Abstract
Retinoic acid (RA) signaling regulates multiple aspects of vertebrate embryonic development and tissue patterning, in part through the local availability of nuclear hormone receptors called retinoic acid receptors (RARs) and retinoid receptors (RXRs). RAR/RXR heterodimers transduce the RA signal, and loss-of-function studies in mice have demonstrated requirements for distinct receptor combinations at different stages of embryogenesis. However, the tissue-specific functions of each receptor and their individual contributions to RA signaling in vivo are only partially understood. Here we use morpholino oligonucleotides to deplete the four known zebra fish RARs (raraa, rarab, rarga, and rargb). We show that while all four are required for anterior–posterior patterning of rhombomeres in the hindbrain, there are unique requirements for rarga in the cranial mesoderm for hindbrain patterning, and rarab in lateral plate mesoderm for specification of the pectoral fins. In addition, the alpha subclass (raraa, rarab) is RA inducible, and of these only raraa expression is RA-dependent, suggesting that these receptors establish a region of particularly high RA signaling through positive-feedback. These studies reveal novel tissue-specific roles for RARs in controlling the competence and sensitivity of cells to respond to RA.
Keywords: Danio rerio, Zebrafish, Nuclear hormone receptor, Retinoic acid, Hindbrain, Vitamin A, RAR, Branchial arch, Forelimb, Rhombomere
Introduction
The vitamin A derivative, retinoic acid (RA), is essential for vertebrate development, including the central nervous system (Glover et al., 2006; Maden, 2007), limbs (Lee et al., 2004), and craniofacial skeleton (Mark et al., 2004). Disruption of RA signaling by vitamin A depletion (VAD; Maden et al., 1996; Clagett-Dame and DeLuca, 2002), genetic disruption of RA synthesis (Niederreither et al., 2000, 2002, 2003, Begemann et al., 2001) or pharmacological blockage of RA receptors (RARs and RXRs, Dupe and Lumsden, 2001; Linville et al., 2004; Mark et al., 2006) suggests a role in patterning along the anterior–posterior (A–P) axis. An anteriorly-declining gradient of RA is thought to act as a morphogen that promotes posterior development. In the developing hindbrain, RA actively induces expression of homeotic genes (hox) in different segments (rhombomeres) in a concentration-dependent manner (Durston et al., 1989; Dupe and Lumsden, 2001;Wendling et al., 2001;White et al., 2007). The output of RA signaling also depends upon the regulated expression and function of RARs and RXRs in cells (Mark et al., 2006), but few studies have investigated their tissue-specific roles during development or in the establishment and maintenance of the RA morphogen gradient.
RARs and RXRs are nuclear hormone receptors that regulate transcription of target genes both in the presence or absence of RA (reviewed in Mark et al., 2006). Receptors heterodimerize and bind a sequence in the regulatory regions of these targets called a retinoic acid response element (RARE). The bound complex recruits co-activators, which enable transcriptional activation. Receptor heterodimers also bind RAREs in the absence of RA, and recruit the co-repressors SMRT and N-CoR to repress gene expression (Koide et al., 2001). Three highly conserved RARs (alpha — RARa, beta — RARb, gamma — RARg) have been studied extensively in mouse, chick, and Xenopus, but functional studies have been limited (Mark et al., 2006). Surprisingly, single RAR knockouts in mice are viable and display relatively mild phenotypes (Li et al., 1993). Only double RAR/RAR or RAR/RXR mutants die in utero and resemble VAD embryos, suggesting partial redundancy between receptors (Taneja et al., 1995; Luo et al., 1996; Subbarayan et al., 1997; Dupe et al., 1999;Wendling et al., 2001; Mark et al., 2006). Evidence for redundancy has also come from treating embryos with pharmacological RAR antagonists, which have graded effects on A–P patterning of the hindbrain depending on antagonist concentration, and also disrupt neurogenesis at later stages (Dupe and Lumsden, 2001; Linville et al., 2004).
Zebrafish have two alpha RARs (raraa and rarab) and two gamma RARs (rarga and rargb) but appear to lack RARbeta. Expression of these receptors in embryos, as well as four zebrafish RXRs, has been reported (Hale et al., 2006; Tallafuss et al., 2006). However, to date there have been no functional studies other than with non-specific chemical antagonists that block all four receptors. Using antisense morpholino oligonucleotides (MO) we show requirements for individual receptors in A–P patterning of the hindbrain, and for rarga in cranial mesoderm and rarab in the pectoral fins. We also show that the alpha subclass of receptors is uniquely RA inducible, suggesting that these two receptors establish regions of particularly high RA signaling through positive-feedback.
Materials and methods
RA and DEAB treatments
Wild type embryos (AB) were collected from natural matings and treated in the dark with all-trans RA (1 µM, Sigma) or the aldehyde dehydrogenase (Aldh) inhibitor diethylaminobenzene (DEAB) in embryo medium at 6 hpf (50% epiboly). Embryos were grown at 28 °C and fixed in 4% paraformaldehyde (PFA) at the appropriate stages.
GFP constructs, morpholinos and RNA injections
Antisense morpholino oligonucleotides (MOs; Gene Tools Inc.)were designed as translational blockers, either to the 5′UTR of raraa (GGT TCA CAT CCA CAC TCT CAT ACAT) and rarga (CCA GAG CCT CCA TAC AGT CGA ACAT), or spanning the translation start site for both rarab (CCA CAA CGT CCA CGC TCT CGTACAT) and rargb (CGT CTC TCATAC CAAGTG GGC TGTT). Second MOs targeting non-overlapping sequences in raraa and rarga caused identical defects, confirming specificity (data not shown). The concentrations used for individual MO injections were: raraa (10 ng), rarab (5 ng), rarga (5 ng) and rargb (2.5 ng), although for double raraa/rarab morphants, 5 ng of each MO was used. In each case, MOs were injected together with the p53 MO to help suppress MO-induced cell death (2 ng; Plaster et al., 2006). All were injected into wild-type embryos at the 1–2 cell stage and the embryos were either fixed in 4% paraformaldehyde for in situ hybridization or alcian blue staining. To test for MO efficiency and specificity, each MO target sequence was fused to GFP, which was used to synthesize capped mRNA (mMessage mMachine Kit, Ambion). This was co-injected with the corresponding MO to detect loss of GFP expression (Suppl. Fig. 2).
Whole-mount in situ hybridization and histology
Whole-mount in situ hybridization was performed as previously described (Thisse et al., 1993). Fragments of raraa, rarab, rarga and rargb were amplified from cDNA (SuperScript III) synthesized from pooled total RNA extracted from embryos of multiple stages (Trizol, Invitrogen) and directionally cloned into pCS2+ to synthesize riboprobes: raraa (ClaI/T7), rarab (EcorI/T7), rarga (BamHI/T7) and rargb (Waxman and Yelon, 2007). Additional riboprobes used were hoxb5a (Prince et al., 1998), valentino (Moens et al., 1998), krox20 (Oxtoby and Jowett, 1993), myoD (Weinberg et al., 1996), and tbx5 (Begemann and Ingham, 2000). Embryos were sectioned after in situ hybridization at 12 µm using a Leica CM3050S Cryostat. Alcian staining was performed at 4 dpf and stained cartilages were dissected and flat-mounted for photography (Javidan and Schilling, 2004).
Quantitative reverse transcription-PCR
Total RNA was extracted from embryos (Trizol, Invitrogen) either injected with all four rar MOs (raraa, 5 ng; rarab, 5 ng; rarya, 5 ng; raryb, 2.5 ng), or treated with 1 µM DEAB along with controls at approximately 7–8 hpf. cDNA was synthesized from these pools of total RNA using the Superscript First-Strand Synthesis System for RT-PCR (Invitrogen), and qRT-PCR was performed using these cDNAs with SYBR Green RT-PCR Master Mix (Roche) on an Opticon DNA Engine (MJ Research).
