Summary
Background
The objective of ECOG 1503 was to determine the response rate of this combination in the second-line treatment of advanced NSCLC.
Methods
Triapine 105 mg/m2 IV on days 1, 8, and 15, and gemcitabine 1,000 mg/m2 on days 1, 8, and 15, of a 28 day cycle.
Results
Eighteen patients enrolled. Three patients were not eligible due to protocol violations. No objective antitumor responses were seen. Three patients (20%) experienced stable disease (90% CI 5.7–44%). Median overall survival: 5.4 months (95% CI 4.2–11.6 months); median time to progression: 1.8 months (95% CI 1.7–3.5 months). Five patients developed acute infusion reactions to Triapine® related to elevated methemoglobinemia. Patients with MDR1 variant genotypes of C3435T experienced superior overall survival compared to non-variants (13.3 vs. 4.3 months, respectively, p=0.023).
Conclusion
This regimen did not demonstrate activity in relapsed NSCLC. Prolonged survival seen with MDR1 variant genotypes is hypothesis-generating.
Keywords: Non-small cell lung cancer, Combination chemotherapy, Ribonucleotide reductase, Single nucleotide polymorphism, ATP binding cassette transporter
Introduction
Investigation of more effective agents and combinations for the treatment of non-small cell lung cancer (NSCLC), the leading cause of cancer-related mortality worldwide, remains paramount. Gemcitabine has well-established anti-tumor activity in the first and second line settings, as well as possible utility as maintenance therapy immediately following completion of first line treatment [1–3]. Its low toxicity profile makes it an ideal candidate for combination therapy.
Triapine® (3-aminopyridine-2-carboxaldehyde thiosemicarbazone, 3-AP, NSC# 663249, Vion Pharmaceuticals, Inc., New Haven, CT) is a potent inhibitor of the M2 subunit of ribonucleotide reductase (RRM2), an enzyme that catalyzes the conversion of ribonucleotide diphosphates to their deoxyribonucleotide counterparts and is, hence, critical for DNA synthesis [4, 5]. Triapine® is an iron chelator that inhibits the enzymatic activity at the tyrosyl radical of the M2 subunit, and has demonstrated preclinical antitumor activity in models of NSCLC [5, 6]. The sequential exposure of NSCLC cell lines to Triapine®, followed by gemcitabine, an inhibitor of the M1 subunit of ribonucleotide reductase (RRM1), has been examined [7, 8]. Pretreatment with Triapine® resulted in increased cellular uptake of gemcitabine, synergistic growth inhibition, increased gemcitabine triphosphate pools, and enhanced incorporation of gemcitabine into DNA [7, 8]. A combination phase I trial of these agents by Yen et al. identified a recommended phase II dose of 105 mg/m2 of Triapine® followed 2 to 4 h later by gemcitabine at 1,000 mg/m2, on days 1, 8, and 15 of a 28 day cycle [9]. One complete response (unknown primary) and two partial responses (bronchioloalveolar carcinoma and esophageal carcinoma) were observed. Pharmacokinetic studies revealed no drug–drug interactions. Treatment-related toxicities included acute reversible hypoxia in three patients felt to be secondary to methemoglobinemia and EKG changes, such as non-specific ST-T wave changes and mild QTc prolongation. In its function as an iron chelator, Triapine® may be redox active, and thus, may secondarily catalyze the formation of methemoglobin. Therefore, Yen et al. recommended that patients with diminished pulmonary function or a history of G6PD deficiency be excluded from treatment with this combination due to their impaired ability to tolerate Triapine®-related transient hypoxia caused by methemoglobinemia [9].
The ATP-binding cassette transporter superfamily is critical in effecting multidrug resistance by promoting efflux of chemotherapeutics out of tumor cells [10, 11]. Two members of this superfamily include P-glycoprotein (P-gp), a product of the multidrug resistance 1 (MDR1) gene, and a 190 kDa membrane transport protein termed multidrug resistance protein (MRP1). Triapine® appears to be a substrate of P-gp and MRP1 [12]. Single nucleotide polymorphism (SNP) germline variations of MDR1 and MRP1 may prove clinically relevant, since certain variations may be associated with reductions in P-gp function [13, 14–18].
