Abstract
Psychotic symptoms in Parkinson’s disease (PD) are relatively common, and in addition to being a disturbance to patients’ daily lives, they have consistently been shown to be associated with poor outcome. Our understanding of the pathophysiology of psychosis in PD has expanded dramatically over the past fifteen years, from an initial interpretation of symptoms as dopaminergic drug side effects to the current view of a complex interplay of extrinsic and disease-related factors. The present article reviews the unique clinical features of psychosis as expressed in PD, associated risk factors, and current theories behind its pathogenesis, including medications, visual processing deficits, sleep disturbances, genetics, and neurochemical and structural abnormalities. Finally, we review both traditional and emergent management strategies for PD psychosis, including antipsychotic agents, cholinesterase inhibitors, electroconvulsive therapy (ECT), and other pharmacological and psychological interventions.
I Introduction
Parkinson’s disease (PD) is no longer conceptualized as a pure motor disorder. Researchers are increasingly attending to and characterizing the non-motor symptoms of the disease such as depression, apathy, dementia and psychosis. While the three cardinal motor symptoms of PD (resting tremor, bradykinesia and rigidity) predominate the clinical picture, the disease ushers in a variety of disturbing concomitant emotional, cognitive, and behavioral features that are reported by some patients and their caregivers to be even more disabling than their primary motor symptoms.1,2 Psychosis is consistently shown to be related to poor outcome. Indeed, research has shown that the presence of psychosis predicts increased caregiver burden,3 nursing home placement4, 5, and mortality.5 Clearly, consideration of psychosis and other non-motor symptoms of PD is necessary for optimal patient management.
Psychosis in PD is characterized by hallucinations (primarily visual), delusions, and other sensory disturbances such as illusions and “sense of presence” hallucinations. These psychotic symptoms occur in 20–40%6, 7 of PD patients and significantly affect patients’ quality of life. Psychotic symptoms are often attributable to anti-parkinsonian medications; however, there is an increasing recognition that the underlying disease process plays a major role in their pathogenesis and expression.
II Clinical features and Risk Factors
The most recent revision of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) acknowledges several definitions of psychosis that vary in breadth from positive symptoms such as disorganized speech or behavior to delusions or prominent hallucinations without insight.8 In the US National Comorbidity Survey, approximately 28% of respondents endorsed psychosis-screening questions, and lifetime prevalences of narrowly (schizophrenia or schizophreniform disorder) and broadly (all nonaffective psychoses) were calculated to be 1.3% and 2.2%, respectively.9
While there are no standardized diagnostic criteria for psychosis in PD, the NINDS and the NIMH recently gathered a work group to promote discourse on the topic.10 Psychosis in PD comprises a specific constellation of clinical features that are different from those typical of other psychotic disorders such as schizophrenia. Specifically, PD develops after a primary diagnosis of PD is made and arises in the context of a clear sensorium and retained insight. It consists primarily of paranoid delusions, visual hallucinations and/or other sensory disturbances, and tends to worsen over time. Psychosis in PD most commonly manifests in visual hallucinations, which can occur at any time of the day, although they are most commonly reported in the evening hours during periods of low stimulation.11 The content of visual hallucinations usually consists of people or animals but may also feature inanimate objects. Visual hallucinations generally last seconds to minutes at a time, and most studies have found them to occur at least once a week, although they can occur much more frequently. Unlike those seen in schizophrenia, auditory hallucinations occur less commonly in PD psychosis, and they typically co-occur with visual hallucinations. They consist more often of nondistinct whispers or music, as opposed to the threatening voices reported in schizophrenia.12 However, some cases of threatening auditory hallucinations have been reported in PD.13 Tactile, olfactory and gustatory hallucinations have also been reported in PD psychosis; however less commonly, and they tend to co-occur with visual hallucinations.14 15
Phenomena such as illusions and “presence” hallucinations have been included in the literature on PD psychosis.16 Illusions refer to a disturbance in perceptual experience, such as mistaking a plant for a person. “Presence” hallucinations involve the feeling that a person or animal is in close physical proximity to the patient. As these features are less likely to disrupt patients’ daily lives, they are less likely to be spontaneously reported.
Delusions and disorganized thinking are also seen in PD psychosis, although they are not as common as visual hallucinations.17 Paranoid thoughts such as spousal infidelity are a common theme, although grandiose, somatic, persecutory, and religious delusions have also been documented. A prospective study of psychotic features in PD failed to identify these latter symptoms in 102 consecutive psychotic PD patients.18 Recent research suggests that the occurrence of delusions, as opposed to hallucinations, may be related to younger age at onset of PD and psychotic symptoms.19
It is important to note that there is currently no well-validated and widely-accepted scale for the assessment of psychosis in PD. The Movement Disorder Society recently gathered a task force to assess strengths and weaknesses of the various scales currently used in the literature. Their review encompassed various disease-specific psychosis scales that are typically used by limited groups of researchers as well as more widely used scales originally developed for assessment of schizophrenic patients. The group concluded that there is a need for a new scale of PD psychosis that covers all relevant content as well as demonstrates adequate mechanistic and psychometric properties.20
Psychotic symptoms in PD typically occur later in the disease. On average, symptoms are reported 10 or more years after the initial diagnosis.21 In its early stages, psychosis in PD occurs within a context of a clear sensorium and retained insight.22 Psychotic symptoms tend to recur and worsen over time, and insight may be lost. 23 Psychosis in PD has often been conceptualized as occurring along a continuum in that experiences such as vivid dreaming and illusions herald more frank hallucinations and delusions, which ultimately lead to florid psychosis and dementia.24 However, recent evidence suggests that this conceptualization may not be accurate.25
The use of dopaminergic medications was the first factor implicated in the development of psychosis in PD, and it appears that dopamine agonists in particular put patients at highest risk. It should be noted that while the use/non-use of drug therapy is a risk factor for psychosis, neither the duration nor the dose of drugs has been found to be.26 27 In addition, multiple other risk factors have now been identified, including cognitive impairment and dementia, increased age, disease duration and severity, depression, and various sleep disturbances.28 29 30 31
III Pathophysiology
There has been considerable interest in identifying the mechanisms underlying psychotic symptoms in Parkinson’s disease. Not all patients will develop them, and it appears that a variety of factors, both intrinsic and extrinsic, contribute to their occurrence. In the following sections, we will review the primary factors research has identified as facilitating the emergence of psychotic symptoms, namely, dopaminergic medications, visual processing deficits, sleep disturbances, and neurochemical and structural abnormalities.
The Role of Dopaminergic Medications
The role of dopamine in the pathogenesis of psychotic symptoms has long been studied. Traditional conceptualizations of the pathophysiology of psychosis in Parkinson’s centered on dopaminergic medications as the causal factor.32, 33 It is known that dopaminergic agonists, such as cocaine and amphetamine, can induce psychotic symptoms. 34 Conversely, the mechanism of action of the various antipsychotic agents used to reduce the occurrence of these symptoms is thought to involve dopamine (D2) receptor occupancy.35
All PD medications (not just levodopa) have been implicated in the appearance of psychotic features, and these features often remit after drug therapy has been reduced or eliminated. 36 Clinical experience supports the view that psychosis develops as a side effect of antiparkinsonian therapy, regardless of the specific drug employed. Indeed, a recent study comparing the relative propensity for levodopa and dopamine agonists to induce psychosis-like behavior in an animal model of PD concluded that both types of medication have similar potential to elicit these symptoms.37 Some research has suggested that adverse drug effects of adjunct therapy, which becomes more common in the treatment of older PD patients who have developed complications associated with long-term levodopa treatment, are more common than with levodopa alone.38
A prominent theory for the mechanism by which PD drugs increase susceptibility to psychotic symptomatology involves hypersensitization of dopamine receptors in the nigrostriatal pathway following chronic stimulation, which may lead to dysfunction of limbic structures responsible for assigning emotional and hedonic significance to sensory input. This dysfunction may result in misattributions of internal stimuli having originated from the external sensory world. 39
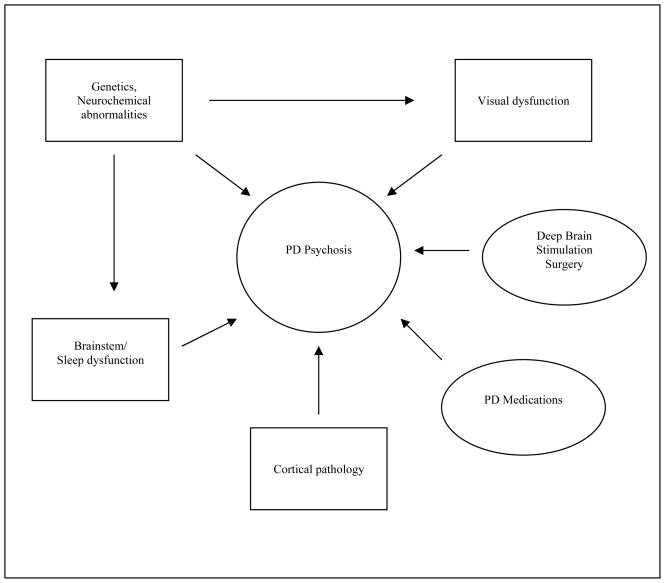
It is now well-accepted that in addition to anti-PD medications, intrinsic, disease-related processes also contribute to the emergence of psychotic symptoms in Parkinson’s disease. Indeed, evidence is accumulating to support the view that dopaminergic medications are neither necessary nor sufficient to account for the expression of psychotic symptomatology. There are reports of hallucinations in PD patients prior to the introduction of levodopa; however, they seemed to occur more frequently after levodopa became available, and early accounts describe hallucinations occuring almost exclusively within the context of depression, dementia, or confusional states.40 41 Unfortunately, it is difficult to to estimate the frequency of hallucinations in the pre-levodopa era due to the lack of prospective studies, the absence of a diagnosis of dementia with Lewy bodies (DLB), and the wide early use of nondopaminergic drugs. Today, psychotic symptoms have also been found to be associated with nondopaminergic agents, including anticholinergics42, amantadine43, and monoamine oxidase-B (MAO-B) inhibitors.44 Also, studies have failed to find a clear relationship between medication dosage and the occurrence or severity of psychosis in patients with PD.45 46 Goetz et al, for example, showed that a high dose levodopa infusion given to five non-demented PD patients with a history of hallucinations induced dyskinesias, but not resurgence of hallucinations. 47 Clearly, non-drug related factors must be involved in the pathogenesis of psychotic symptoms. Recent research has implicated such factors as visual processing abnormalities, sleep dysfunctions, and structural and neurochemical changes (see Figure 1).
Figure 1.
Pathophysiology of psychosis in Parkinson’s disease: an interplay between environmental and intrinsic factors.