Confocal microscopy and transgenic embryos
RARE-YFP homozygous transgenic embryos (Perz-Edwards et al., 2001) were obtained through natural matings and injected at the 1–2 cell stage with either raraa, rarab, rarga or rargb MOs. Morphants were raised to 26–30 hpf in embryo medium containing phenylthiourea (PTU) to inhibit pigmentation, anesthetized with tricaine (MS-222; Sigma), and mounted in 0.7% agarose prepared in embryo medium on bridged coverslips and imaged with a Zeiss LSM 510 confocal microscope at 2.95 µm intervals.
Mosaic analysis
Wild-type donor embryos were injected at the 1–2 cell stage with 3% biotinylated dextran/3% tetramethylrhodamine (Molecular Probes). Sibling embryos to be used as hosts were simultaneously injected with 5 ng of the rarab MO. At 4 hpf, cells from the labeled donors were transplanted to the margins of the unlabeled, morphant hosts, into a fate map position that gives rise to mesendoderm (Begemann et al., 2001). These mosaic embryos were grown to 32–36 hpf, during which time the cells were tracked by rhodamine fluorescence, and then fixed with 4% paraformaldehyde. Biotin was visualized using the Vectastain ABC kit as previously described before proceeding with in situ hybridization (Westerfield, 1998).
Results
Tissue-specific expression patterns of zebrafish rars
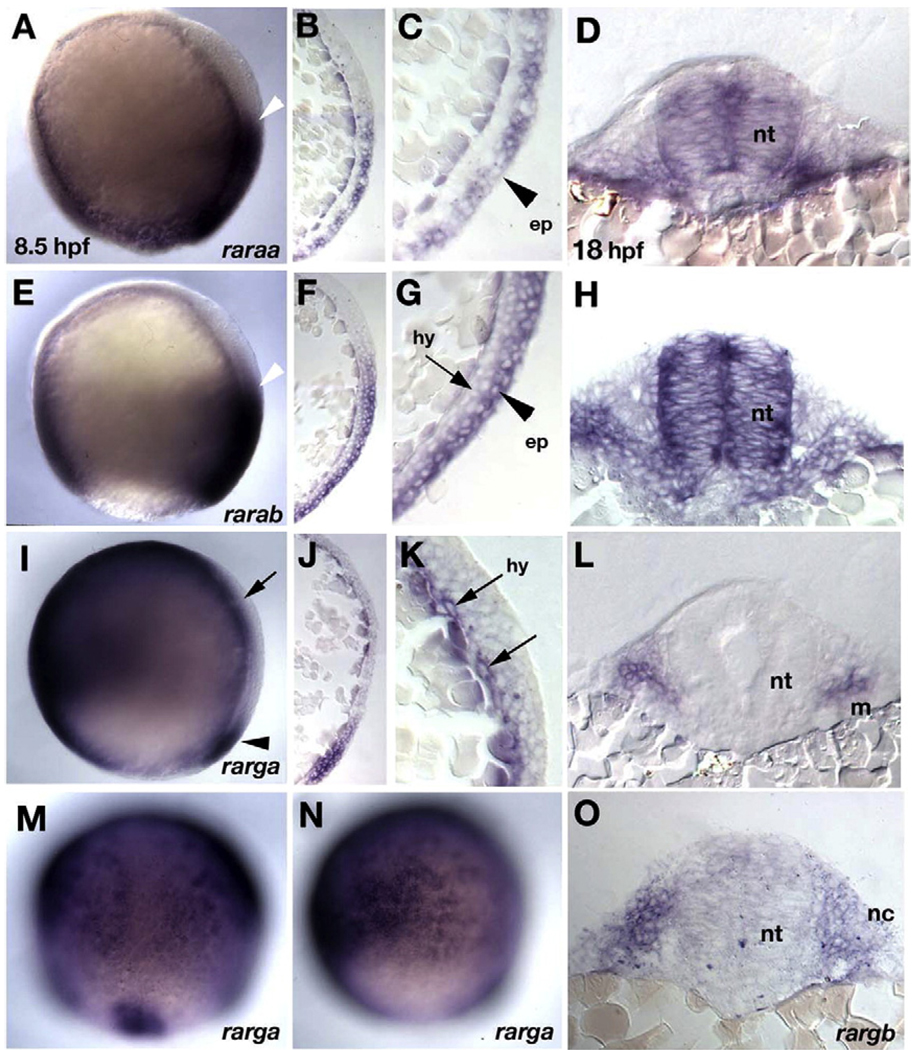
Several previous studies described the expression of zebrafish rars (Joore et al., 1994; Stafford et al., 2006; Hale et al., 2006;Waxman and Yelon, 2007). However, these did not examine germ-layer specific expression of each receptor during gastrulation, when RA signaling is required for A–P patterning. To investigate this issue, we first examined expression of all four known zebrafish rar receptors (raraa, rarab, rarga, and rargb) by whole-mount in situ hybridization and in sectioned material during gastrula stages (Fig. 1). Distinct expression patterns were detected for three out of the four RARs at 7–9 h post-fertilization [hpf] (Figs. 1A, E, I). Expression of the fourth and most recently discovered receptor, rargb, was ubiquitous at gastrula stages, with stronger expression on the dorsal side (data not shown; Waxman and Yelon, 2007). Parasagittal sections of whole mounts revealed that expression of raraa at these stages was restricted to the posterior epiblast (future ectoderm; including weak expression in the enveloping layer, EVL) and excluded from the hypoblast (future mesoderm and endoderm, Figs. 1B, C). Expression of rarab was detected posteriorly in both layers, but much stronger in the epiblast (Figs. 1F, G). In contrast, rarga expression was detected anteriorly in the hypoblast, in a pattern complementary to raraa and rarab (Fig. 1I) as well as at the dorsal margin (Fig.1M) and in non-neural ectoderm (Fig. 1N). Hypoblast expression was visible in sections as a thin layer of cells next to the yolk (Figs. 1J, K). These expression patterns are summarized in Suppl. Fig. 1K.
Fig. 1.
Germ layer-specific expression of zebrafish rars. In situ hybridization in whole mounts (A, E, I, M, N) and parasagittal sections (B, C, F, G, J, K) at 8.5 hpf (90% epiboly), and transverse sections at 18 hpf (18 somites) at hindbrain levels (D, H, L, O). (A–D) raraa. (A) Expression is in posterior ectoderm in the gastrula, with an anterior border in the presumptive hindbrain (white arrowhead). (B–D) Sections reveal that expression is restricted to epiblast, shown at higher magnification in panel C (arrowhead), and persists in the neural tube (D). (E–H) rarab. (E) Expression is in posterior ectoderm in the gastrula, with an anterior border in the presumptive hindbrain (white arrowhead), similar to raraa. (F–H) Sections reveal strong expression in epiblast (arrowhead in panel G), weaker in hypoblast, which persists in the neural tube and pharyngeal arches (H). (I–N) rarga. (I, M, N) Expressed anteriorly and ventrally during gastrulation and at the dorsal margin (arrowheads in panels I and M). Panel M shows a dorsal view and panel N is focused laterally to show expression in ventral epiblast (presumptive epidermis). (J–L) Sections near the dorsal side reveal expression restricted to the hypoblast (arrows in panel K) and later in cranial mesoderm (L). (O) rargb. Expression is ubiquitous at early stages (not shown), and by 18 hpf sections reveal expression in pharyngeal arch mesenchyme. Abbreviations: ep, epiblast; hy, hypoblast; m, mesoderm; nc, neural crest; nt, neural tube.