We sought to describe the clinical efficacy and tolerability of combination therapy with Triapine® and gemcitabine in the second line setting of advanced NSCLC. In addition, we hypothesized that polymorphic variations of MDR1 and MRP1 may impact the clinical efficacy and toxicity associated with Triapine®.
Patients and methods
Patient selection
Patients at least 18 years of age with histologically confirmed advanced (stage IIIB with pleural or pericardial effusion, stage IV, or recurrent) NSCLC whose disease had progressed during or after treatment with no more than one prior cytotoxic combination chemotherapy regimen and who gave informed consent according to institutional and FDA guidelines were eligible for this study provided that the following criteria were met: Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1; brain metastases, if present, must have been clinically stable after treatment with surgery and/or radiotherapy; adequate bone marrow, liver and renal function; life expectancy of at least 3 months; measureable disease per RECIST criteria; no prior treatment with gemcitabine, no treatment with chemotherapy or radio-therapy within 3 weeks of enrollment and must have recovered from the adverse effects of prior treatment to baseline or grade 1 or less; no other active malignancy; absence of HIV positivity receiving combination antiretroviral therapy; and no uncontrolled intercurrent illness, including dementia, active psychosis, pulmonary disease requiring supplemental oxygen, history of myocardial infarction within the prior 6 months or active cardiac disease. Further, nursing or pregnant women, persons with a history of G6PD deficiency, persons with a history of allergic reactions attributed to compounds of similar chemical or biologic composition to Triapine® or gemcitabine, or persons who used any investigational agent in the month prior to enrollment were excluded. This protocol was approved through institutional ethics review boards of participating ECOG sites.
Treatment plan
Triapine® was supplied by Vion Pharmaceuticals, Inc. (50 mg/vial; 5 mg/ml) and distributed by the Cancer Therapy Evaluation Program, the Division of Cancer Treatment and Diagnosis, National Cancer Institute. Triapine® was further diluted in D5W (final concentration between 0.01–2 mg/ml) in non-polyvinyl chloride (non-PVC) bags and administered as a 2 h infusion through non-PVC tubing.
Treatment consisted of Triapine® 105 mg/m2 IV over 2 h on days 1, 8, and 15 and gemcitabine 1,000 mg/m2 over 30 min on days 1, 8, and 15, of a 28 day cycle. Gemcitabine was administered between 4 and 8 h after completion of the Triapine® infusion. Treatment was given only if the ANC≥1,500/μL and platelets ≥100,000/μL on the first day of a new cycle. Dose modifications and delays were specified in the protocol. Treatment was continued for a maximum of six cycles until disease progression, unacceptable toxicity, or withdrawal of consent. Patients who required more than two dose reductions due to toxicity were removed from the study. All toxicities (except alopecia) must have resolved to grade 1 or less prior to the start of the next cycle. All dose reductions were permanent. Patients were to be followed until disease progression or for a maximum of 2 years from the date of registration.
Triapine® iron complexes are believed to be redox active, and thus, may secondarily catalyze hemoglobin oxidation to methemoglobin. Clinical monitoring for methemoglobinemia was performed every 30 min during the Triapine® infusion on day 1 of cycle 1, and every 60 min for up to 4 h after completion of the infusion. Isolated, mild hypoxemia was treated with supplemental oxygen. The Triapine® infusion was stopped if moderate or severe dyspnea or hypotension (SBP <85 mm Hg) developed. Asymptomatic methemoglobinemia (<5%) did not prompt dose reduction. Patients were removed from protocol treatment with persistent symptoms of hypoxia, dyspnea, or hypotension, or persistent, elevated methemoglobinemia (>15%).
Disease assessment
The objective antitumor response rate (RR) was determined using computed tomography imaging at baseline and after every other cycle of treatment. Bone scans and brain imaging were performed only if clinically indicated. Additional baseline assessment included a history and physical, complete blood count, comprehensive chemistry panel, serum iron studies, ECOG PS, an electrocardiogram, a methemoglobin level, and screening of high risk groups (patients of African, Asian, or Mediterranean origin/ancestry) for G6PD deficiency. In addition, a complete blood count with differential was obtained on days 8 and 15 of each cycle. On day 1 of cycle 1, a methemoglobin level was obtained prior to treatment, at the end of the Triapine® infusion, and then just prior to the gemcitabine administration.