DBS Surgery
Many researchers have noted that DBS surgery may improve psychotic symptoms in Parkinson’s disease, presumably due to the resultant reduction in dopaminergic medications.48 However, there have also been reports of stimulation-induced psychotic symptoms as well as transient manic psychosis following DBS surgery, which may be more common in older patients.49 50 51
Visual Processing Deficits
Research has suggested that PD patients who experience visual hallucinations may also suffer from deficits in visual processing. These patients have been found to have lower visual acuity52, deficits in color and contrast recognition53, and greater ocular pathology54, including cataracts, retinal disease and glaucoma, as compared to non-hallucinating PD patients. Dopamine deficiency at the level of the retina is also found in PD55 and has been linked to the occurrence of visual hallucinations in both dementia with Lewy bodies (DLB)56 and PD57. These deficits may serve to facilitate the onset of visual hallucinations in PD.
While structural MRI studies of hallucinating PD patients have yielded little insight into specific occipital lobe or deep white matter lesions58, more recent studies have used fMRI to identify functional abnormalities in the processing of visual stimuli among hallucinators. Specifically, Stebbins et al found that hallucinating PD patients indexed more frontal (BA 44, 6) and subcortical (caudate nucleus) activation and less visual cortical activation than non-hallucinating PD patients.59 These authors postulated that PD hallucinators suffer from a weakening of retinal-striatal-cortical signals, which may lead to the disinhibition of “top-down” processing and the subsequent release of internally-generated images into areas that normally represent externally-generated percepts. Another fMRI study documented a similar pattern of increased activation of visual association cortex (BA 19) coupled with decreased activation of primary visual cortex (BA 17) in PD patients experiencing visual hallucinations.60 Barnes et al report deficient reality monitoring in PD hallucinators in that these patients were more likely than non-hallucinating PD patients and elderly controls to believe that mental images were real external stimuli.61 Currently, studies are underway to determine the effects of pharmacological treatments for hallucinations on functional visual networks.
Sleep Disorders
Psychosis in Parkinson’s has also been linked to sleep disturbances. Sleep complaints such as insomnia62 and daytime sleepiness63 are common in PD. The “continuum hypothesis” of psychosis in PD asserts that sleep disturbances in PD lead to altered dream phenomena, which later lead to frank daytime hallucinations and delusions. Indeed these early phenomena occur frequently in PD. A study found that 48% of PD patients experienced some sort of altered dream phenomena such as vivid dreams, nightmares, and reports suggestive of REM disorder or night terrors.64 30.7% of patients in an early retrospective study were reported to experience vivid dreams.65 One study designed to test the continuum hypothesis found that sleep fragmentation, altered dream phenomena, and hallucinations/illusions were not independent; however, no interaction was found between sleep fragmentation and hallucinations/illusions, which the authors explained by suggesting that the three phenomena are distinct but often overlapping.66
A prominent view of the relationship between sleep disturbances and PD psychosis involves a disruption in rapid eye movement (REM) sleep. Polysomnographic studies of Parkinson patients have found a relationship between visual hallucinations and short, fragmented REM sleep. Specifically, patients experiencing hallucinations evidenced lower sleep efficiency and reduced total REM sleep time and percentage, as compared to patients not experiencing hallucinations.67 Visual hallucinations in PD have been purported by some to represent a narcolepsy-like phenomena involving the intrusion of REM dream imagery into the waking state.68 This intrusion may be related to a reduction in acetylcholine, which leads to the disinhibition of the dream images and their release into the waking state.69
Neurochemical Abnormalities
Of the brain’s neurotransmitters, dopamine has been most consistently linked to psychosis in PD as described above. However, serotonin and acetylcholine and the interaction between these and dopaminergic systems may also play a role in the emergence of psychotic symptoms. Evidence for serotonin’s contribution comes from the observation that several pharmacological agents that reduce serotonergic activity improve psychotic symptoms. For example, the efficacy of the second-generation antipsychotics has been proposed to be related to their relatively high affinity for serotonin receptors compared to dopamine receptors. Also, ondansetron, a 5-HT3 receptor antagonist, has been found to be successful in improving psychotic symptoms in PD70 71. Conversely, serotonergic agonists have been found to induce both delirium and psychosis.72
Arguments against a role for serotonin in the development of PD psychosis come from an early autopsy study that have found that brains of PD patients with psychosis contain less serotonin in the brainstem as compared to PD patients without psychosis.73 However, it is possible that reduced serotonin could lead to post-synaptic hypersensitivity.74 Also, levodopa may increase serotonergic activity.75
It has long been known that PD involves a cholinergic deficit, specifically in the nucleus basalis of Meynert.76 This deficit is even more apparent in cognitively impaired and demented PD patients, and cognitive dysfunction and dementia have consistently been identified as risk factors for the development of psychosis in PD.77 Additionally, a cholinergic deficit has been linked to psychosis in dementia with Lewy bodies (DLB).78 Finally, anticholinergic drugs used to treat the motor symptoms of PD can lead to the emergence of psychotic symptoms, and research is showing that cholinesterase inhibitors may represent an alternative to antipsychotic agents for the treatment of PD psychosis. Overall, it appears that PD psychosis involves the dysregulation of a combination of neurotransmitter systems.
Structural Abnormalities
Lewy body deposition has long been known to be associated with dementia in Parkinson’s disease, which has been identified as risk factor for PD psychosis. One study found a strong correlation between the distribution of Lewy bodies in the temporal lobe, specifically in the amygdale and parahippocampus, and well-formed visual hallucinations in Lewy Body diseases such as dementia with Lewy Bodies and Parkinson’s disease dementia. 79 These temporal lobe Lewy bodies are also associated with an earlier onset of hallucinations in this disease. A large-scale autopsy study of 788 cases of parkinsonism showed a high specificity of visual hallucinations for Lewy body diseases (92.9%).80 A recent clinico-pathological comparison of 10 PD patients with a history of visual hallucinations and 10 PD controls revealed a significantly greater Lewy body burden in the amygdala and cortical areas in the hallucinating patients. 81
Genetics
Recent research has increasingly attended to genetic contributions to Parkinson’s disease and its associated neuropsychiatric symptoms. Family history of dementia appears to be a significant risk factor for the development of hallucinations in patients with PD.82 Furthermore, preliminary evidence suggests that certain genetic profiles may be associated with the development of psychosis in PD. For example, studies have identified the APOE epsilon4 allele as a significant risk factor for drug-induced visual hallucinations in PD83 and an earlier appearance of psychosis in PD.84 However, other studies have failed to document an association between APOE4 and PD psychosis.85 86 More recently, a post-mortem analysis reported an association between PD-related hallucinations and the tau H1H1 genotype.87 Further research is necessary to elucidate the role of genetics in the expression of psychotic symptoms in PD.
IV Management
Before treating psychosis in PD, it is important to rule out an underlying medical illnesses as the cause of the symptoms. For example, urinary and pulmonary infections, metabolic and endocrine imbalances, cerebral hypoperfusion states, and psychosocial stressors can lead to delirium and psychotic symptoms, especially in geriatric patients88.
If the patient is in the early state of the disease, it is likely the psychotic symptoms are attributable to a pre-existing psychiatric disorder or another Parkinsonian syndrome such as dementia with Lewy bodies (DLB). In one study, all patients identified as having early-onset (within three months of initiating levodopa therapy) hallucinations were later found to carry a diagnosis other than or in addition to Parkinson’s disease that could account for their psychotic symptoms89.
Another important consideration in the management of PD psychosis is the issue of polypharmacy, which has been shown to be an independent risk factor for the development of psychotic symptoms in PD90. Not only dopaminergic medications, but also narcotics, hypnotics, antidepressants and anxiolytics may contribute to the expression of psychosis.
Medication Reduction
It is generally accepted that the most effective first-line strategy in the treatment of psychosis in Parkinson’s disease is a reduction in anti-PD medications. If the patient is on multiple medications, most authorities would recommend the gradual removal of anti-PD drugs in the following order: anticholinergics, selegiline, amantadine, dopamine agonists, then catechol-O-methyltransferase (COMT) inhibitors, and lastly, levodopa.91 92 Clinicians should also consider using the short-acting formulation of levodopa rather than the continued-release or long-acting version because the former carries a lower risk for the accumulation of adverse side effects. If the reduction in anti-PD medications to the lowest dose tolerable without the exacerbation of motor symptoms does not improve psychosis, the addition of an antipsychotic agent should be considered.
Antipsychotic Agents
Compared with first-generation or traditional antipsychotics, second-generation antipsychotics (SGAs) produce fewer and less severe extrapyramidal side effects and serum prolactin elevations, presumably due to dual serotonin-dopamine antagonism.93 94 As a result, SGAs, or “atypical” antipsychotics, are typically the treatment of choice for combating psychosis associated with PD. However, the Food and Drug Administration (FDA) of the United States has mandated that all manufacturers provide a boxed warning on product labels stating that SGAs have been found to be associated with a higher risk of mortality when used in elderly patients with dementia95. This finding is particularly relevant in the management of PD psychosis considering the age and frailty of the late-stage PD patients who typically experience psychosis. Since the mechanism by which SGAs caused increased mortality has not been fully elucidated, they will likely continue to be the most common pharmacological agents used in the treatment of PD psychosis. However, studies using alternative therapies such as cholinesterase inhibitors are currently underway.
Six non-traditional or “atypical” antipsychotic drugs have been marketed in the US: clozapine, risperidone, olanzapine, quetiapine, ziprasidone, and aripiprazole. The differences between these agents lie in their relative tendencies to worsen motor symptoms, and the clinician’s choice of an antipsychotic agent is based largely on its unique side effect profile. Given the adverse consequences associated with the use of neuroleptics, the duration of treatment is an important consideration. Hallucinations tend to persist in Parkinson’s disease, and the existence of hallucinations at baseline is a strong predictor of their existence at follow-up evaluations96. One study that attempted to wean psychosis-free PD patients off of their antipsychotic medications was aborted after enrollment of only six patients because 5/6 subjects experienced rebound psychosis. Furthermore, 3 out of the 5 patients experienced a worsening of psychotic episodes in the form of paranoid delusions or threatening auditory hallucinations97. Research is needed to confirm whether antipsychotic medications should be continued after they are initiated.
Clozapine
Clozapine, a dibenzodiazepine derivative, is the only SGA fully recommended for the treatment of psychosis in PD according to a 2007 meta-analysis98. Numerous double-blind placebo-controlled as well as open-label trials have been conducted that demonstrate its efficacy and tolerability in PD patients.