Complementary patterns of expression of alpha and gamma subclasses persisted at later stages (Hale et al., 2006). In whole mounts, both raraa and rarab expression were restricted along the A–P axis of the neural plate at 11–15 hpf, with raraa expressed up to the rhombomere 6/7 (r6/7) boundary, and rarab up to the r1/2 boundary (Suppl. Figs. 1A–F). In contrast, rarga expression was limited to the cranial mesoderm and tailbud (Suppl. Figs. 1G, H). These distinct patterns of expression were confirmed in sections of whole mounts at 18 hpf (Fig. 1) – raraa and rarab retained expression in the neural tube (Figs. 1D, H) while rarab, rarga and rargb were expressed in the pharyngeal arches (Figs. 1L, O; Waxman and Yelon, 2007) – and persisted at 48 hpf (Suppl. Figs. 1C, F, I, J). Expression domains of alpha and gamma subclasses of RARs overlap in a few places, including the pectoral fin buds, where rarab is expressed proximally, rarga distally, and rargb throughout the fin mesenchyme (insets in Suppl. Figs. 1C, F, I, J). These expression patterns are summarized in Suppl. Fig. 1K. From these results we infer that RA signaling in the neural ectoderm in zebrafish is mediated predominantly by the alpha subclass of RARs, while signaling in other tissues requires both alphas and gammas.
Functional requirements for rars in the hindbrain
Expression of raraa and rarab in the neural plate suggests that these two receptors mediate the A–P patterning functions of RA in the hindbrain. To test this hypothesis, we designed antisense morpholino oligonucleotides (MOs) targeted to the translation start sites or 5′UTR of each of the four rars. We confirmed the binding efficiency of each receptor MO by generating fusion constructs containing the MO target sequence upstream of eGFP and co-injected these with the corresponding MOs, all of which efficiently inhibited GFP expression (Suppl. Fig. 2). We also confirmed specificity of the raraa and rarga MO-induced defects with second MOs targeting non-overlapping sequences (data not shown).
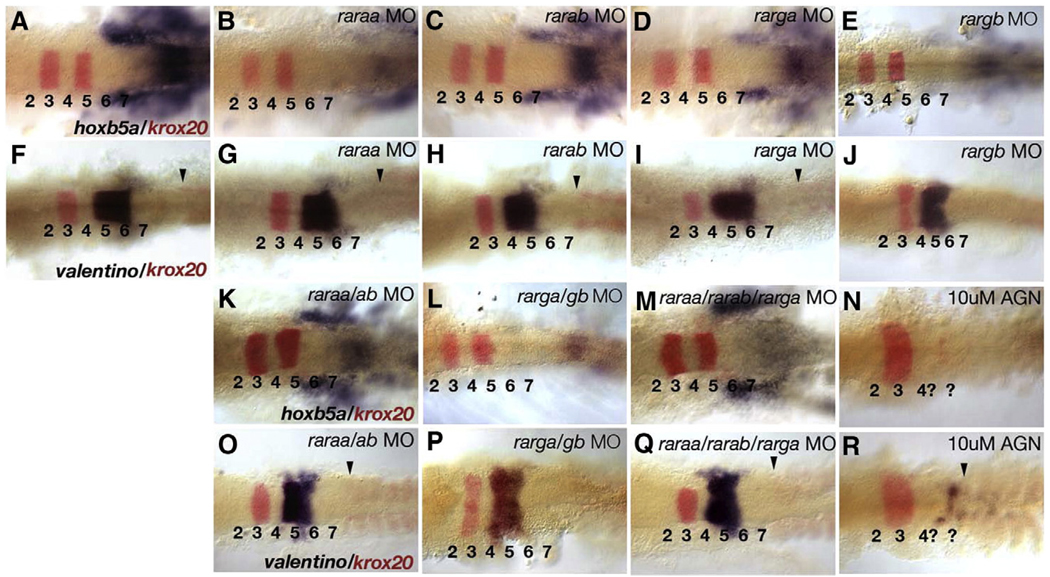
A–P patterning of hindbrain rhombomeres in RAR morphants was assessed by expression of hoxb5a in the anterior spinal cord (Prince et al., 1998; Bruce et al., 2001), krox20 in r3 and r5 (Oxtoby and Jowett, 1993), and valentino in r5 and r6 (val; Moens et al., 1998) (Fig. 2). Embryos treated with the pan-RAR antagonist, AGN 193109, which are severely deficient in RA signaling, lacked hoxb5a and val expression as well as the r5 stripe of krox20 (Figs. 2N, R). In contrast, embryos injected with raraa or rarab MOs alone showed a reduction of hoxb5a expression (Figs. 2A–C), but little or no effect on expression of krox20 or val (Figs. 2F–H). Double morphants for raraa and rarab also continued to express hoxb5a (22/22; Fig. 2K) and val (35/38; Fig. 2O) and their hindbrains were not strongly anteriorized, suggesting that these are not the only essential receptors for RA in the hindbrain.
Fig. 2.
Redundant requirements for rars in hindbrain patterning. Dorsal views of flat-mounted hindbrains at 15 hpf (12-somite), anterior to the left, labeled by two-color in situ hybridization for either hoxb5a/krox20 (A–E, K–N) or valentino/krox20 (F–J, O–R). Hindbrain rhombomeres are numbered (1–7), arrowheads indicate the location of the first somite. (A, F) Controls. (B–E) Embryos injected with MOs targeting raraa (B), rarab (C) rarga (D) or rargb (E) show reduced hoxb5a expression in posterior neural tube and mesoderm (blue), but no defects in krox20 expression in r3 or r5 (red). (G–J) Embryos injected with each of the four MOs alone show no defects in valentino (val) expression, or in the position of somite 1 (arrowheads). (K, L) Combined injection of raraa and rarab MOs (K; N=22), or rarga and rargb MOs (L; N=25) causes a reduction in hoxb5a expression. (M) Combined injection of the rarga MO with both raraa and rarab MOs (N=25) causes severe reduction in hoxb5a expression in the neural tube, but mesodermal expression persists. These triple morphants retain the r5 stripe of krox20 expression unlike embryos treated with a pan-RAR antagonist, AGN 193109 (N). (O–Q) raraa/rarab double morphants (O; 35/38), rarga/rargb double morphants (P; N=20), and raraa/rarab/rarga triple morphants (Q; N=22) all show slight reductions in val expression, while its expression is almost completely eliminated by AGN treatments (R; N=25).