MDR1 genotyping
The methods of assaying peripheral blood at baseline for the common MDR1 and MRP1 polymorphisms (C1236T, G2677T, and C3435T) have been described [14].
Statistical considerations
The primary endpoint of this study was the best overall confirmed objective RR, defined as the percentage of patients experiencing complete responses (CRs) or partial responses (PRs). Secondary objectives included the rate of stable disease (SD), time to disease progression (TTP), duration of response, overall survival (OS), and safety and tolerability of this combination. All treated patients were evaluable for toxicity assessments. Eligible patients must have received treatment to have been evaluable for response and survival.
In this phase II trial, a true objective RR of at least 25% was considered evidence for further exploration of this regimen. A true RR≤5% was considered evidence of minimal activity not worthy of further study. A two-stage design was employed to terminate the study early if the combination appeared to elicit minimal response. The first stage planned to accrue 20 patients, with 18 patients expected to be eligible. If at least two responses were observed among the initial 18 eligible patients, 28 additional patients were to be entered assuming 25 would be eligible. This regimen would be considered worthy of further study if at least seven total responses were observed among the 43 eligible patients. If the RR of Triapine® and gemcitabine was at least 25%, this design yielded a 92% chance of recommending the regimen for further study. For a response rate of less than 5%, the probability of accepting the regimen was less than 0.005.
Response was evaluated using RECIST criteria. Patients who were unevaluable for response were included in the denominator when computing the rates of RR and SD. Duration of response was defined as the time from the onset of response (CR or PR, whichever status was recorded first) to first documentation of disease progression. Patients with responses but without documented disease progression were censored at the time of last disease evaluation. Patients without responses were excluded from this analysis.
Overall survival was defined as the time from registration to death from any cause. Patients who were alive were censored at the date last known alive. Time to progression was defined as the time from registration to first documentation of disease progression. Patients without documented progression were censored at the time of last known free of progression. If such a date was not available, patients were censored at the time of registration. Toxicities were graded per the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Exact binomial 90% confidence intervals (CIs) were computed for the rates of RR (CR + PR) and SD. The Kaplan and Meier method was used for event–time distributions. Descriptive statistics were used to characterize patient demographics, disease characteristics, and adverse events. Fisher’s exact test was used to evaluate associations between genotype and toxicity for MDR1 and MRP1 polymorphisms. The log-rank test was employed to examine the differences in OS/TTP between genotypes for various MDR1 and MRP1 polymorphisms. All p-values are two-sided. A level of 5% was considered statistically significant.
Results
Patient characteristics and treatment
A total of 18 patients enrolled between July 2004 and January 2005. The study was closed at the first interim analyses in August 2005 due to a lack of efficacy. Table 1 displays the patient demographics. The median age was fairly young, at 61.1 years (range 53.3–79.6). Eighty percent of the patients were male. The median time from completing prior chemotherapy was 4.1 months (range 0.8–14.4 months). Two-thirds of the patients had received prior radiation therapy. All patients received protocol treatment. Nevertheless, among those enrolled, three were ineligible (two patients had baseline scans done prior to the study-defined interval, and the third patient had been treated with more than one prior chemotherapy regimen). Consequently, all efficacy analyses were based on 15 eligible, treated patients. Toxicity analyses included all 18 treated patients. The median number of cycles administered was two. No patients completed the maximum six cycles of treatment. Six patients discontinued treatment due to disease progression, six due to toxicity, two due to patient refusal, and one due to non-compliance.
Table 1.