The only negative study in the literature on clozapine in PD was also the very first double-blind, placebo-controlled trial.99 In that study, 3 out of 6 patients experienced worsened parkinsonism; however, the titration schedule used was taken from the schizophrenia literature and reached 150mg/d. At higher dosages, subjects reported severe sedation, which may partially explain the exaggeration of PD motor symptoms. It is now known that PD patients can experience therapeutic benefit at doses as low as 6.25mg/d.100
Two randomized, double-blind, placebo-controlled trials of low-dose clozapine in PD were published in 1999.101 The US trial demonstrated significant improvement in symptoms as measured by four different psychosis scales as well as no decline in motor function. Additionally, there was a significant improvement in tremor. Many patients experienced complete resolution of psychotic symptoms in just one day, and the majority experienced significant improvement in one to two weeks. Only one subject discontinued treatment as a result of a decline in white blood cell (WBC) count. The French trial reported similar results. A meta-analysis reported an average improvement rate of 85% with acceptable tolerance.102
Analysis of longitudinal data (26 months) from 59 out of the original 60 patients enrolled in the US trial revealed that 25% had died, and 42% had been placed in nursing homes. 8 patients who were not demented at baseline (MMSE > 25) were carrying a clinical diagnosis of dementia at follow-up.103 69% of patients continued to hallucinate; however, persisting hallucinations at long-term follow-up did not predict mortality, nursing home placement, or dementia. Of the 40 patients still on an antipsychotic agent, 23 were still receiving clozapine. Of those on clozapine, seven required repeat WBC counts, and two patients withdrew from therapy as a result.
With regard to long-term efficacy and safety, one retrospective analysis of 39 parkinsonism patients reported that over the course of 60 months, 85% of patients experienced a continued partial/good response to clozapine, and 13% experienced a complete resolution of psychotic symptoms.104 Data from a five-year follow-up study of 32 patients with PD revealed that 9 patients had discontinued treatment with clozapine because of improvement in psychosis, while three patients discontinued treatment because of somnolence.105
Despite its demonstrated efficacy, clozapine is often avoided because of its potential for producing agranulocytosis, which is thought to occur in 0.38% (in a sample of over 99,000 US patients with schizophrenia).106 For this reason, the use of clozapine must be accompanied by weekly WBC count monitoring during the first six months of use and bi-weekly monitoring thereafter. Other side effects of clozapine include sedation, orthostatic hypotension and sialorrhea, which are more common.107 The use of second-generation antipsychotics in schizophrenic patients has also been associated with a “metabolic syndrome”, which features insulin resistance, weight gain, dyslipidemia, and abnormal glucose metabolism.108 However, this syndrome has not been reported in PD patients.
In summary, the data support the use of low-dose clozapine for the treatment of psychosis in PD. However, because of the strict monitoring required during treatment with clozapine, research is underway to identify a more practical agent for the treatment of psychosis in PD.
Risperidone
Although risperidone, which is chemically dissimilar to clozapine, was released in the US as an “atypical” antipsychotic, it has been shown to behave more like “typical” neuroleptics in that it exhibits a dose-dependent incidence of extrapyramidal side effects and prolactin elevation.109 110 111 The vast majority of trials with risperidone in patients with parkinsonism are open-label. Overall, the reports demonstrate significant improvement in psychosis. However, results regarding motor side effects vary greatly across studies. Some describe severe motor worsening in each risperidone-treated patient.112 113 Others report no change in motor functioning.114,115 116 Still others report mixed results.117 118 119 120 A meta-analysis of 82 risperidone-treated PD patients reported that 33% experienced a worsening of motor symptoms.121 Only one double-blind study of risperidone in PD has been conducted; however, it only included 10 patients.122 In that study, patients were randomized to receive either clozapine or risperidone. The risperidone group, but not the clozapine group, experienced a significant improvement in psychosis, as measured by the psychosis cluster on the Brief Psychiatric Rating Scale (BPRS); however, mean improvement did not differ between the clozapine and risperidone groups.
The variability in results among risperidone studies is largely attributable to the open-label nature of most reports. However, clinicians may also have differed in their ability to assess parkinsonism, and different studies utilized different titration schedules and durations of observations. Due to numerous reports of motor worsening in PD patients treated with risperidone and the agent’s “typical” antipsychotic behavior, many movement disorders specialists are reluctant to use this agent to treat PD psychosis.
Olanzapine
Olanzapine is more chemically similar to clozapine than is risperidone, and it appears to be more “atypical” in that the dosage levels required to induce catalepsy are higher than those that would be used in treating humans, and it does not cause significant prolactin secretion.123
The very first study of olanzapine in PD was open label and demonstrated that the agent was effective in treating drug-induced psychosis in 15 non-demented patients without motor worsening. A 2003 review of the literature estimated motor deterioration occurred in about 40% of Parkinson’s patients.124
Four double-blind trials of olanzapine in PD have been published. Three were placebo controlled, and one compared olanzapine to clozapine. The latter trial was aborted after six out of 7 of the olanzapine-treated subjects experienced a significant decline in motor functioning.125 All three placebo-controlled trials demonstrated no significant improvements in PD psychosis and a worsening of motor functioning.126 127 In summary, olanzapine appears ineffective in the treatment of psychosis in PD and can lead to intolerable motor deterioration even at low doses.
Quetiapine
Among the SGAs, quetiapine, a dibenzothiazepine, has the closest structural resemblance to clozapine. It is a strong 5-HT2 receptor antagonist and a moderate D2 receptor antagonist, and it shows only low affinity for the muscarinic receptor. It does not block apomorphine-induced stereotypy, alter prolactin levels, or show a risk of agranulocytosis.
Numerous open-label studies on quetiapine for the treatment of psychosis in PD have been reported, two of which were retrospective analyses. One report found that 35 out of 43 PD patients experienced a significant improvement in psychotic symptoms when treated with quetiapine, and only five patients (all of whom were demented) experienced a mile deterioration in motor functioning. No patients discontinued treatment.128 In 2003, Fernandez et al reported that 78 out of 106 PD patients treated with quetiapine at a single movement disorders center remained on the agent for a mean duration of 15 months, and 87 (82%) of those patients experienced partial or complete resolution of psychosis.129 Mild motor worsening was reported over 15 months by 32% of the patients, and these patients tended to be more demented.
Two double-blind trials of quetiapine in PD reported no significant improvement in psychosis. The first trial, in which 31 patients were randomized (2:1) to quetiapine or placebo, may not have been adequately powered to detect a treatment effect.130 In the second trial, 30 patients were randomized to quetiapine and 28 patients were randomized to placebo.131 Of the 15 patients who discontinued the medication in the quetiapine group, 10 patients reported a lack of efficacy. The authors suggest that this large dropout rate may have influenced their results. These results are surprising given that open-label studies of over 400 patients have reported overall efficacy and tolerance of quetiapine in PD.
Another double-blind trial of quetiapine in PD did show efficacy in treating psychosis. 40 patients were randomized to either quetiapine or clozapine. Both groups experienced a significant improvement in psychotic symptoms, and the groups did not differ in mean improvement.132
A recent study reported on 35 PD patients with psychosis who were followed over 24 months.133 15 (43%) patients responded to quetiapine over the 24-month period. 11 of those patients were still receiving quetiapine at 24 months, 3 discontinued the medication due to improvement in their psychosis, and 3 discontinued the medication due to financial reasons. There were no reports of worsening motor symptoms.
There have been some reports suggesting that quetiapine may have a positive effect on sleep architecture. An open-label trial of quetiapine for the treatment of insomnia in Parkinson’s disease found a significant improvement in the severity of insomnia, as assessed with the Pittsburg Sleep Quality Index (PSQI) and a significant reduction in daytime sleepiness, as assessed with the Epworth Sleepiness Scale (ESS) with no worsening of motor symptoms.134 Given the known association between sleep disturbances, including insomnia and daytime sleepiness, and the development of visual hallucinations in PD, studies are currently underway to investigate the relationship between quetiapine’s effects on both sleep and psychosis in PD.
In summary, quetiapine appears to be less effective than clozapine for the treatment of PD psychosis. It does not improve tremor, and it may induce mild motor worsening.135 However, unlike olanzapine and risperidone, no declines in motor functioning reported in PD patients have required hospitalization. Additionally, quetiapine does not carry an associated risk of agranulocytosis, and thus it does not require the vigilant monitoring that clozapine requires.
Ziprasidone
Compared with other second-generation antipsychotics, ziprasidone has a greater affinity for serotonin 5-HT2 receptors than for dopamine D2 receptors136. Its use has been limited because of its effects on the heart’s electrical cycle, but there has been no reported cases of ziprasidone causing torsades de pointes as a result of the drug’s side effect of prolonging the QT interval.137 Available data on the use of ziprasidone to treat PD psychosis is limited.
Two case reports suggest that ziprasidone may be a relatively safe treatment for psychosis in PD, especially when other antipsychotic agents have proven ineffective or caused intolerable side effects. One patient’s delusions and multiple-modality hallucinations resolved with ziprasidone, and his motor functioning improved at a final dose of 80mg/day.138 The second patient experienced a resolution of both hallucinations and delusions without adverse effects at a final dose of 120mg/day.139
Two case series on ziprasidone in PD have been published. The first, conducted in Spain, was an open-label trial in 12 PD patients.140 On average, psychosis improved by 58.4% (mean dose 24 mg/day) at one month and 72% (mean dose 32 mg/day) at 12 weeks, as measured by the Neuropsychiatric Inventory. Two patients discontinued treatment due to sedation or deterioration of gait. UPDRS motor scores did not worsen significantly, despite the deterioration of gait observed. The second report, conducted in Germany, was an open-label trial of intramuscular injections of ziprasidone (10–20 mg) for acute agitation in 5 PD patients.141 Mean improvement in symptoms was 24.6 points on the Brief Psychotic Rating Scale, and no worsening of parkinsonism was reported.
While it appears that ziprasidone may represent a relatively safe and effective treatment of PD psychosis in some patients, after reviewing data on ziprasidone for the treatment of schizophrenia, a panel of expert psychiatrists concluded that its extrapyramidal side effects profile is “better than risperidone, the same as olanzapine but not quite as good at quetiapine or clozapine”.142
Aripiprazole
Unlike first- and second-generation antipsychotics, which are D2 antagonists, aripiprazole is a partial agonist at both D2 and 5-HT1 receptors and has been referred to as the first of the third generation of antipsychotic agents.143 144 145 Additionally, it is believed to carry a relatively low risk of extrapyramidal side effects due to its high 5-HT2/D2 affinity ratio. However, available data on aripiprazole suggest that its efficacy and tolerability in PD patients is variable.
Several case reports on aripiprazole in PD have been published. The first reports a drastic deterioration in motor symptoms, including severe akinesia and anarthria, with no change in hallucinations or delusions.146 Another patient experienced similar motor complications with aripiprazole, although auditory hallucinations and delusions were completely resolved.147 Treatment with aripiprazole was discontinued after the patient experienced akinesia and rigidity. A third report describes three patients with PD psychosis.148 All three patients experienced improvement in psychosis, as measured by the Brief Psychiatric Rating Scale and the Clinical Global Impression scale, and no adverse events were reported.