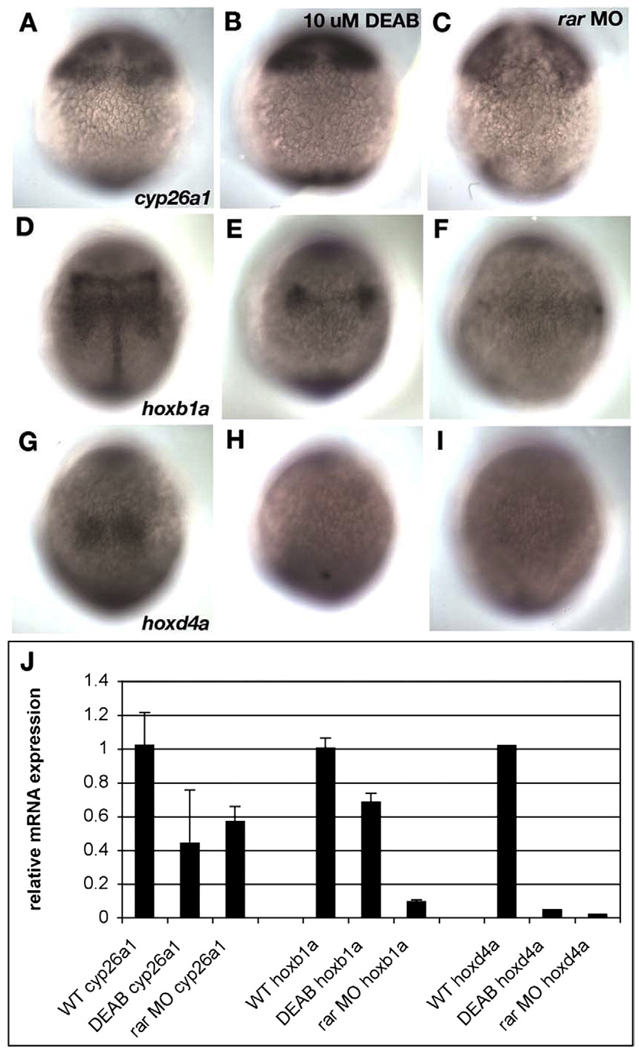
Similarly, injections of rarga and rargb MOs, either alone or in combination, reduced hoxb5a (Figs. 2D, E, L) but not val expression (Figs. 2I, J, P) and when combined with raraa and rarab MOs these only slightly enhanced the reduction in hoxb5a (Figs. 2M, Q). This suggests that all four receptors are required for hindbrain patterning. Unfortunately, embryos injected with all four receptor MOs had widespread cell death and did not survive long enough to assay gene expression in the hindbrain, even when co-injected with a p53-targeted MO to block apoptosis (data not shown; Plaster et al., 2006). Therefore, we examined expression of RA target genes in these embryos during gastrulation by in situ and qRT-PCR (Fig. 3). Quadruple rar morphants (injected with all four MOs and hereafter referred to as rar-MO) were compared to embryos treated with the Aldh inhibitor, DEAB, for changes in hoxb1a expression in presumptive hindbrain (which requires RA), and cyp26a1 in presumptive forebrain–midbrain (which is largely RA independent; White et al., 2007). hoxb1a expression was strongly reduced in DEAB-treated and even more so in rar-MO embryos, compared to controls (Figs. 3D–F), while cyp26a1 expression appeared unchanged (Figs. 3A–C). Expression of another RA target, hoxd4a, was absent in both DEAB-treated and rar-MO embryos (Figs. 3G–I), suggesting that it is more sensitive to RA levels than hoxb1a. These results were further supported by qRT-PCR analyses with RNA isolated from siblings of the embryos used for in situ hybridization (Fig. 3J). Depletion of all four rars eliminates hoxb1a and hoxd4a expression, and reduces expression of cyp26a1 (White et al., 2007). These results suggest partial functional redundancy among the four RARs in hindbrain development.
Fig. 3.
Depleting all four rars eliminates hox expression in the hindbrain. (A–I) Dorsal views of late gastrula stage embryos (8.5 hpf), labeled by whole mount in situ hybridization for cyp26a1 (A–C), hoxb1a (D–F), and hoxd4a (G–I). Anterior is to the top. Controls (left column), DEAB-treated (middle column) or quadruple morphants (rar-MO) injected with MOs targeting raraa, rarab, rarga and rargb. (J) Histogram summarizing results of quantitative PCR (qPCR) to detect relative amounts of the same three gene products, using RNA isolated from the siblings of embryos used for panels A–I. (A–C) No changes were detected in the pattern of cyp26a1 expression in presumptive forebrain and midbrain in DEAB-treated (B) or rar-MO quadruple morphants (C), but total levels were significantly reduced in both cases (J). (D–F) hoxb1a expression in presumptive hindbrain was dramatically reduced by DEAB treatments (E) and eliminated by rar-MO (F), and this was confirmed by qPCR (J). (G–I) hoxd4a expression was virtually eliminated by both DEAB treatments (H) and rar-MO (I), which was confirmed by qPCR (J).
rarga functions in pharyngeal arch development
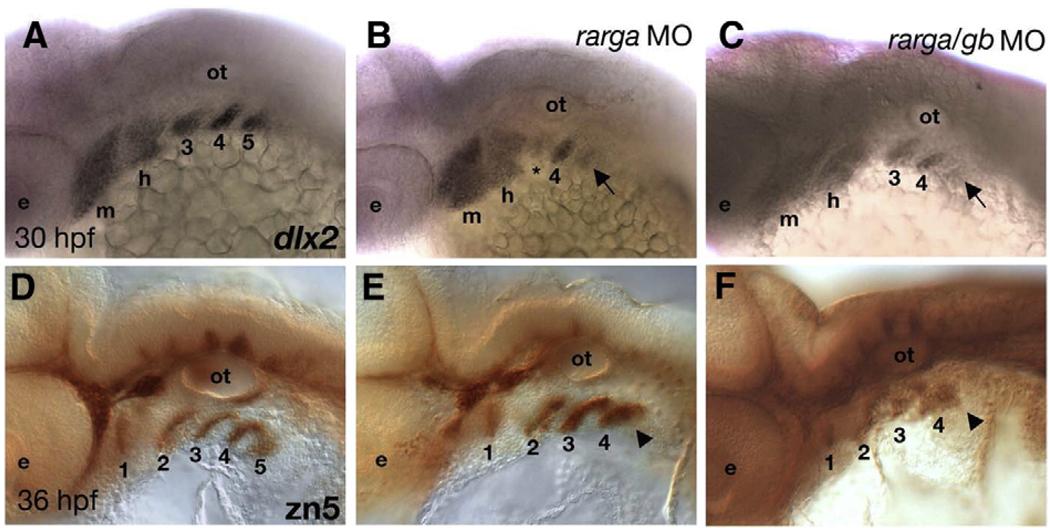
RA signaling is also required for development of the craniofacial skeleton, including the pharyngeal arches. Posterior arches are reduced or lost in aldh1a2 mutant zebrafish (Begemann et al., 2001) and mice (Niederreither et al., 2000). All four zebrafish rars are expressed in the arches — raraa, rarab and rargb in NC cells that form the pharyngeal skeleton, and rarga in mesodermal cells that form the pharyngeal muscles and endothelia (Figs. 1, 2; Schilling and Kimmel, 1994). Injection of an rarga MO caused reductions in the arch skeleton, similar to aldh1a2 mutants, but raraa and rarab MOs had no affect on the arches (data not shown). dlx2 expression was reduced in migrating NC precursors of the skeleton in arches 3–5 in rarga morphants (28/30; Figs. 4A, B). Co-injection of the rarga MO with the rargb MO did not substantially increase the severity of the arch defects (35/35; Fig. 4C). rarga morphant embryos also lacked the 5th endodermal pouch, as revealed by immunostaining with the zn5 antibody that recognizes the cell surface adhesion molecule, DM-GRASP (Figs. 4D–F; Trevarrow et al., 1990). These results indicate a unique role for rarga in development of the pharyngeal arches. Based on its expression in the cranial mesoderm, these data suggest that the requirements for rarga in neural crest and endoderm within the arches may be, at least in part, indirect.
Fig. 4.