Patient characteristics
| Number | Percent | |
|---|---|---|
| Sex | ||
| Male | 12 | 80.0 |
| Female | 3 | 20.0 |
| Race | ||
| White | 13 | 86.7 |
| Black | 2 | 13.3 |
| PS | ||
| 0 | 3 | 20.0 |
| 1 | 12 | 80.0 |
| Weight loss in previous 6 months | ||
| <5% | 1 | 6.7 |
| 5 to <10% | 4 | 26.7 |
| >=20% | 1 | 6.7 |
| Unknown | 1 | 6.7 |
| Disease stage at entry | ||
| IIIB (not recurrent) | 1 | 6.7 |
| IV (not recurrent) | 10 | 66.7 |
| Recurrent | 4 | 26.7 |
| Histology | ||
| Squamous carcinoma | 5 | 33.3 |
| Adenocarcinoma | 6 | 40.0 |
| Non-small cell lung cancer, NOS | 4 | 26.7 |
| Prior chemotherapy | 15 | 100.0 |
| Prior radiation therapy | 10 | 66.7 |
| Prior surgery | 4 | 26.7 |
| Age (median and range) | 61.1 | 53.3–79.6 |
Toxicity
Table 2 lists toxicities of all grades at least possibly related to study treatment that affected at least two patients. No patients died on study. The most common grade 3/4 toxicities were leucopenia (grade 3–8 patients; grade 4–1 patient), neutropenia (grade 3–8 patients; grade 4–2 patients), acute, reversible hypoxia (grade 3–4 patients), dyspnea (grade 3–2 patients; grade 4–1 patient), fatigue (grade 3–3 patients), and vomiting (grade 3–2 patients). There were no episodes of febrile neutropenia. Five patients experienced acute reactions to Triapine® during the initial infusion. These reactions were characterized by reversible elevations in methemoglobin, transient chest tightness, and hypoxia, reaching grade 3 in three of the five patients. Two of these three patients were removed from protocol treatment. In total, six patients discontinued treatment due to adverse events experienced while on study. These events included leukopenia, neutropenia, and hypoxia stemming from acute reactions to Triapine® infusions.
Table 2.
Treatment-related toxicity
| Toxicity Type | Treatment Arm A (n=18) |
|||
|---|---|---|---|---|
| Grade | ||||
| 1, 2 | 3 | 4 | 5 | |
| (n) | (n) | (n) | (n) | |
| Hemoglobin | 16 | – | – | – |
| Hemolysis | 3 | – | – | – |
| Leukocytes | 4 | 8 | 1 | – |
| Neutrophils | 3 | 8 | 2 | – |
| Platelets | 10 | 1 | 1 | – |
| Hematologic-other | 1 | 1 | – | – |
| Hypotension | 3 | – | – | – |
| Fatigue | 12 | 3 | – | – |
| Sweating | 2 | – | – | – |
| Weight loss | 2 | – | – | – |
| Alopecia | 5 | – | – | – |
| Injection site reaction | 2 | – | – | – |
| Rash/desquamation | 3 | – | – | – |
| Anorexia | 8 | 1 | – | – |
| Constipation | 9 | – | – | – |
| Diarrhea w/o prior colostomy | 4 | – | – | – |
| Muco/stomatitis by exam, oral cavity | 2 | – | – | – |
| Nausea | 11 | 1 | – | – |
| Vomiting | 5 | 2 | – | – |
| Hypoalbuminemia | 8 | – | – | – |
| Alkaline phosphatase | 5 | – | – | – |
| ALT, SGPT | 3 | – | – | – |
| AST, SGOT | 3 | – | – | – |
| Hypocalcemia | 2 | – | – | – |
| Creatinine | 2 | – | – | – |
| Hyperglycemia | 10 | – | – | – |
| Hypokalemia | 2 | – | – | – |
| Hyponatremia | 3 | – | – | – |
| Neuropathy-sensory | 5 | – | – | – |
| Dyspnea | 7 | 2 | 1 | – |
| Hypoxia | 2 | 4 | – | – |
Treatment efficacy
No patient experienced either a CR or a PR. Eight patients (53%) experienced disease progression, while three patients (20%; 95% CI 5.7–44.0%) had SD. Four patients (27%) were unevaluable for response: one withdrew from study treatment prior to the first disease assessment, two patients had disease assessment scans performed too early, and one patient had inadequate follow-up measurements on repeat imaging and then died.