To date, two open-label trials of aripiprazole in PD have been published. The first trial included 8 patients experiencing visual hallucinations and/or paranoid delusions.149 Only 2 out of the 8 patients showed a complete improvement in psychosis, as measured by the BPRS. The remaining 6 patients discontinued treatment due to inefficacy (2 patients), motor deterioration (2 patients), lack of improvement in motor functioning that had been compromised after treatment with olanzapine (1 patient), or intolerable restlessness and confusion (1 patient). The second open-label trial involved 14 patients and reported similar results.150 In this study, 6 out of the 14 patients experienced an improvement in psychosis. The remaining 8 subjects discontinued treatment due to worsened parkinsonism (3 patients), exacerbated psychosis (2 patients), worsened parkinsonism and psychosis (2 patients), and inefficacy (1 patient).
Available data suggests that while aripiprazole may be efficacious for some patients, it carries a high risk of adverse effects and results are variable.
Other treatments
Ondansetron
Ondansetron, an antiemetic, was tested as a possible treatment for schizophrenia due to its action as an antagonist at the 5-HT3 receptors, but it has not been found to be efficacious for this purpose. However, there are several early reports that it may be efficacious for the treatment of psychosis in Parkinson’s disease without the worsening of motor symptoms, presumably due to its high selectivity. Two open-label trials conducted by Zoldan et al. reported that a total of 40 patients experienced an improvement in psychosis. Side effects were limited and included headache (1 patient) and constipation (7 patients).151 152 However, these positive results have not been reproduced by others.153 Furthermore, the cost of the drug has kept it from being tried further in the PD population.
Cholinesterase inhibitors
Tacrine
The first published trial of a cholinesterase inhibitor in PD involved tacrine for the treatment of PD dementia in patients experiencing confusion and visual hallucinations.154 In this open-label design, 5 out of 7 demented patients experienced a complete resolution of psychotic symptoms, and the remaining 2 patients improved. No patients experienced a decline in motor functioning. However, Ott and Lannon reported an exacerbation of parkinsonism in one PD patient treated with tacrine.155 Due to hepatic toxicity, tacrine is rarely used for the treatment of PD psychosis.
Donepezil
Several open-label studies of donepezil in Parkinson’s disease have been reported. One trial involved 8 non-demented patients experiencing visual hallucinations with or without delusions, and the authors reported a significant decrease in psychotic symptoms, as measured by the Parkinson Psychosis Rating Scale (PPRS).156 Two patients experienced a clinically significant worsening of motor functioning. Another open-label trial included 6 demented PD patients with psychotic symptoms.157 5 out of the 6 patients experienced at least a moderate improvement in psychosis and the remaining patient experienced a mild improvement. No adverse motor side effects were reported. A third study reported on 3 patients with visual hallucinations.158 The authors report that all 3 patients experienced an improvement in their hallucinations without worsening of motor functioning. However, one of the 3 patients developed delusions, which resolved after treatment was terminated.
A randomized, placebo-controlled trial of donepezil in PD patients with psychosis enrolled 22 demented PD patients.159 There was no difference between patients receiving donepezil or placebo in psychosis, as measured by the BPRS. However, this trial excluded patients with more severe psychosis. A double-blind, placebo-controlled trial of donepezil in demented PD patients studied 14 subjects for 20 weeks.160 No patients experienced a decline in motor functioning. With regard to psychotic symptomatology, 3 patients showed improvement in scores for delusions, 2 for hallucinations, and 9 for agitation. However, none of these improvements reached statistical significance, likely due to low baseline scores and a small sample size.
Galantamine
Galantamine is unique from other cholinesterase inhibitors in that it also acts on nicotinic Ach receptors. There is evidence to suggest that activity at these receptors may prevent the down-regulation of acetylcholine that may occur after treatment with cholinesterase inhibitors.161 Thus, increasing activity at nicotinic receptor located on presynaptic terminals of dopamine neurons in the striatum may facilitate the release of dopamine and exert a positive effect on motor symptoms. There is only one reported open label trial of galantamine in psychotic PD patients. In that study, 7 out of the 9 patients experiencing hallucinations experienced an improvement in their hallucinations with 3 of those 7 experiencing a complete resolution of symptoms. Furthermore, parkinsonism improved in 6 out of the 9 patients. However, the remaining 3 experienced a worsening of tremor.
Rivastigmine
Rivastigmine is unique from the other cholinesterase inhibitors in that in addition to its action on acetylcholinesterase, it also inhibits butyrylcholinesterase. In an open-label trial of rivastigmine in12 PD patients with psychosis, the drug was well-tolerated, MMSE scores improved from 20.8 to 25.4, UPDRS motor scale scores did not change, and caregiver distress improved.162 Importantly, mean NPI scores improved on the subscales measuring hallucinations and sleep disturbances, although not on the delusion subscale. No worsening of tremor or parkinsonism was noted. A case series of four patients with visual hallucinations found that rivastigmine improved hallucinations and was well-tolerated.163
Recently, results from a large, double-blind, placebo-controlled trial of rivastigmine for the treatment of visual hallucinated in demented PD patients were reported.164 The trial enrolled 188 hallucinating patients and 348 non-hallucinators. Patients experiencing hallucinations improved significantly in overall NPI score as well as on the individual item for agitation/aggression. In addition, hallucinating patients experienced a greater therapeutic benefit from rivastigmine than did non-hallucinating patients, most likely due to the more rapid decline in the hallucinating placebo group. Additional double-blind, controlled trials of rivastigmine for the treatment of visual hallucinations in PD are needed and are currently underway.
Electroconvulsive Therapy (ECT)
ECT has been found to be an effective treatment for psychiatric disorders such as medication-resistant depression and schizophrenia. Research regarding its use in Parkinson’s disease is limited to case reports; however, it appears that ECT may be useful in reducing psychotic symptoms in PD, especially when there is concurrent depression and/or pharmacologic therapies have been unsuccessful. Most patients in the literature experienced a reduction in psychotic symptoms following ECT treatment.165 166 167 Reports also indicate that ECT may temporarily improve motor symptoms. Because ECT treatment may require hospitalization and side effects include cognitive deficits and confusion, it should only be considered when trials with pharmacological treatments have proved unsuccessful.
Antidepressants
Early studies suggested that some antidepressants may induce or exacerbate psychotic symptoms.168 169 However, there is emerging evidence that certain antidepressant medications, such as clomipramine and citalopram, may actually improve psychotic symptoms, especially in patients with concurrent depression.170 171 The literature to date consists only of case reports, and further studies, including controlled trials, are needed.
Psychological Approaches
Managing psychosis and its behavioral and emotional consequences through non-pharmacological methods should also be discussed with the patient and his/her family. Psychosis in PD tends to persist, and it may not resolve with aggressive pharmacological treatments. In addition, psychosis is related to disturbing emotional problems such as depression. Self management skills and structured psychological interventions are both important, albeit understudied, strategies in optimizing psychosis management.
Diederich et al found that 36 out of 46 hallucinating PD patients surveyed reported using self-driven coping strategies such as cognitive, interactive, and visual techniques, to manage their symptoms.172 While the study did not include a measure to formally evaluate the effectiveness of these strategies, patients who reported using them reported that their hallucinations were “bothersome or depressing” only 39% of the time, compared to 60% of the time for those who did not report using coping strategies. This difference did not reach significance. Further studies should be conducted to further explicate the most beneficial forms of self-driven coping strategies for different types of patients.
Structured psychological interventions, including cognitive-behavioral therapy, supportive therapy, and psychoeducation, have also been useful in the treatment of PD psychosis.173 174 175 While most of the evidence for the effectiveness of these interventions comes from the schizophrenia literature, the results are promising, and future research should aim to investigate their feasibility and effectiveness in Parkinson’s populations. Since early psychosis in PD typically involves retained insight, these patients may potentially benefit even more from such techniques as cognitive-behavioral therapy.
V Conclusion
Psychotic symptoms are a common feature of PD and are related to disease duration and severity, dementia, depression, and age. The pathophysiology of psychosis is now known to involve an interaction between extrinsic, drug-related and intrinsic, disease-related components such as neurochemical (dopamine, serotonin, acetycholine, etc) and structural abnormalities, visual processing deficits, sleep dysregulation, and genetics. The treatments for PD psychosis are as numerous as its proposed origin, and can include various pharmacological and other types of strategies. First-line treatment traditionally involves the removal of or reduction in anti-PD medications; however, persistent symptoms may require the addition of another drug. While clozapine and quetiapine appear to be the most efficacious of the second- and third-generation antipsychotics, their side effects have prompted an explosion of research into alternative agents, the most promising of which appears to be cholinesterase inhibitors such as rivastigmine. Future research is required in order to elucidate the unique contributions of the various proposed mechanisms responsible for the emergence of psychotic symptoms of PD and to identify the safest and most beneficial treatment strategies.
Footnotes
Disclosure Statement:
Hubert H. Fernandez, MD has, over the past 5 years, been a paid consultant, paid speaker or performed clinical research under contract with:
Amarin, Allergan, AstraZeneca, Aventis, Boehringer Ingelheim, Boston Life Sciences, Biogen Idec, Cephalon, Easai, Elan, Forest Laboratories, GlaxoSmithKlein, Huntington Study Group, Ipsen, Kyowa, Merck KgaA, Merz, MylanBertek, National Parkinson Foundation, Neurotrax, NIH/NINDS, Novartis, Parkinson Study Group, Solstice, Solvay, Teva, United Biosource Corporation, Valeant, and Vernalis; but has no owner interest in any pharmaceutical company.
No funding has been received for the conduct of this study and/or the preparation of this manuscript.
Contributor Information
Laura B. Zahodne, Department of Clinical and Health Psychology, Graduate Assistant, Department of Neurology, University of Florida/McKnight Brain Institute, Gainesville, FL.
Hubert H. Fernandez, Clinical Trials in Movement Disorders, Co-Director, Movement Disorders Center, Director, Neurology Residency Training Program, University of Florida/McKnight Brain Institute, Gainesville, FL.