Depletion of rarga alone disrupts posterior pharyngeal arches. Lateral views, anterior to the left. (A, D) Controls. (B, E) rarga morphants (C, F) rarga/rargb double morphants. (A–C) Whole mount in situ hybridization to detect dlx2 mRNA reveals reductions in migrating neural crest cells in arches 3–5 (arrows) in both single (B; 28/30) and double (C; 35/35) morphants. (D–F) Whole mount, immunostaining with the zn-5 antibody reveals reductions in the pharyngeal pouch endoderm (particularly pouch #5, arrowheads) in these arches, but no defects in zn5+ cells in the hindbrain or cranial ganglia in single (E) and double (F) morphants. Abbreviations: e, eye; m, mandibular; h, hyoid; ot, otic vesicle.
rarab functions in pectoral fin budding
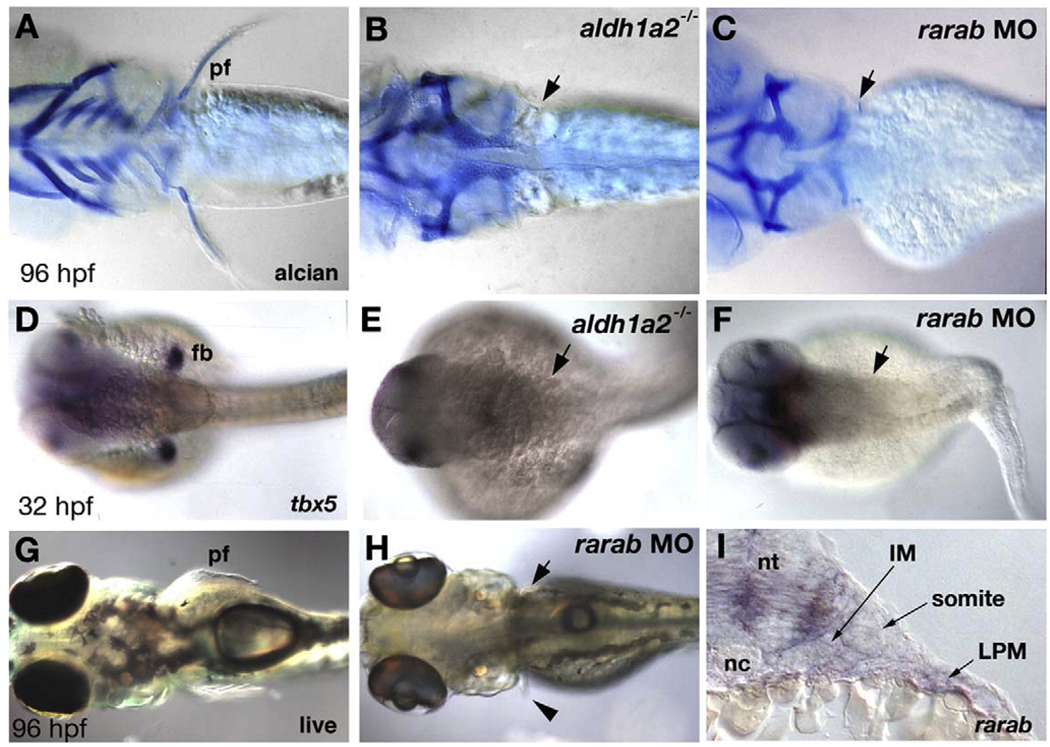
RA is also a key player in vertebrate limb development. Zebrafish embryos treated with RAR-antagonists (Gibert et al., 2006; data not shown) or mutant for aldh1a2 (Begemann et al., 2001) lack pectoral fin buds and fail to express tbx5, a T-box containing protein that specifies forelimb identity (Begemann and Ingham, 2000, Ahn et al., 2002). rarab, rarga and rargb are all expressed in the pectoral fin buds (Fig. 2). Therefore, we tested their functions with MOs and stained the MO-injected larvae for tbx5 in the fin buds at 32 hpf (Figs. 5D–F) as well as cartilage at 5 dpf (Figs. 5A–C). In wild type controls, cartilaginous endoskeletal discs protrude into the fins on both sides of the larvae, and these are lost in aldh1a2−/− mutants (n=12; Figs. 5A, B). Similarly, injection of rarab-MO caused a loss of fin cartilage, while the scapulocoracoid cartilage of the pectoral girdle, to which the fin is normally attached, was unaffected (24/24; Fig. 5C). Like aldh1a2−/− mutants, rarab morphant embryos lacked tbx5 expression in the fin bud mesenchyme at 32 hpf, suggesting a defect in budding (7/7; Figs. 5E, F). rarab morphants were otherwise healthy when compared to uninjected larvae at 4 dpf, lacking only their fin blades (12/14; Figs. 5G, H). In some cases, small buds formed unilaterally (1/14; Fig. 5H, arrow). In contrast, raraa, rarga or rargb MOs caused no fin defects (data not shown). This suggests a central role for the rarab receptor in pectoral fin development.
Fig. 5.
Depletion of rarab alone disrupts pectoral fin budding. (A–C) Alcian blue stained cartilage in larvae (4 dpf). Ventral views, anterior to the left. (A) In controls the pectoral fins protrude laterally just posterior to the skull. (B, C) Lack of fins and fin cartilages in both aldh1a2 mutants (B, arrow; N=12) and rarab morphants (C, arrow; N=24). (D–F) tbx5 expression at 32 hpf, detected by whole mount in situ hybridization. Dorsal views, anterior to the left. (D) In controls, tbx5 labels the bilateral pair of early pectoral fin buds, which are absent in aldh1a2 mutants (E, arrow) and rarab morphants (F, arrow; N=7). (G, H) Live 96 hpf larvae, dorsal views, anterior to the left. rarab morphants occasionally form a small pectoral fin on one side (H, arrowhead). (K) Transverse sections at forelimb levels at 24 hpf reveal rarab expression in the neural tube and lateral plate mesoderm, but absent from the adjacent somite or intermediate mesoderm. Abbreviations: fb, fin bud; IM, intermediate mesoderm; LPM, lateral plate mesoderm; pf, pectoral fin; nc, notochord; nt, neural tube.
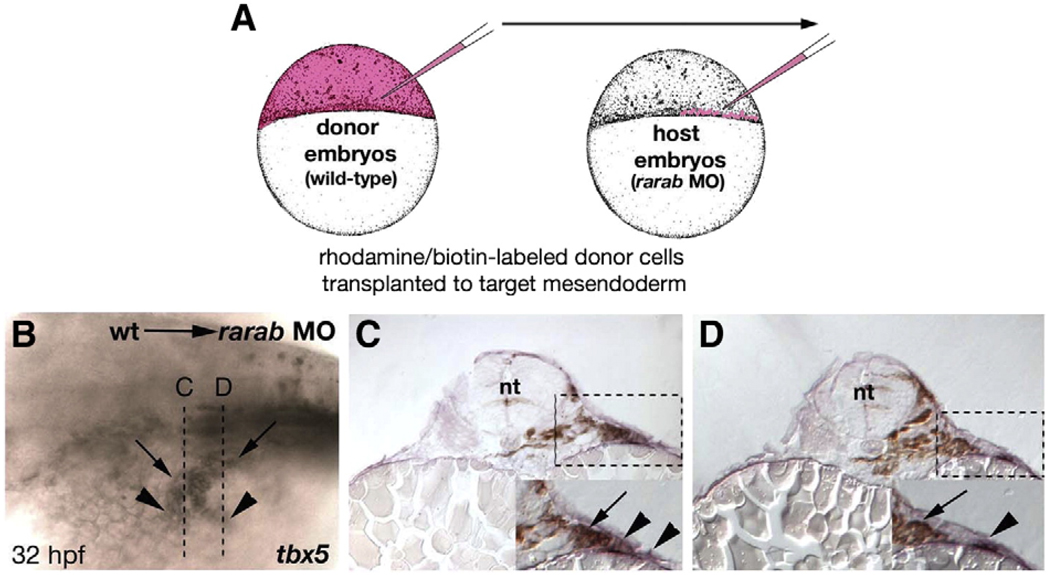
rarab is expressed in the lateral plate mesoderm (LPM), which contributes to the pectoral fins, but not in paraxial and intermediate mesoderm (IM; Fig. 5I). To determine if the pectoral fin defects in rarab morphants are due to cell autonomous requirements for this receptor in LPM, we performed mosaic rescue studies. We transplanted wild-type cells from labeled donors into rarab-morphant hosts and confined them to mesoderm by targeting the transplants to the margin of the blastoderm at 4–5 hpf (Fig. 6A). These were typically confined to one side of the animal and extended across many segments along the A–P axis (Begemann et al., 2001). Wild-type cells transplanted into the LPM rescued tbx5 expression (Figs. 6B–D) and fin outgrowth unilaterally in rarab morphants, either partially (15/50) or fully (2/50). These results indicate cell autonomous requirements for rarab in the LPM for pectoral fin development.
