002/cncr.21007. [DOI] [PubMed] [Google Scholar]
- 17.Kong FM, Hayman JA, Griffith KA, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;65:1075–1086. doi: 10.1016/j.ijrobp.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 18.Hayman JA, Martel MK, Ten Haken RK, et al. Dose escalation in non-small-cell lung cancer using three-dimensional conformal radiation therapy: update of a phase I trial. J Clin Oncol. 2001;19:127–136. doi: 10.1200/JCO.2001.19.1.127. [DOI] [PubMed] [Google Scholar]