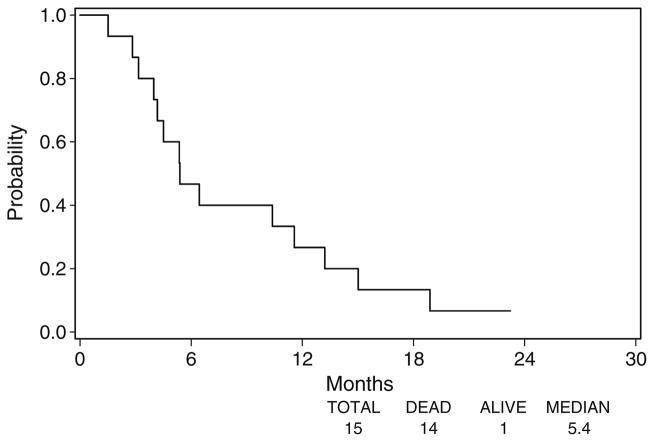
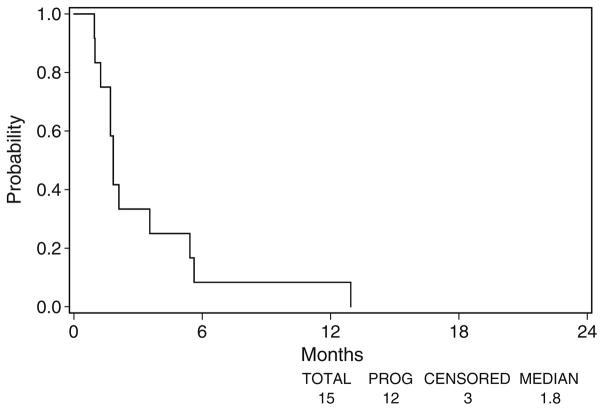
Fourteen of the 15 eligible patients have died. Twelve patients eventually experienced disease progression. The three patients without disease progression were missing disease assessment data at their last disease assessment, and were censored at the time of registration. Figure 1 displays the median OS of 5.4 months (95% CI 4.2–11.6 months) and Fig. 2 displays the median TTP of 1.8 months (95% CI 1.7–3.5 months).
Fig. 1.
Overall survival
Fig. 2.
Time to progression
MDR1 and MRP1 genotyping
Genotyping for MDR1 polymorphisms C1236T, G2677T, C3435T, and MRP1 G>C was performed on 14 of 15 eligible patients. MDR1 and MRP1 polymorphism frequencies were consistent with Hardy-Weinberg predictions: C1236T (CC: 0.14; CT: 0.64; TT: 0.22), G2677T (GG: 0.14; GT: 0.64; TT: 0.22), C3435T (CC: 0.14; CT: 0.43; TT: 0.43), MRP > G/C (GG: 0.64; GC: 0.22; CC: 0.14).
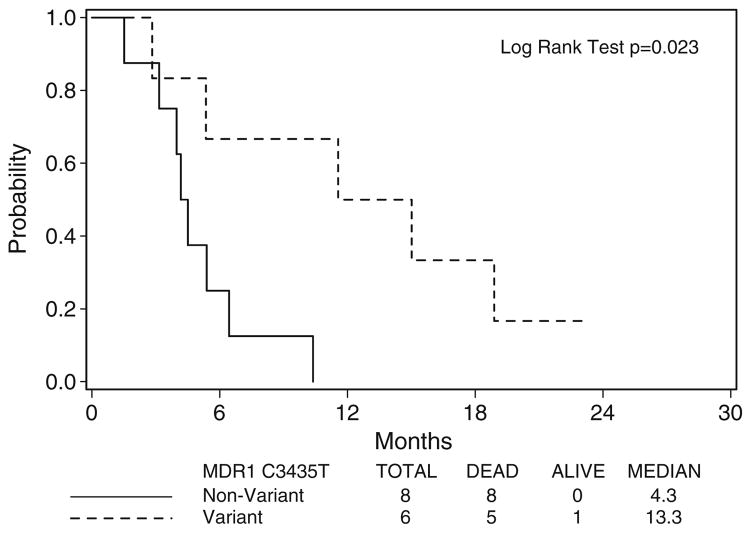
For the analyses of survival and toxicity, genotypes were grouped as variant (homozygous) versus non-variant (heterozygous and wild-type). Patients with variant genotypes of C3435T experienced statistically superior OS compared to non-variants (median OS 13.3 vs. 4.3 months, respectively, p=0.023, as seen in Fig. 3). Fisher’s exact test indicated that non-variant MRP1 genotypes (67%) developed grade ≥3 gastrointestinal toxicity (anorexia and vomiting) more frequently than variants (p=0.033). No statistically significant difference in TTP or any other incidence of toxicity, including acute reactions to Triapine® infusions, was observed between genotypes for any polymorphism. Due to the small number of patients who underwent MDR1 and MRP1 genotyping (N=14), these analyses were exploratory in nature. Further, no statistical adjustment for the multiple comparisons was performed.