References
- 1.Witjas T, Kaphan E, Azulay JP, et al. Nonmotor fluctuations in Parkinson’s disease: frequent disabling. Neurology. 2002 Aug 13;59(3):408–13. doi: 10.1212/wnl.59.3.408. [DOI] [PubMed] [Google Scholar]
- 2.Global Parkinson’s Disease Survey Steering Committee. Factors impacting on quality of life in Parkinson’s disease: results from an international survey. Mov Disord. 2002;17:60–7. doi: 10.1002/mds.10010. [DOI] [PubMed] [Google Scholar]
- 3.Carter JH, Stewart BJ, Archbold PG, et al. Living with a person who has Parkinson’s disease: the spouse’s perspective by stage of disease. Parkinson’s Study Group. Mov Disord. 1998;13:20–8. doi: 10.1002/mds.870130108. [DOI] [PubMed] [Google Scholar]
- 4.Aarsland D, Larsen JP, Tandbert E. Predictors of nursing home placement in PD: a population-based prospective study. J Am Geriatr Soc. 2000;48:938–42. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 5.Goetz CG, Stebbins GT. Risk factors for nursing home placement in advanced Parkinson’s disease. Neurology. 1993;43:2227–9. doi: 10.1212/wnl.43.11.2227. [DOI] [PubMed] [Google Scholar]
- 6.Fenelon G, Mahieux F, Huron R, Ziegler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000 Apr;123(Pt 4):733–45. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Ramos JR, Ortoll R, Paulson GW. Visual hallucinations associated with Parkinson disease. Arch Neurol. 1996 Dec;53(12):1265–8. doi: 10.1001/archneur.1996.00550120077019. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic and Statistical Manual for Mental Disorders. 4 [Google Scholar]
- 9.Kendler KS, Gallagher TJ, Abelson JM, Kessler RC. Lifetime prevalence, demographic risk factors, and diagnostic validity of nonaffective psychosis as assessed in a US community sample. The National Comorbidity Survey. Archives of General Psychiatry. 1996;53:1022–31. doi: 10.1001/archpsyc.1996.01830110060007. [DOI] [PubMed] [Google Scholar]
- 10.Ravina B, Marder K, Fernandez HH, et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS/NIMH work group. [DOI] [PubMed] [Google Scholar]
- 11.Fenelon G, Mahieux F, Huron R, Ziegler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123 (Pt 4):733–45. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- 12.Inzelberg R, Kipervasser S, Korczyn AD. Auditory hallucinations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;64:533–5. doi: 10.1136/jnnp.64.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Factor SA, Molho ES. Threatening auditory hallucinations and Cotard syndrome in Parkinson disease. Clin Neuropharmacol. 2004;27:205–7. doi: 10.1097/01.wnf.0000144040.20600.c1. [DOI] [PubMed] [Google Scholar]
- 14.Marsh L, Williams JR, Rocco M, Grill S, Munro C, Dawson TM. Psychiatric comorbidities in patients with Parkinson disease and psychosis. Neurology. 2004;63:293–300. doi: 10.1212/01.wnl.0000129843.15756.a3. [DOI] [PubMed] [Google Scholar]
- 15.Aarsland D, Ballard C, Larsen JP, McKeith I. A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson’s disease with and without dementia. Int J Geriatr Psychiatry. 2001;16:528–36. doi: 10.1002/gps.389. [DOI] [PubMed] [Google Scholar]
- 16.Fenelon G, Mahieux F, Huron R, Ziegler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123 (Pt 4):733–45. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- 17.Sadock BJ, Sadock VA, editors. Kaplan and Sadock ’s comprehensive textbook of psychiatry. 8 rev. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 18.Holroyd S, Currie L, Wooten GF. Prospective study of hallucinations and delusions in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2001;70:734–738. doi: 10.1136/jnnp.70.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiziltan G, Ozekmekci S, Ertan S, Ertan T, Erginoz E. Relationship between age and subtypes of psychotic symptoms in Parkinson’s disease. J Neurol. 2007 April;254(4):448–52. doi: 10.1007/s00415-006-0388-4. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez HH, Aarsland D, Fenelon G, et al. Report of a movement Disorder Society Task Force on Rating Scales in Parkinson’s disease. Scales to assess psychosis in Parkinson’s disease: critique and recommendations. [DOI] [PubMed] [Google Scholar]
- 21.Papapetropoulos S. Drug-induced psychosis in Parkinson disease: phenomenology and correlations among psychosis rating instruments. Clin Neuropharmacol. 2006;29:59. doi: 10.1097/00002826-200601000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Wolters EC, Berendse HW. Management of psychosis in Parkinson’s disease. Curr Opin Neurol. 2001;14:499–504. doi: 10.1097/00019052-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Goetz CG, Fan W, Leurgans S, Bernard B, Stebbins GT. The malignant course of "benign hallucinations" in Parkinson disease. Arch Neurol. 2006;63:713–6. doi: 10.1001/archneur.63.5.713. [DOI] [PubMed] [Google Scholar]
- 24.Moskovitz C, Moses H, Klawans HL. Levodopa-induced psychosis: a kindling phenomenon. Am J Psychiatry. 1978;135:669–75. doi: 10.1176/ajp.135.6.669. [DOI] [PubMed] [Google Scholar]
- 25.Pappert EJ, Goetz CG, Niederman FG, et al. Sleep fragmentation, and altered dream phenomena in PD. Mov Disord. 1999;14:117–21. doi: 10.1002/1531-8257(199901)14:1<117::aid-mds1019>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Aarsland D, Larsen JP, Cummins JL, Laake K. Prevalence and clinical correlates of psychotic symptoms in Parkinson disease: a community-based study. Arch Neurol. 1999;56 (5):595–601. doi: 10.1001/archneur.56.5.595. [DOI] [PubMed] [Google Scholar]
- 27.Merims D, Shabtai H, Korczyn AD, Peretz C, Wizman N, Giladi N. Antiparkinsonian medication is not a risk factor for the development of hallucinations in Parkinson’s disease. J Neural Transm. 2004;111(10–11):1447–53. doi: 10.1007/s00702-004-0209-9. [DOI] [PubMed] [Google Scholar]
- 28.Fenelon G, Mahieux F, Huron R, Ziegler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123 (Pt 4):733–45. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- 29.Giladi N, Treves TA, Paleacu D, et al. Risk factors for dementia, depression, and psychosis in longstanding Parkinson’s disease. Neural Transm. 2000;107 (1):59–71. doi: 10.1007/s007020050005. [DOI] [PubMed] [Google Scholar]
- 30.Doe De Maindreville A, Fenelon G, Mahieux F. Hallucinations in Parkinson’s disease: a follow-up study. Mov Disord. 2005 Feb;20(2):212–7. doi: 10.1002/mds.20263. [DOI] [PubMed] [Google Scholar]
- 31.Papapetropoulos S, Argyriou AA, Ellul J. Factors associated with drug-induced visual hallucinations in Parkinson’s disease. J Neurol. 2005 Oct;252(10):1223–8. doi: 10.1007/s00415-005-0840-x. [DOI] [PubMed] [Google Scholar]
- 32.Friedman JH. The management of the levodopa psychoses. Clin Neuropharmacol. 1991;14:283–95. doi: 10.1097/00002826-199108000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Factor SA, Molho ES, Podskalny GD, Brown D. Parkinson’s disease: drug-induced psychiatric states. Adv Neurol. 1995;65:115–38. [PubMed] [Google Scholar]
- 34.Glenthøj BY, Hemmingsen R. Dopaminergic sensitization: implications for the pathogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:23–46. doi: 10.1016/s0278-5846(96)00158-3. [DOI] [PubMed] [Google Scholar]
- 35.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157 (4):514–20. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 36.Goetz CG, Tanner CM, Klawans HL. Pharmacology of hallucinations induced by long-term drug therapy. Am J Psychiatry. 1982;139 (4):494–7. doi: 10.1176/ajp.139.4.494. [DOI] [PubMed] [Google Scholar]
- 37.Fox SH, Visanji NP, Johnston TH, Gomez-Ramirez J, Voon V, Brotchie JM. Dopamine receptor agonists and levodopa and inducing psychosis-like behavior in the MPTP primate model of Parkinson disease. Arch Neurol. 2006 Sept;63:1343–4. doi: 10.1001/archneur.63.9.1343. [DOI] [PubMed] [Google Scholar]
- 38.Chan DK. The art of treating Parkinson disease in the older patient. Aust Fam Physician. 2003 Nov;32(11):927–31. [PubMed] [Google Scholar]
- 39.Wolters EC. Intrinsic and extrinsic psychosis in Parkinson’s disease. J Neurol. 2001;248(Suppl 3):III/22–III/27. doi: 10.1007/pl00007822. [DOI] [PubMed] [Google Scholar]
- 40.Fenelon G, Goetz CG, Karenberg A. Hallucinations in Parkinson disease in the prelevodopa era. Neurology. 2006;66:93–8. doi: 10.1212/01.wnl.0000191325.31068.c4. [DOI] [PubMed] [Google Scholar]
- 41.Rondot P, de Recondo J, Coignet A, Ziegler M. Mental disorders in Parkinson’s disease after treatment with L-DOPA. Adv Neurol. 1984;40:259–69. [PubMed] [Google Scholar]
- 42.Tanner CM, Vogel C, Goetz CG, Klawans HL. Hallucinations in Parkinson’s disease: a population study. Ann Neurol. 1983;12:136. (abstract) [Google Scholar]
- 43.Postma JU, Van Tilburg W. Visual hallucinations and delirium during treatment with amantadine (Symmetrel) J Am Geriatr Soc. 1975 May;23(5):212–5. doi: 10.1111/j.1532-5415.1975.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 44.Klein C, Kompf D, Pulkowski U, Moser A, Vieregge P. A study of visual hallucinations in patients with Parkinson’s disease. J Neurol. 2007 Jun;244(6):371–7. doi: 10.1007/s004150050104. [DOI] [PubMed] [Google Scholar]
- 45.Holroyd S, Currie L, Wooten GF. Prospective study of hallucinations and delusions in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2001;70:734–8. doi: 10.1136/jnnp.70.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merims D, Shabtai H, Korczyn AD, Peretz C, Weizman N, Giladi N. Antiparkinsonian medication is not a risk factor for the development of hallucinations in Parkinson’s disease. J Neural Transm. 2004;111:1447–53. doi: 10.1007/s00702-004-0209-9. [DOI] [PubMed] [Google Scholar]