Fig. 6.
Grafts of wild-type mesoderm rescue tbx5 expression in rarab morphants. (A) Cell transplantation technique. Donor embryos were injected at the one-cell stage with both a fixable biotinylated dextran and a fluorescent rhodamine-dextran (red). Hosts were simultaneously injected with the rarab MO. At 4 hpf small groups of cells were transferred from donors to the margin (which forms the mesendoderm) of hosts, which were raised to 32 hpf, and stained for biotin (brown, grafted cells) and tbx5 (blue, pectoral fin). (B) Whole mount, anterior to the left, showing partial unilateral rescue of tbx5 expression — arrowheads indicate tbx5-expressing cells, and arrows indicate locations of wild-type, donor cells. Rescue was either partial (15/50) or completely restored the fin (2/50). Dotted lines indicate locations of transverse sections in panels C and D. (C, D) Transverse sections through the fin fields of mosaic embryos, showing the absence of tbx5 expression on the untransplanted, left side and tbx5+ cells in the LPM (arrowheads) on the right side in close proximity to biotin-labeled, donor cells (arrows). Abbreviations: nt, neural tube; wt, wild type.
Regulation of rar expression by RA
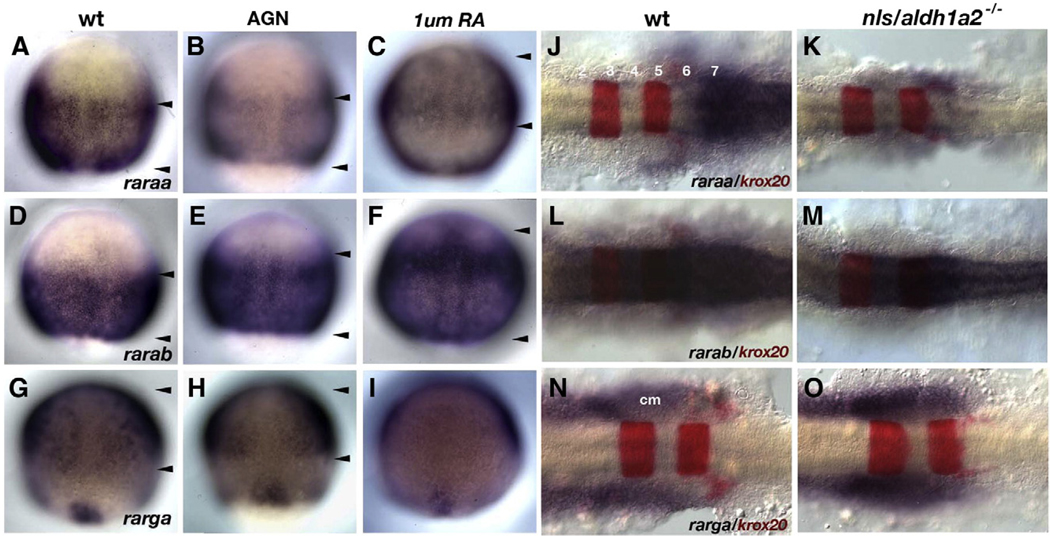
RA directly regulates RAR transcription in other species (De The et al., 1990; Hoffman et al., 1990; Leroy et al., 1991a,b). To determine if RA signaling activates expression of the four zebrafish rars, we treated embryos with either a pan-RAR antagonist, AGN 193109 (Allergan, Inc.), or with RA during gastrulation (Fig. 7). raraa expression was dramatically reduced in the posterior neural plate during gastrulation and at later stages in the hindbrain in embryos treated with 10 µMAGN(Figs. 7A, B; 32/32 treated embryos) and in aldh1a2−/− mutants (Figs. 7J, K; 24/24 mutants). These results suggest that RA initiates and maintains raraa expression. Conversely, high RA concentrations (1 µM) caused raraa expression to shift anteriorly (Fig. 7C, arrowheads; 23/23).
Fig. 7.
rars are differentially regulated by RA. (A–I) Whole mount in situ hybridization for three of the four rars during gastrulation (8.5 hpf). Dorsal views, anterior to the top, of wild-types (A, D, G), AGN-treated (B, E, H), and RA-treated (C, F, I) embryos. Arrowheads indicate approximate borders of receptor expression along the anterior–posterior axis. (A–C) Only raraa expression is reduced by AGN treatment (B) and induced by RA (C). (D–F) AGN has no effect on rarab expression (E) though expression is upregulated anteriorly by RA (F). (G–I) AGN treatments also have little effect on rarga expression (H), but surprisingly RA treatments downregulate expression (I). (J–O) Expression of rars in the hindbrain at 18 hpf. Dorsal views, anterior to the left, of flat-mounted hindbrains showing rar expression (blue) and krox20 in r3 and r5 (red). Similar to the gastrula studies, only raraa expression is reduced in aldh1a2 mutants (K) while other receptors appear unaffected (M, O). Abbreviations: cm, cranial mesoderm.
In contrast, expression of the other three rars was unaffected by similar AGN and RA treatments (rarab — Figs. 7D, E, L, M; rarga — Figs. 7G, H, N, O [N=25 in each case]). 1 µM RA upregulated rarab expression in the anterior neuroectoderm (Fig. 7F, arrowheads; 23/25), but appeared to inhibit rarga expression (Fig. 7I; 19/22). Given its unique sensitivity to RA, raraa may provide a mechanism for amplifying RA signaling in this region of the embryo through positive feedback.
RARs and tissue sensitivity to RA
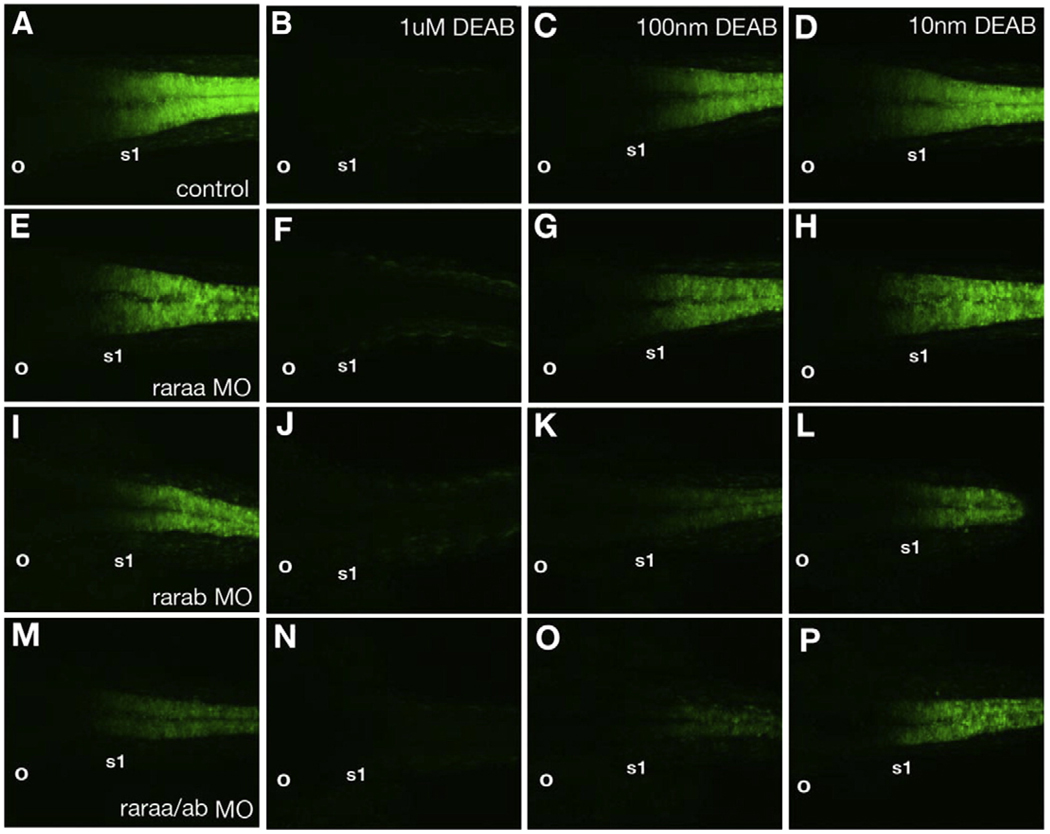
To examine RA sensitivity in more detail, we depleted each rar with MOs and simultaneously treated the embryos with DEAB, to inhibit RA synthesis (Fig. 8). These experiments were performed in transgenic zebrafish embryos expressing yellow fluorescent protein (YFP) driven by RA response elements (RARE-YFP; Perz-Edwards et al., 2001), as a direct reporter for RA signaling. At 24 hpf this labels the posterior hindbrain and spinal cord up to the r6/r7 boundary, adjacent to the first somite (s1; Fig. 8A), and expression was completely eliminated with treatments of 1 µM DEAB (Figs. 8B, F, J, N; N>10 in each case). Lower concentrations of DEAB (0.1 µM) caused a posterior shift in the domain of RARE-YFP expression, relative to the first somite (Figs. 8C, D; 9/10), and similar shifts were seen in rarab morphants (Fig. 8I; 10/11). Injection of the raraa MO caused little to no change in the severity of the defect in RARE-YFP expression caused by treatments with 0.1 µM or 0.01 µM DEAB (Figs. 8G, H; 5/5). In contrast, depletion of rarab dramatically enhanced the severity at both concentrations (Figs. 8 K, L; 6/6). raraa/rarab double morphants were similar to rarab morphants alone (Figs. 8O, P; 10/10). Thus while raraa is more strongly induced by RA, rarab is more essential in the neural tube for direct activation of an RARE. This correlates with the distinct expression patterns of the two receptors, with rarab throughout the hindbrain and raraa restricted to r7 and the anterior spinal cord where the highest levels of RA signaling are achieved.