Fig. 3.
Overall survival by MDR1 C3435T
Discussion
Eastern Cooperative Oncology Group trial 1503 was closed at its first interim analyses due to lack of efficacy. This lack of efficacy was also seen in Ma, et al., a multicenter trial of this regimen in this same population performed in Hong Kong and Singapore [19]. Time to progression in Ma, et al. was slightly longer, at 3.3 months.
Further, our regimen proved moderately toxic, with 6 of 15 patients discontinuing treatment due to toxicity. Neutropenia appeared more commonly in our study, compared to either the phase I study of this combination or Ma, et al., possibly related to prior myelosuppresive radiation therapy that two-thirds of our patients had received [9, 19]. Further, five patients (28%) developed acute, symptomatic infusion reactions to Triapine®, consisting of reversible elevated methemoglobinemia, chest tightness, and hypoxia. This syndrome was described in the phase I experience of this regimen, such that those investigators recommended that patients with poor pulmonary, or G6PD deficiency, not receive treatment [9]. Despite the fact that these exclusion criteria were included in ECOG 1503, 28% of our patients still developed this Triapine® infusion reaction. This syndrome was also seen in one-third of patients enrolled in Ma, et al., strongly suggesting that this population is not suited for this regimen [19].
A growing body of evidence suggests that ribonucleotide reductase expression may influence response and survival in a number of malignancies. Over-expression of RRM1 in in vitro and in vivo models of lung cancer was associated with a less malignant phenotype and suppression of tumor growth [20, 21]. This was evident clinically, as survival increased correspondingly with RRM1 levels in resected specimens of NSCLC [22]. RRM1 is also the predominant cellular determinant of efficacy of gemcitabine, in that over-expression is associated with in vitro drug resistance [23, 24]. Likewise, clinical response to gemcitabine correlates inversely with pretreatment RRM1 expression [25]. However, data examining the relationship between expression of RRM2 and treatment outcomes are less consistent, with laboratory experiments that both increase or decrease RRM2 expression yielding increased sensitivity of cancer cell lines to gemcitabine [26, 27]. This contradiction may relate to the fact that, while expression of RRM1 remains constant through the cell cycle, the expression of the RRM2 gene is tightly controlled during cell cycle progression, with a sharp induction at the end of the G1 phase [24]. Thus, inconsistencies in the timing of sampling may contribute to the seemingly conflicting predictive value of RRM2 seen thus far. Clinically, Souglakos et al. found that low levels of RRM2 mRNA were associated with response to treatment of patients with NSCLC to gemcitabine and docetaxel [28]. The value of RRM2 as a biomarker to predict treatment response should be considered in future studies with Triapine®.
Our exploratory analyses of MDR1 and MRP1 polymorphisms revealed that the variant alleles of the MDR1 gene were associated with prolonged survival and that non-variant alleles of MRP1 were associated with increased treatment-related anorexia and vomiting. It is reasonable to speculate that individuals with variant alleles may have decreased efflux of Triapine®, resulting in higher tumor concentrations, and improved response. Likewise, those with the non-variant alleles may have increased efflux, higher plasma concentrations, and increased toxicity. These hypothesis-generating findings require confirmation with a larger sample size and pharmacokinetic data to evaluate possible associations with increased drug exposure.
In summary, our findings mirror those of Ma et al., in that this regimen did not demonstrate activity in a non-selected patient population with relapsed NSCLC. In addition, the syndrome of acute methemoglobinemia related to Triapine® infusion reactions makes this an impractical regimen for this population. However, the prolonged survival of patients with the variant genotypes of MDR1, compared to those without, suggest that this combination should be explored in those patients.
Acknowledgments
The authors thank the patients who participated in this trial, as well as the investigators who enrolled patients, and the data managers, and the clinical and research nurses who brought this trial to completion. This study was conducted by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by the University of Wisconsin Paul P. Carbone Comprehensive Cancer Center K12 CA087716 and 5 P30 CA014520-35, and by the Public Health Service Grants CA23318, CA66636, CA21115, CA21076, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Contributor Information
Anne M. Traynor, Email: amt@medicine.wisc.edu, University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, University of Wisconsin School of Medicine and Public Health, K6/568 CSC, #5669, 600 Highland Avenue, Madison, WI 53792, USA
Ju-Whei Lee, Eastern Cooperative Oncology Group and the Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Boston, MA, USA.