- 47.Goetz CG, Pappert EJ, Blasucci LM, et al. Intravenous levodopa in hallucinating Parkinson's disease patients: high-dose challenge does not precipitate hallucinations. Neurology. 1998;50:515–17. doi: 10.1212/wnl.50.2.515. [DOI] [PubMed] [Google Scholar]
- 48.Chen CC, Lee ST, Wu T, Chen CJ, Chen MC, Lu CS. Short-term effects of bilateral subthalamic stimulation for advanced Parkinson’s disease. Chang Gung Med J. 2003 May;26(5):344–51. [PubMed] [Google Scholar]
- 49.Herzog J, Volkmann J, Krack P, et al. Two-year follow-up of subthalamic deep brain stimulation in Parkinson’s disease. Mov Disord. 2003 Nov;18(11):1332–7. doi: 10.1002/mds.10518. [DOI] [PubMed] [Google Scholar]
- 50.Castelli L, Perozzo P, Zibetti M, et al. Chronic deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: effects on cognition, mood, anxiety and personality traits. Eur Neurol. 2006;55(3):136–44. doi: 10.1159/000093213. [DOI] [PubMed] [Google Scholar]
- 51.Vesper J, Haak S, Ostertag C, Nikkhah G. Subthalamic nucleus deep brain stimulation in elderly patients – analysis of outcome and complications. BMC Neurol. 2007 Mar 16;7:7. doi: 10.1186/1471-2377-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holroyd S, Currie L, Wooten GF. Prospective study of hallucinations and delusions in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2001;70:734–8. doi: 10.1136/jnnp.70.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diedrich NJ, Goetz CG, Raman R, et al. Poor visual discrimination and visual hallucinations in Parkinson’s disease. Clin Neuropharmacol. 1998;21:289–95. [PubMed] [Google Scholar]
- 54.Fenelon G, Mahieux F, Huron R, Ziegler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123 (4):733–45. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- 55.Bodis-Wollner I. Visual deficits related to dopamine deficiency in experimental animals and Parkinson’s disease patients. Trends Neurosci. 1990 Jul;13(7):296–302. doi: 10.1016/0166-2236(90)90113-o. [DOI] [PubMed] [Google Scholar]
- 56.Maurage CA, Ruchoux MM, de Vos R, Surguchov A, Destee A. Retinal involvement in dementia with Lewy bodies: a clue to hallucinations? Ann Neurol. 2003;54:542–7. doi: 10.1002/ana.10730. [DOI] [PubMed] [Google Scholar]
- 57.Onofrj M, Bonanni L, Albani G, et al. Visual hallucinations in Parkinson’s disease: Clues to separate origins. J Neurol Sci. 2006;248:143–50. doi: 10.1016/j.jns.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 58.Kraft E, Winkelmann J, Trenkwalder C, et al. Visual hallucinations: white matter lesions and disease severity in Parkinson’s disease. Acta Neurol Scand. 1999;98:1–6. doi: 10.1111/j.1600-0404.1999.tb07365.x. [DOI] [PubMed] [Google Scholar]
- 59.Stebbins GT, Goetz CG, Carillo MC, et al. Altered cortical visual processing in PD with hallucinations. Neurology. 2004;63:1409–16. doi: 10.1212/01.wnl.0000141853.27081.bd. [DOI] [PubMed] [Google Scholar]
- 60.Holroyd S, Wooten GF. Preliminary fMRI evidence of visual system dysfunction in Parkinson’s disease patients with visual hallucinations. J Neuropsychiatry Clin Neurosci. 2006;18 (3):402–4. doi: 10.1176/jnp.2006.18.3.402. [DOI] [PubMed] [Google Scholar]
- 61.Barnes J, Boubert L, Harris J, et al. Reality monitoring and visual hallucinations in Parkinson’s disease. Neuropsychologia. 2003;41:565–74. doi: 10.1016/s0028-3932(02)00182-3. [DOI] [PubMed] [Google Scholar]
- 62.Larsen JP, Tandberg E. Sleep disorders in patients with Parkinson’s disease. CNS Drugs. 2001;15 (4):267–75. doi: 10.2165/00023210-200115040-00002. [DOI] [PubMed] [Google Scholar]
- 63.Pacchetti C, Manni R, Zangaglia R, et al. Relationship between hallucinations, delusions, and rapid eye movement sleep behavior disorder in Parkinson’s disease. Movement Disorders. 2005;20 (11):1439–48. doi: 10.1002/mds.20582. [DOI] [PubMed] [Google Scholar]
- 64.Pappert EJ, Goetz CG, Niederman FG, et al. Sleep fragmentation, and altered dream phenomena in PD. Mov Disord. 1999;14:117–21. doi: 10.1002/1531-8257(199901)14:1<117::aid-mds1019>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 65.Moskovitz C, Moses H, Klawans HL. Levodopa-induced psychosis: a kindling phenomenon. Am J Psychiatry. 1978;135:669–75. doi: 10.1176/ajp.135.6.669. [DOI] [PubMed] [Google Scholar]
- 66.Pappert EJ, Goetz CG, Niederman FG, et al. Sleep fragmentation, and altered dream phenomena in PD. Mov Disord. 1999;14:117–21. doi: 10.1002/1531-8257(199901)14:1<117::aid-mds1019>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 67.Comella CL, Tanner CM, Ristanovic RK. Polysomnographic sleep measures in Parkinson’s disease patients with treatment-induced hallucinations. Ann Neurol. 1993;34:710–4. doi: 10.1002/ana.410340514. [DOI] [PubMed] [Google Scholar]
- 68.Arnulf I, Knofal E, Merino-Andreu M, et al. Parkinson’s disease and sleepiness. An integral part of PD. Neurology. 2002;58:1019–24. doi: 10.1212/wnl.58.7.1019. [DOI] [PubMed] [Google Scholar]
- 69.Perry E, Walker M, Grace J, et al. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273–80. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- 70.Zoldan J, Friedberg G, Goldberg-Stern H, et al. Ondansetron for hallucinosis in advanced Parkinson's disease. Lancet. 1993;341(8844):562–3. doi: 10.1016/0140-6736(93)90327-d. [DOI] [PubMed] [Google Scholar]
- 71.Zoldan J, Friedberg G, Livneh M, et al. Psychosis in advanced Parkinson's disease: treatment with ondansetron, a 5-HT3 receptor antagonist. Neurology. 1995;45 (7):1305–8. doi: 10.1212/wnl.45.7.1305. [DOI] [PubMed] [Google Scholar]
- 72.Van der Mast RC, Fekkes D. Serotonin and amino acids: partners in delirium pathophysiology? Semin Clin Neuropsychiatry. 2000;5 (2):125–31. doi: 10.153/SCNP00500125. [DOI] [PubMed] [Google Scholar]
- 73.Birkmayer W, Riederer P. Responsibility of extrastriatal areas for the appearance of psychotic symptoms (clinical and biochemical human post-mortem findings) J Neural Transm. 1975;37 (2):175–82. doi: 10.1007/BF01663632. [DOI] [PubMed] [Google Scholar]
- 74.Nausied APA, Tanner CM, Klawans HL. Serotonergically active agents in levodopa-induced psychiatric toxicity reactions. Adv Neurol. 1983;37:23–32. [PubMed] [Google Scholar]
- 75.Melamed E, Zoldan J, Friedberg G, Ziv I, Weizmann A. Involvement of serotonin in clinical features of Parkinson's disease and complications of L-DOPA therapy. Adv Neurol. 1996;69:545–50. [PubMed] [Google Scholar]
- 76.Whitehouse PJ. Clinical and neurochemical consequences of neuronal loss in the nucleus basalis of Meynert in Parkinson's disease and Alzheimer's disease. Adv Neurol. 1987;45:393–7. [PubMed] [Google Scholar]
- 77.Kuhl DE, Minoshima S, Fessler JA, et al. In vivo mapping of cholinergic terminals in normal aging, Alzheimer's disease, and Parkinson's disease. Ann Neurol. 1996;40(3):399–410. doi: 10.1002/ana.410400309. [DOI] [PubMed] [Google Scholar]
- 78.Perry EK, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273–80. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- 79.Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002 Feb 1;125(2):391–403. doi: 10.1093/brain/awf033. [DOI] [PubMed] [Google Scholar]
- 80.Williams DR, Lees AJ. Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: a retrospective autopsy study. Lancet Neurol. 2005;4:605–10. doi: 10.1016/S1474-4422(05)70146-0. [DOI] [PubMed] [Google Scholar]
- 81.Papapetropoulos S, McCorquodale DS, Gonzalez J, et al. Cortical and amygdalar Lewy body burden in Parkinson’s disease patients with visual hallucinations. Parkinsonism Relat Disord. 2006 May;122(4):253–6. doi: 10.1016/j.parkreldis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 82.Paleacu D, Schechtman E, Inzelberg R. Association between family history of dementia and hallucinations in Parkinson disease. Neurology. 2005 May;64(10):1712–5. doi: 10.1212/01.WNL.0000161872.85903.8E. [DOI] [PubMed] [Google Scholar]
- 83.De la Fuente-Fernandez R, Nunez MA, Lopez E. The apolipoprotein E epsilon 4 allele increases the risk of drug-induced hallucinations in Parkinson’s disease. Clin Neuropharmacol. 1999 July-Aug;22(4):226–30. [PubMed] [Google Scholar]
- 84.Feldman B, Chapman J, Korczyn AD. Apolipoprotein epsilon4 advances appearance of psyhosis in patients with Parkinson’s disease. Acta Neurol Scand. 2006 Jan;113(1):14–7. doi: 10.1111/j.1600-0404.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- 85.Goetz CG, Burke PF, Leurgans S, Berry-Kravis E, Blasucci LM, Raman R, Zhou L. Genetic variation analysis in Parkinson disease patients with and without hallucinations: case-control study. Arch Neurol. 2001 Feb;58(2):209–13. doi: 10.1001/archneur.58.2.209. [DOI] [PubMed] [Google Scholar]
- 86.Camicioli R, Rajput A, Rajput M, Reece C, Payami H, Hao C, Rajput A. Apolipoprotein E epsilon4 and catechol-O-methyltransferase alleles in autopsy-proven Parkinson’s disease: relationship to dementia and hallucinations. Mov Disord. 2005 Aug;20(8):989–94. doi: 10.1002/mds.20481. [DOI] [PubMed] [Google Scholar]
- 87.Papapetropoulos S, Farrer MJ, Stone JT, et al. Phenotypic associations of tau and ApoE in Parkinson’s disease. Neuroscience Letters. 2007;414:141–4. doi: 10.1016/j.neulet.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 88.Wint DP, Okun MS, Fernandez HH. Psychosis in Parkinson’s disease. J Geriatr Psychiatry Neurol. 2004:127–36. doi: 10.1177/0891988704267457. [DOI] [PubMed] [Google Scholar]
- 89.Goetz CG, Vogel C, Tanner CM, et al. Early dopaminergic drug-induced hallucinations in parkinsonian patients. Neurology. 1998;51 (3):811–4. doi: 10.1212/wnl.51.3.811. [DOI] [PubMed] [Google Scholar]