Fig. 8.
Depletion of raraa or rarab alters sensitivity to exogenous RA. Confocal images of RARE-YFP transgenics at 20 hpf. Dorsal views, anterior to the left. (A) Controls express YFP up to an anterior border between r6 and r7 in the hindbrain (adjacent to somite 1), and in the anterior spinal cord. (B) YFP expression is lost in embryos treated with 1 µM DEAB, and reduced/shifted posteriorly at lower DEAB concentrations (C, D). (E–H) raraa morphants. Untreated morphants express YFP similar to controls (E), and lack expression when treated with 1 µM DEAB (F), but show little change in expression at lower DEAB concentrations (G, H). (I–L) rarab morphants show reduced YFP expression that shifts posteriorly (I), lack expression when treated with 1 µM DEAB (J) and show stronger reduction/shifts in expression when treated with lower DEAB concentrations than controls (K, L). (M–P) raraa/rarab double morphants also show reduced/shifted YFP expression (M), and almost completely lose expression when treated with 0.1 µMDEAB (O). Abbreviations: o, otic vesicle; s1, somite #1.
Discussion
RARs show remarkable structural and functional conservation across vertebrates. We have shown requirements for all four zebrafish RARs in the hindbrain, as well as distinct roles for rarab and rarga in the pectoral fins and pharyngeal arches, respectively. These defects resemble loss of function phenotypes in the RA signaling pathway in a variety of vertebrate model systems. In addition, we show: 1) previously unrecognized functions for RARs in the mesoderm, 2) differences between alpha and gamma RAR subtypes in their regulation by RA, and 3) divergent functions for duplicate RARs within each subtype. We propose a model (Fig. 9) in which the early expression of different RARs establishes anterior boundaries of RA responsiveness during gastrulation, both in the hindbrain and cranial mesendoderm, and later establishes a second domain of high RA signaling at the brain/spinal cord junction. This region of higher signaling also induces budding of the forelimbs.
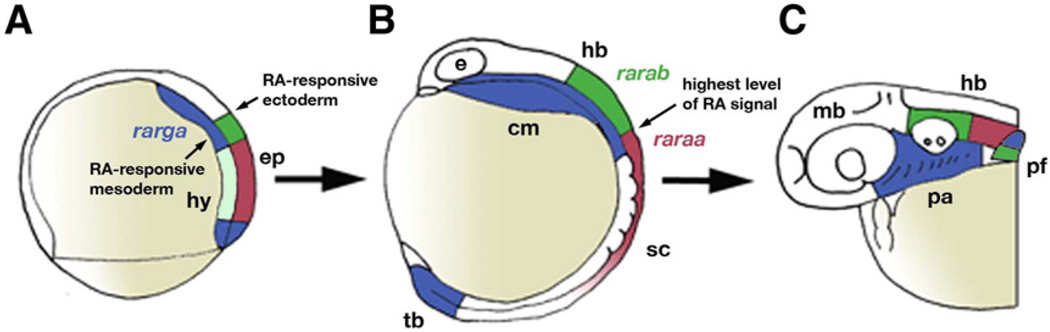
Fig. 9.
Model for roles of zebrafish RARs. Expression of raraa (red), rarab (green) and rarga (blue) at gastrula (A), 12 hpf (B) and 36 hpf (C). Lateral views, anterior to the left. (A) From the onset of their expression, raraa and rarab define anterior limits of responsiveness to RA in the ectoderm, while rarga mediates responses in cranial mesoderm that have secondary consequences for hindbrain patterning. (B) By neurula stages, raraa expression defines a second domain of stronger RA signal, with an anterior border near the head-trunk boundary. (C) rarga plays a crucial role in pharyngeal arch development, while rarab, which is expressed in the proximal pectoral fin bud, is crucial for fin budding. Abbreviations: cm, cranial mesoderm; e, eye; ep, epiblast; hb, hindbrain; hy, hypoblast; mb, midbrain; np, neural plate; pa, pharyngeal arches; pf, pectoral fin; sc, spinal cord; tb, tailbud.
Unique and redundant functions for RARs
RARs and RXRs transduce RA signals as heterodimers, which are developmentally regulated (reviewed in Mark et al., 2006). Mechanisms by which different heterodimers combine to control distinct target genes could explain many of the pleiotropic effects of RA. Our studies suggest partial redundancy both within and between subtypes of RARs. Similarly, single RAR null mutant mice are viable, while compound loss-of-function mutations in RARα/RARβ (Dupe et al., 1999) and RARα/RARγ (Wendling et al., 2001), more closely resemble an RA-deficient phenotype.
Compound mutants or morphants, however, may be incorrectly interpreted as reflecting redundancy if genes function in different cell types that contribute to the same tissues. For example, alpha and gamma subclasses of RARs in zebrafish are expressed in complementary domains in the embryo – alphas predominantly in the CNS and endoderm, gammas predominantly in mesoderm – yet both are required to pattern the hindbrain (Fig. 9). Similarly, RA signaling-deficient zebrafish (Begemann et al., 2001; Grandel et al., 2002; Kopinke et al., 2006) and mice (Lohnes et al., 1993; 1994; Mendelsohn et al., 1994a,b; Matt et al., 2003; Niederreither et al., 2003; Mark et al., 2004) lack pharyngeal arches 3–6. Expression of an RARE-lacZ reporter in mice suggests that RA signals directly to both endoderm and mesoderm that contribute to the arches (Niederreither et al., 2003). RA also signals directly to the endoderm in zebrafish (Stafford et al., 2006) and is essential for formation of segmental pouches in the pharyngeal endoderm, which may secondarily cause many of the NC defects seen in RA-deficient embryos (Wendling et al., 2000; Kopinke et al., 2006).