Gerald K. Bayer, Green Bay Oncology, Saint Vincent Hospital, Green Bay, WI, USA
John M. Tate, Roger Maris Cancer Center, Merit Care Hospital CCOP, Fargo, ND, USA
Sachdev P. Thomas, Illinois Cancer Care, Peoria, IL, USA
Miroslaw Mazurczak, Sanford Clinic Oncology, Sanford Hospital, Sioux Falls, SD, USA.
David L. Graham, Carle Cancer Association, Urbana, IL, USA
Jill M. Kolesar, University of Wisconsin Paul P. Carbone Comprehensive Cancer Center, University of Wisconsin School of Medicine and Public Health, K6/568 CSC, #5669, 600 Highland Avenue, Madison, WI 53792, USA
Joan H. Schiller, Division of Hematology and Oncology, Department of Internal Medicine, University of Texas at Southwestern Medical Center, Dallas, TX, USA
References
- 1.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 2.Crino L, Mosconi AM, Scagliotti G, et al. Gemcitabine as second-line treatment for advanced non-small-cell lung cancer: a phase II trial. J Clin Oncol. 1999;17:2081–2085. doi: 10.1200/JCO.1999.17.7.2081. [DOI] [PubMed] [Google Scholar]
- 3.Brodowicz T, Krzakowski M, Zwitter M, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer. 2006;52:155–163. doi: 10.1016/j.lungcan.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Murren J, Modiano M, Clairmont C, et al. Phase I and pharmacokinetic study of triapine, a potent ribonucleotide reductase inhibitor, administered daily for five days in patients with advanced solid tumors. Clin Cancer Res. 2003;9:4092–4100. [PubMed] [Google Scholar]
- 5.Yu Y, Wong J, Lovejoy DB, et al. Chelators at the cancer coalface: desferrioxamine to Triapine and beyond. Clin Cancer Res. 2006;12:6876–6883. doi: 10.1158/1078-0432.CCR-06-1954. [DOI] [PubMed] [Google Scholar]
- 6.Finch RA, Liu M, Grill SP, et al. Triapine (3-amino-pyridine-2-carboxaldehyde- thiosemicarbazone): a potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem Pharmacol. 2000;59:983–991. doi: 10.1016/S0006-2952 (99)00419-0. [DOI] [PubMed] [Google Scholar]
- 7.Sigmond J, Kamphuis JA, Laan AC, et al. The synergistic interaction of gemcitabine and cytosine arabinoside with the ribonucleotide reductase inhibitor triapine is schedule dependent. Biochem Pharmacol. 2007;73:1548–1557. doi: 10.1016/j.bcp.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, King I, Belcourt M, et al. Triapine, a ribonucleotide reductase inhibitor, enhances incorporation of gemcitabine into DNA and cytoxicity to KB cells. Proc AACR-EORTC conference. 2002;38:S26. (abstract 71) [Google Scholar]
- 9.Yen Y, Margolin K, Doroshow J, et al. A phase I trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone in combination with gemcitabine for patients with advanced cancer. Cancer Chemother Pharmacol. 2004;54:331–342. doi: 10.1007/s00280-004-0821-2. [DOI] [PubMed] [Google Scholar]
- 10.Kaufman DC, Chabner BA. Clinical strategies for cancer treatment: the role of drugs. In: Chabner BA, Longo DL, editors. Cancer chemotherapy and biotherapy: principles and practice. 3. Lippincott, Williams, and Wilkins; Philadelphia: 2001. pp. 1–16. [Google Scholar]
- 11.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Rappa G, Lorico A, Liu MC, et al. Overexpression of the multidrug resistance genes mdr1, mdr3, and mrp in L1210 leukemia cells resistant to inhibitors of ribonucleotide reductase. Biochem Pharmacol. 1997;54:649–655. doi: 10.1016/S0006-2952(97) 00210-4. [DOI] [PubMed] [Google Scholar]
- 13.Kerb R, Hoffmeyer S, Brinkmann U. ABC drug transporters: hereditary polymorphisms and pharmacological impact in MDR1, MRP1 and MRP2. Pharmacogenomics. 2001;2:51–64. doi: 10.1517/14622416.2.1.51. [DOI] [PubMed] [Google Scholar]