- 90.Henderson MJ, Mellers JDC. Psychosis in Parkinson's disease: “between a rock and a hard place. Int Rev Psychiatry. 2000;12:319–34. [Google Scholar]
- 91.Fernandez HH, Friedman JF. The role of atypical antipsychotics in the treatment of movement disorders. CNS Drugs. 1999;11 (6):467–83. [Google Scholar]
- 92.Friedman JH, Fernandez HH. The non-motor problems of Parkinson’s disease. Neurology. 2000;6:18–27. [Google Scholar]
- 93.Noel JM. ASHP therapeutic position statement on the use of second-generation antipsychotic medications in the treatment of adults with psychotic disorders. AM J Health-Syst Pharm. 2007;64:863–76. doi: 10.2146/ajhp070051. [DOI] [PubMed] [Google Scholar]
- 94.Keltner NL, Johnson V. Aripiprazole: A third generation of antipsychotics begins? Perspectives in Psychiatric Care. 2002;38(4):157–9. doi: 10.1111/j.1744-6163.2002.tb01566.x. [DOI] [PubMed] [Google Scholar]
- 95.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294 (15):1934–43. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 96.Goetz CG, Leurgans S, Pappert EJ, Raman R, Stemer AB. Prospective longitudinal assessment of hallucinations in Parkinson’s disease. Neurology. 2001;57:2078–82. doi: 10.1212/wnl.57.11.2078. [DOI] [PubMed] [Google Scholar]
- 97.Fernandez HH, Trieschmann ME, Okun MS. Rebound psychosis: effect of discontinuation of antipsychotics in Parkinson’s disease. Movement Disorders. 2005;20 (1):104–15. doi: 10.1002/mds.20260. [DOI] [PubMed] [Google Scholar]
- 98.Frieling H, Hillemacher T, Ziegenbein M, Neundorfer B, Bleich S. Treating dopamimetic psychosis in Parkinson’s disease: structured review and meta-analysis. Eur Neuropsychopharmacol. 2007 Feb;17(3):165–71. doi: 10.1016/j.euroneuro.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 99.Wolters EC, Hurwitz TA, Mak E, et al. Clozapine in the treatment of parkinsonian patients with dopaminomimetic psychosis. Neurology. 1990;40 (5):832–4. doi: 10.1212/wnl.40.5.832. [DOI] [PubMed] [Google Scholar]
- 100.French Clozapine Parkinson Study Group. Clozapine in drug-induced psychosis in Parkinson's disease. Lancet. 1999;353 (9169):2041–2. [PubMed] [Google Scholar]
- 101.Parkinson Study Group. Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson's disease. N Engl J Med. 1999;340 (10):757–63. doi: 10.1056/NEJM199903113401003. [DOI] [PubMed] [Google Scholar]
- 102.Friedman JH, Factor SA. Atypical antipsychotics in the treatment of drug-induced psychosis in Parkinson’s disease. Mv Disord. 2000;151:201–11. doi: 10.1002/1531-8257(200003)15:2<201::aid-mds1001>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 103.Factor SA, Feustel PJ, Friedman, et al. Longitudinal outcome of Parkinson’s disease patients with psychosis. Neurology. 2003;60:1756–61. doi: 10.1212/01.wnl.0000068010.82167.cf. [DOI] [PubMed] [Google Scholar]
- 104.Fernandez HH, Friedman JH, Lansang MC, et al. Diabetes mellitus among parkinsonian patients treated chronically with clozapine. Parkinsonism Relat Disord. 2004;10 (7):439–41. doi: 10.1016/j.parkreldis.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 105.Klein C, Gordon J, Pollak L, et al. Clozapine in Parkinson's disease psychosis: 5-year follow-up review. Clin Neuropharmacol. 2003;26 (1):8–11. doi: 10.1097/00002826-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 106.Honigfeld G, Arellano F, Sethi J, et al. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(Suppl 3):3–7. [PubMed] [Google Scholar]
- 107.Pollak P, Tison F, Rascol O, et al. Clozapine in drug induced psychosis in Parkinson's disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75 (5):689–95. doi: 10.1136/jnnp.2003.029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Holt RI, Peveler RC, Byrne CD. Schizophrenia, the metabolic syndrome and diabetes. Diabet Med. 2004;21 (6):515–23. doi: 10.1111/j.1464-5491.2004.01199.x. [DOI] [PubMed] [Google Scholar]
- 109.Grant S, Fiton A. Risperidone: a review of its pharmacology and therapeutic potential in the treatment of schizophrenia. Drugs. 1994;43:456–60. doi: 10.2165/00003495-199448020-00009. [DOI] [PubMed] [Google Scholar]
- 110.Rosebush PI, Mazurek MF. Neurologic side effects in neuroleptic-naive patients treated with haloperidol or risperidone. Neurology. 1999;52 (4):782–5. doi: 10.1212/wnl.52.4.782. [DOI] [PubMed] [Google Scholar]
- 111.Rustembegovic A, Sofic E, Wichart I. Serum prolactin, leptin, lipids and lipoprotein levels during antipsychotic treatment in Parkinson’s disease and related psychosis. Med Arh. 2006;60 (4):211–2. [PubMed] [Google Scholar]
- 112.Ford B, Lynch T, Greene P. Risperidone in Parkinson's disease. Lancet. 1994;344 (8923):681. doi: 10.1016/s0140-6736(94)92114-8. [DOI] [PubMed] [Google Scholar]
- 113.McKeith IG, Ballard CG, Harrison RW. Neuroleptic sensitivity to risperidone in Lewy body dementia. Lancet. 1995;346 (8976):699. doi: 10.1016/s0140-6736(95)92307-1. [DOI] [PubMed] [Google Scholar]
- 114.Allen RL, Walker Z, D'Ath PJ, et al. Risperidone for psychotic and behavioural symptoms in Lewy body dementia. Lancet. 1995;346 (8968):185. doi: 10.1016/s0140-6736(95)91245-2. [DOI] [PubMed] [Google Scholar]
- 115.Meco G, Alessandria A, Bonifati V, et al. Risperidone for hallucinations in levodopa-treated Parkinson's disease patients. Lancet. 1994;343 (8909):1370–1. doi: 10.1016/s0140-6736(94)92511-9. [DOI] [PubMed] [Google Scholar]
- 116.Workman RH, Jr, Orengo CA, Bakey AA, et al. The use of risperidone for psychosis and agitation in demented patients with Parkinson's disease. J Neuropsychiatry Clin Neurosci. 1997;9 (4):594–7. doi: 10.1176/jnp.9.4.594. [DOI] [PubMed] [Google Scholar]
- 117.Rich SS, Friedman JH, Ott BR. Risperidone versus clozapine in the treatment of psychosis in six patients with Parkinson's disease and other akinetic-rigid syndromes. J Clin Psychiatry. 1995;56 (12):556–9. [PubMed] [Google Scholar]
- 118.Mohr E, Mendis T, Hildebrand K, et al. Risperidone in the treatment of dopamine-induced psychosis in Parkinson's disease: an open pilot trial. Mov Disord. 2000;15 (6):1230–7. doi: 10.1002/1531-8257(200011)15:6<1230::aid-mds1026>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 119.Meco G, Alessandri A, Giustini P, et al. Risperidone in levodopa-induced psychosis in advanced Parkinson's disease: an open-label, long-term study. Mov Disord. 1997;12 (4):610–2. doi: 10.1002/mds.870120423. [DOI] [PubMed] [Google Scholar]
- 120.Ford B, Lynch T, Greene P. Risperidone in Parkinson's disease. Lancet. 1994;344 (8923):681. doi: 10.1016/s0140-6736(94)92114-8. [DOI] [PubMed] [Google Scholar]
- 121.Factor SA, Molho ES, Friedman JH. Risperidone and Parkinson’s disease [letter] Mov Disord. 2001;12:364–9. doi: 10.1002/mds.1258. [DOI] [PubMed] [Google Scholar]
- 122.Ellis T, Cudkowicz ME, Sexton PM, et al. Clozapine and risperidone treatment of psychosis in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 2000;12 (3):364–9. doi: 10.1176/jnp.12.3.364. [DOI] [PubMed] [Google Scholar]
- 123.Moore NA, Tye NC, Axton MS, et al. The behavioral pharmacology of olanzapine, a novel "atypical" antipsychotic agent. J Pharmacol Exp Ther. 1992;262 (2):545–51. [PubMed] [Google Scholar]
- 124.Fernandez HH, Trieschmann ME, Friedman JH. The treatment of psychosis in Parkinson’s disease: safety considerations. Drug Saf. 2003;26:643–59. doi: 10.2165/00002018-200326090-00004. [DOI] [PubMed] [Google Scholar]
- 125.Goetz CG, Blasucci LM, Leurgans S, et al. Olanzapine and clozapine: comparative effects on motor function in hallucinating PD patients. Neurology. 2000;55 (6):789–94. doi: 10.1212/wnl.55.6.789. [DOI] [PubMed] [Google Scholar]
- 126.Breier A, Sutton VK, Feldman PD, et al. Olanzapine in the treatment of dopamimetic-induced psychosis in patients with Parkinson's disease. Biol Psychiatry. 2002;52 (5):438–45. doi: 10.1016/s0006-3223(02)01392-6. [DOI] [PubMed] [Google Scholar]
- 127.Ondo WG, Levy JK, Vuong KD, et al. Olanzapine treatment for dopaminergic-induced hallucinations. Mov Disord. 2002;17 (5):1031–5. doi: 10.1002/mds.10217. [DOI] [PubMed] [Google Scholar]
- 128.Reddy S, Factor SA, Molho ES, et al. The effect of quetiapine on psychosis and motor function in parkinsonian patients with and without dementia. Mov Disord. 2002;17 (4):676–81. doi: 10.1002/mds.10176. [DOI] [PubMed] [Google Scholar]
- 129.Fernandez HH, Trieschmann ME, Burke MA, et al. Long-term outcome of quetiapine use for psychosis among Parkinsonian patients. Mov Disord. 2003;18 (5):510–4. doi: 10.1002/mds.10374. [DOI] [PubMed] [Google Scholar]
- 130.Ondo WG, Tintner R, Voung KD, et al. Double-blind, placebo-controlled, unforced titration parallel trial of quetiapine for dopaminergic-induced hallucinations in Parkinson's disease. Mov Disord. 2005;20 (8):958–63. doi: 10.1002/mds.20474. [DOI] [PubMed] [Google Scholar]
- 131.Rabey JM, Prokhorov T, Miniovich A, et al. The effect of quetiapine in Parkinson's disease (PD) psychotic patients: A double-blind labeled study of three months duration. Mov Disord. 2005;20 (Suppl 10):S46. doi: 10.1002/mds.21116. [DOI] [PubMed] [Google Scholar]
- 132.Morgante L, Epifanio A, Spina E, et al. Quetiapine and clozapine in parkinsonian patients with dopaminergic psychosis. Clin Neuropharmacol. 2004;27 (4):153–6. doi: 10.1097/01.wnf.0000136891.17006.ec. [DOI] [PubMed] [Google Scholar]
- 133.Klein C, Prokhorov T, Miniovich A, Dobronevsky E, Rabey JM. Long-term follow-up (24 months) of quetiapine treatment in drug-induced Parkinson disease psychosis. Clin Neuropharmacol. 2006 Jul-Aug;29(4):215–9. doi: 10.1097/01.WNF.0000228176.98582.93. [DOI] [PubMed] [Google Scholar]
- 134.Juri C, Chana P, Tapia J, Kuntsmann C, Parrao T. Quetiapine for insomnia in Parkinson disease: results from an open-label trial. Clin Neuropharmacol. 2005;28:185–7. doi: 10.1097/01.wnf.0000174932.82134.e2. [DOI] [PubMed] [Google Scholar]
- 135.Fernandez HH, Trieschmann ME, Friedman JH. The treatment of psychosis in Parkinson’s disease: safety considerations. Drug Saf. 2003;26:643–59. doi: 10.2165/00002018-200326090-00004. [DOI] [PubMed] [Google Scholar]
- 136.Stahl SM, Shayegan DK. The psychopharmacology of ziprasidone: receptor-binding properties and real-world psychiatric practice. J Clin Psychiatry. 2003;64(Suppl 19):6–12. [PubMed] [Google Scholar]
- 137.Glassman AH. Schizophrenia, antipsychotic drugs, and cardiovascular disease. J Clin Psychiatry. 2005;66(Suppl 6):5–10. [PubMed] [Google Scholar]
- 138.Connemann BJ, Schonfeldt-Lecuona C. Ziprasidone in Parkinson’s disease psychosis. Can J Psychiatry. 2004;49 (1):73. doi: 10.1177/070674370404900119. [DOI] [PubMed] [Google Scholar]
- 139.Shiah I-S, Lin C-L, Mao W-C, Luu S-U. Ziprasidone in the treatment of Parkinson’s disease psychosis. Eur Psychiatry. 2006;21:578–9. doi: 10.1016/j.eurpsy.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 140.Gomez-Esteban JC, Zarranz JJ, Velasco F, et al. Use of ziprasidone in parkinsonian patients with psychosis. Clin Neuropharmacol. 2005;28 (3):111–4. doi: 10.1097/01.wnf.0000164297.91643.ff. [DOI] [PubMed] [Google Scholar]
- 141.Oechsner M, Korchounov A. Parenteral ziprasidone: a new atypical neuroleptic for emergency treatment of psychosis in Parkinson's disease? Hum Psychopharmacol. 2005;20 (3):203–5. doi: 10.1002/hup.682. [DOI] [PubMed] [Google Scholar]
- 142.Weiden PJ, Iqbal N, Mendelowitz AJ, et al. Best clinical practice with ziprasidone: update after one year of experience. J Psychiatr Pract. 2002;8 (2):81–97. doi: 10.1097/00131746-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 143.McGavin JK, Goa KL. Aripiprazole. CNS Drugs. 2002;16 (11):779–86. doi: 10.2165/00023210-200216110-00008. [DOI] [PubMed] [Google Scholar]
- 144.Stahl SM. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 1, “Goldilocks” actions at dopamine receptors. J Clin Psychiatry. 2001 Nov;62(11):841–2. doi: 10.4088/jcp.v62n1101. [DOI] [PubMed] [Google Scholar]
- 145.Stahl SM. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 2: illustrating their mechanism of action. J Clin Psychiatry. 2001 Dec;62(12):923–4. doi: 10.4088/jcp.v62n1201. [DOI] [PubMed] [Google Scholar]
- 146.Schonfeldt-Lecuona C, Connemann BJ. Aripiprazole and Parkinson’s disease psychosis. Am J Psychiatry. 2004;161:373–4. doi: 10.1176/appi.ajp.161.2.373-a. [DOI] [PubMed] [Google Scholar]
- 147.Wickremaratchi M, Morris HR. Aripiprazole associated with severe exacerbation of Parkinson’s disease. Movement Disorders. 2006;21 (9):1538–9. doi: 10.1002/mds.21025. [DOI] [PubMed] [Google Scholar]
- 148.Lopez-Meza E, Ruiz-Chow A, Ramirez-Bermudez J. Aripiprazole in psychosis associated with Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2005;p17 (3):421–2. doi: 10.1176/jnp.17.3.421. [DOI] [PubMed] [Google Scholar]
- 149.Fernandez HH, Trieschmann ME, Friedman JH. Aripiprazole for drug-induced psychosis in Parkinson disease: preliminary experience. Clin Neuropharmacol. 2004;27:4–5. doi: 10.1097/00002826-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 150.Friedman JH, Berman RM, Goetz CG, et al. Open-label flexible-dose pilot study to evaluate the safety and tolerability of aripiprazole in patients with psychosis associated with Parkinson’s disease. Movement Disorders. 2006;21 (12):2078–81. doi: 10.1002/mds.21091. [DOI] [PubMed] [Google Scholar]
- 151.Zoldan J, Friedberg G, Goldberg-Stern H, et al. Ondansetron for hallucinosis in advanced Parkinson's disease. Lancet. 1993;341 (8844):562–3. doi: 10.1016/0140-6736(93)90327-d. [DOI] [PubMed] [Google Scholar]
- 152.Zoldan J, Friedberg G, Livneh M, et al. Psychosis in advanced Parkinson's disease: treatment with ondansetron, a 5-HT3 receptor antagonist. Neurology. 1995;45 (7):1305–8. doi: 10.1212/wnl.45.7.1305. [DOI] [PubMed] [Google Scholar]
- 153.Eichhorn TE, Brunt E, Oertel WH. Ondansetron treatment of L-dopa-induced psychosis. Neurology. 1996;47 (6):1608–9. doi: 10.1212/wnl.47.6.1608-b. [DOI] [PubMed] [Google Scholar]
- 154.Hutchinson M, Fazzini E. Cholinesterase inhibition in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1996;61 (3):324–5. doi: 10.1136/jnnp.61.3.324-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ott B, Lannon M. Exacerbation of parkinsonism by tacrine. Clin Neuropharmacol. 1992;15 (4):322–5. doi: 10.1097/00002826-199208000-00008. [DOI] [PubMed] [Google Scholar]
- 156.Fabbrini G, Barbanti P, Aurilia C, et al. Donepezil in the treatment of hallucinations and delusions in Parkinson's disease. Neurol Sci. 2002;23 (1):41–3. doi: 10.1007/s100720200022. [DOI] [PubMed] [Google Scholar]
- 157.Bergman J, Lerner V. Successful use of donepezil for the treatment of psychotic symptoms in patients with Parkinson's disease. Clin Neuropharmacol. 2002;25 (2):107–10. doi: 10.1097/00002826-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 158.Kurita A, Ochiai Y, Kono Y, et al. The beneficial effect of donepezil on visual hallucinations in three patients with Parkinson's disease. J Geriatr Psychiatry Neurol. 2003;16 (3):184–8. doi: 10.1177/0891988703256054. [DOI] [PubMed] [Google Scholar]
- 159.Ravina B, Putt M, Siderowf A, et al. Donepezil for dementia in Parkinson's disease: a randomised, double blind, placebo controlled, crossover study. J Neurol Neurosurg Psychiatry. 2005;76 (7):934–9. doi: 10.1136/jnnp.2004.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aarsland D, Laake K, Larsen J, et al. Donepezil for cognitive impairment in Parkinson’s disease: a randomized controlled study. J Neurol Neurosurg Psychiatry. 2002;72:708–12. doi: 10.1136/jnnp.72.6.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aarsland D, Hutchinson M, Larsen JP. Cognitive, psychiatric and motor response to galantamine in Parkinson’s disease with dementia. Int J Geriatr Psychiatry. 2003;18:937–41. doi: 10.1002/gps.949. [DOI] [PubMed] [Google Scholar]
- 162.Reading PJ, Luce AK, McKeith IG. Rivastigmine in the treatment of parkinsonian psychosis and cognitive impairment: preliminary findings from an open trial. Mov Disord. 2001;16:1171–4. doi: 10.1002/mds.1204. [DOI] [PubMed] [Google Scholar]
- 163.Bullock R, Cameron A. Rivastigmine for the treatment of dementia and visual hallucinations associated with Parkinson’s disease: a case series. Curr Med Res Opin. 2002;18 (5):258–64. doi: 10.1185/030079902125000813. [DOI] [PubMed] [Google Scholar]
- 164.Burn D, Emre M, McKeith I, De Deyn PP, Aarsland D, Hsu C, Lane R. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21 (11):1899–907. doi: 10.1002/mds.21077. [DOI] [PubMed] [Google Scholar]
- 165.Factor SA, Molho ES, Brown DL. Combined clozapine and electroconvulsive therapy for the treatment of drug-induced psychosis in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1995;7 (3):304–7. doi: 10.1176/jnp.7.3.304. [DOI] [PubMed] [Google Scholar]
- 166.Hurwitz TA, Calne DB, Waterman K. Treatment of dopamimetic psychosis in Parkinson’s disease with electroconvulsive therapy. Can J Neurol Sci. 1988;15 (1):32–4. doi: 10.1017/s0317167100027141. [DOI] [PubMed] [Google Scholar]
- 167.Ozer F, Meral H, Aydin B, et al. Electroconvulsive therapy in drug-induced psychiatric states and neuroleptic malignant syndrome. J ECT. 2005 June;21(2):125–7. doi: 10.1097/01.yct.0000159325.08303.45. [DOI] [PubMed] [Google Scholar]
- 168.Lauterbach EC. Dopaminergic hallucinosis with fluoxetine in Parkinson's disease. Am J Psychiatry. 1993;150 (11):1750. doi: 10.1176/ajp.150.11.1750a. [DOI] [PubMed] [Google Scholar]
- 169.Normann C, Hesslinger B, Frauenknecht S, Berger M, Walden J. Psychosis during chronic levodopa therapy triggered by the new antidepressive drug mirtazapine. Pharmacopsychiatry. 1997;30 (6):263–5. doi: 10.1055/s-2007-979504. [DOI] [PubMed] [Google Scholar]
- 170.Meco G, Bernardi S. Antidepressant use in treatment of psychosis with comorbid depression in Parkinson’s disease. Prof Neuropsychopharmacol Biol Psychiatry. 2007 Jan 30;31(1):311–3. doi: 10.1016/j.pnpbp.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 171.Voon V, Lang AE. Antidepressants in the treatment of psychosis with comorbid depression in Parkinson disease. Clin Neuropharmacol. 2004;27 (2):90–2. doi: 10.1097/00002826-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 172.Diederich NJ, Pieri V, Goetz CG. Coping strategies for visual hallucinations in Parkinson’s disease. Movement Disorders. 2003;18 (7):831–8. doi: 10.1002/mds.10450. [DOI] [PubMed] [Google Scholar]
- 173.McGorry PD. Psychoeducation in first-episode psychosis: a therapeutic process. Psychiatry. 1995 Nov;58(4):313–28. doi: 10.1080/00332747.1995.11024736. [DOI] [PubMed] [Google Scholar]
- 174.Tarrier N, Wykes T. Is there evidence that cognitive behaviour therapy is an effective treatment for schizophrenia? A cautious or cautionary tale? Behav Res Ther. 2004 Dec;42(12):1377–407. doi: 10.1016/j.brat.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 175.Jenner JA, van de Willige G, Wiersma D. Effectiveness of cognitive therapy with coping training for persistent auditory hallucinations: a retrospective study of atttenders of a psychiatric out-patient department. Acta Psychiatr Scand. 1998 Nov;98(5):384–9. doi: 10.1111/j.1600-0447.1998.tb10103.x. [DOI] [PubMed] [Google Scholar]