- 14.Attia S, Kolesar J, Mahoney MR, et al. A phase 2 consortium (P2C) trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) for advanced adenocarcinoma of the pancreas. Invest New Drugs. 2008;26:369–379. doi: 10.1007/s10637-008-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzolini C, Paus E, Buclin T, et al. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004;75:13–33. doi: 10.1016/j.clpt.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Kim RB, Leake BF, Choo EF, et al. Identification of functionally variant MDR1 alleles among European Americans and African Americans. Clin Pharmacol Ther. 2001;70:189–199. doi: 10.1067/mcp.2001.117412. [DOI] [PubMed] [Google Scholar]
- 17.Pan JH, Han JX, Wu JM, et al. MDR1 single nucleotide polymorphism G2677T/A and haplotype are correlated with response to docetaxelcisplatin chemotherapy in patients with non-small-cell lung cancer. Respiration. 2009 doi: 10.1159/000158454. [DOI] [PubMed] [Google Scholar]
- 18.Rudin CM, Liu W, Desai A, et al. Pharmacogenomic and pharmacokinetic determinants of erlotinib toxicity. J Clin Oncol. 2008;26:1119–1127. doi: 10.1200/JCO.2007.13.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma B, Goh BC, Tan EH, et al. A multicenter phase II trial of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine) and gemcitabine in advanced non-small-cell lung cancer with pharmacokinetic evaluation using peripheral blood mononuclear cells. Invest New Drugs. 2008;26:169–173. doi: 10.1007/s10637-007-9085-0. [DOI] [PubMed] [Google Scholar]
- 20.Gautam A, Bepler G. Suppression of lung tumor formation by the regulatory subunit of ribonucleotide reductase. Cancer Res. 2006;66:6497–6502. doi: 10.1158/0008-5472.CAN-05-4462. [DOI] [PubMed] [Google Scholar]
- 21.Gautam A, Li ZR, Bepler G. RRM1-induced metastasis suppression through PTEN-regulated pathways. Oncogene. 2003;22:2135–2142. doi: 10.1038/sj.onc.1206232. [DOI] [PubMed] [Google Scholar]
- 22.Zheng Z, Chen T, Li X, et al. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med. 2007;356:800–808. doi: 10.1056/NEJMoa065411. [DOI] [PubMed] [Google Scholar]
- 23.Davidson JD, Ma L, Flagella M, et al. An increase in the expression of ribonucleotide reductase large subunit 1 is associated with gemcitabine resistance in non-small cell lung cancer cell lines. Cancer Res. 2004;64:3761–3766. doi: 10.1158/0008-5472.CAN-03-3363. [DOI] [PubMed] [Google Scholar]
- 24.Bepler G, Kusmartseva I, Sharma S, et al. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J Clin Oncol. 2006;24:4731–4737. doi: 10.1200/JCO.2006.06.1101. [DOI] [PubMed] [Google Scholar]
- 25.Bepler G, Sharma S, Cantor A, et al. RRM1 and PTEN as prognostic parameters for overall and disease-free survival in patients with non-small-cell lung cancer. J Clin Oncol. 2004;22:1878–1885. doi: 10.1200/JCO.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Wong SJ, Myette MS, Wereley JP, et al. Increased sensitivity of hydroxyurea-resistant leukemic cells to gemcitabine. Clin Cancer Res. 1999;5:439–443. [PubMed] [Google Scholar]
- 27.Duxbury MS, Ito H, Zinner MJ, et al. RNA interference targeting the M2 subunit of ribonucleotide reductase enhances pancreatic adenocarcinoma chemosensitivity to gemcitabine. Oncogene. 2004;23:1539–1548. doi: 10.1038/sj.onc.1207272. [DOI] [PubMed] [Google Scholar]
- 28.Souglakos J, Boukovinas I, Taron M, et al. Ribonucleotide reductase subunits M1 and M2 mRNA expression levels and clinical outcome of lung adenocarcinoma patients treated with docetaxel/gemcitabine. Br J Cancer. 2008;98:1710–1715. doi: 10.1038/sj. bjc.6604344. [DOI] [PMC free article] [PubMed] [Google Scholar]