Embryos depleted of all four RARs with MOs have more severe defects than even the most severe vitamin A deficiencies. One explanation for this may be that RARs both activate and repress their targets, depending on cellular context. Activation is ligand-dependent, and lack of ligand can lead to excess target gene repression. Embryos treated with RAR antagonists (such as AGN used here) lose the activation of RA target-genes because the antagonist increases the affinity of co-repressors (N-CoR/Smrt) for the RAR/RXR heterodimer complex (Koide et al., 2001). These antagonists are sometimes referred to as “inverse agonists”. In contrast, MOs block RAR expression, eliminating both activating and repressing forms. Our results suggest that both functions of RARs are essential for early development.
Functions of RARs in forelimb development
RA is primarily synthesized in the paraxial (somitic) and lateral plate mesoderm (Begemann et al., 2001; Cui et al., 2003; Gibert et al., 2006) and plays important roles in somite formation (Rancourt et al., 1995). Rarab, rarga and rargb are all expressed in the LPM of the pectoral fin bud, and we have shown a central role for rarab in limb budding. To test which tissues require rarab we transplanted morphant cells into the LPM of the fin bud of wild type hosts and found cell autonomous requirements for rarab in this mesoderm. Similarly, selective depletion of raraa and rarab expression in endodermal cells in the zebrafish disrupts the specification of pancreatic beta cells (Stafford et al., 2006).
Pectoral fin development in zebrafish requires RA produced in the paraxial mesoderm (Gibert et al., 2006; Linville et al., 2004). Previous studies have suggested that this acts on adjacent mesodermal populations to induce wnt2b expression in intermediate mesoderm (IM) (Mercader et al., 2006) and tbx5 expression in the LPM. Tbx5 is required for the migration of future fin LPM into the fin bud (Ahn et al., 2002; Garrity et al., 2002; Agarwal et al., 2003; Rallis et al., 2003). We show rarab expression in the LPM but not in IM, suggesting that the LPM responds directly to RA (Fig. 6). Injection of an rarab MO eliminated fin budding and tbx5 expression, similar to aldh1a2−/− mutants (Begemann et al., 2001) and tbx5 morphants (Ahn et al., 2002). Thus, despite co-expression of several receptors in the fin bud, only rarab is required. Other receptors may act later in fin outgrowth or differentiation.
The LPM gives rise to the limb skeleton, while mesodermal cells of the hypaxial myotome form limb muscles (Ahn et al., 2002). The position of the forelimb field along the A–P axis is controlled by hox genes in the LPM, particularly hoxb5, hoxc6 and hoxc8, all of which are regulated by RA signaling. Hoxb5−/− mutant mice have defects in the shoulder girdle (Rancourt et al., 1995). In contrast, the pectoral girdle is unaffected in both tbx5 and rarab morphant zebrafish; only the fin blade fails to form (Ahn et al., 2002). This suggests that rarab is not required to specify the forelimb field, but rather maintains expression of tbx5 and promotes budding. Other RARs may mediate an earlier cascade of hox expression in the LPM that positions the fin field (Waxman and Yelon, in preparation).
Autoregulation through the rar alpha subclass and sensitivity to RA
In zebrafish, raraa requires RA for expression, but other rars are either completely RA independent or much less sensitive. In mammals, RA upregulates all three RARs, in some cases directly (De The et al., 1990; Hoffman et al., 1990; Sucov et al., 1990; Leroy et al., 1991a,b; Kamei et al., 1993; Serpente et al., 2005). Autoregulation within the pathway can impart a differential sensitivity to RA in regions where raraa is expressed (i.e. in r7 of the hindbrain and in the anterior spinal cord; Fig. 9). This domain correlates precisely with expression of the RARE-YFP transgenic reporter, which is thought to reflect sustained, high levels of RA signaling (Perz-Edwards et al., 2001).
Differential regulation of RARs may contribute to the robustness of the RA signal (White et al., 2007). By treating rar morphant zebrafish with DEAB we found that rarab has more influence on the sensitivity of the embryo to RA levels. These data also reveal functional differences between two close duplicates within the alpha subclass in zebrafish (Hale et al., 2006). In contrast, several rars in the chick require RA for their expression (Cui et al., 2003). All three mammalian RARs have alternative isoforms generated by two different promoters (P1 and P2) and alternative splicing, but only the P2 promoter in RARα and RARβ contains an RARE and is RA-regulated (Mollard et al., 2000; Mark et al., 2006).
Evolution of the RAR gene family
Recent comparative studies of the genomic organization of the RAR and RXR families in zebrafish and other vertebrates suggest that these animals lack a true RARβ receptor (Joore et al., 1994; Hale et al., 2006; Tallafuss et al., 2006). Instead, zebrafish have duplicate alpha and gamma genes. MOs targeting raraa cause hindbrain and pharyngeal defects, similar to mouse RARβ mutants. In addition, raraa expression appears relatively late during gastrulation at 8 hpf while both rarab and rarga are maternally provided (Hale et al., 2006 and unpublished results). Similarly, mouse RARα and RARγ are widely expressed at primitive streak stages (E6.5) prior to the onset of RA synthesis in both embryonic and extra-embryonic tissues, while RARβ expression is not detected until E7.5 in the neuroectoderm and adjacent mesenchyme (Ang and Duester, 1997). raraa is also the only zebrafish rar that is not expressed in the forelimbs, similar to mouse RARβ. Thus, based on its expression and function in zebrafish, raraa appears to have arisen as a recent duplicate in teleosts that has subsequently acquired a beta-like function.
RARs and their roles in A–P patterning appear to have arisen in the deuterostome lineage and are absent in protostomes (e.g. nematodes and arthropods) (Marletaz et al., 2006). Studies of comparative ligand-binding as well as expression in non-vertebrate chordates, such as amphioxus, suggests that the ancestral RAR was most closely related to the RARbeta subclass (Escriva et al., 2006). Its expression defines a region of competence that extends throughout the presumptive hindbrain region. This combined with localized degradation of RA anteriorly by the Cyp26 enzymes may have led to the emergence of the ancestral A–P patterning system in which RA signaling is graded from posterior to anterior (White et al., 2007). The incorporation of additional RARs into the A–P patterning system in vertebrates with different boundaries of expression and RA sensitivities, may have been crucial in the evolution of the sharp boundaries of gene expression that define the segmental organization of the hindbrain and pharyngeal arches.
Supplementary Material
Acknowledgments
We would like to thank members of the Schilling lab for comments on the manuscript. This work was supported by NIH grant R01NS41353.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ydbio.2008.09.022.
References
- Agarwal P, Wylie JN, Galceran J, Arkhitko O, Li C, Deng C, Grosschedl R, Bruneau BG. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. doi: 10.1242/dev.00191. [DOI] [PubMed] [Google Scholar]
- Ahn DG, Kourakis MJ, Rohde LA, Silver LM, Ho RK. T-box gene tbx5 is essential for formation of the pectoral limb bud. Nature. 2002;417:754–758. doi: 10.1038/nature00814. [DOI] [PubMed] [Google Scholar]
- Ang HL, Duester G. Initiation of retinoid signaling in primitive streak mouse embryos: spatiotemporal expression patterns of receptors and metabolic enzymes for ligand synthesis. Dev. Dyn. 1997;208:536–543. doi: 10.1002/(SICI)1097-0177(199704)208:4<536::AID-AJA9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Begemann G, Ingham PW. Developmental regulation of Tbx5 in zebrafish embryogenesis. Mech. Dev. 2000;90:299–304. doi: 10.1016/s0925-4773(99)00246-4. [DOI] [PubMed] [Google Scholar]
- Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- Bruce AE, Oates AC, Prince VE, Ho RK. Additional hox clusters in the zebrafish: divergent expression patterns belie equivalent activities of duplicate hoxB5 genes. Evol. Dev. 2001;3:127–144. doi: 10.1046/j.1525-142x.2001.003003127.x. [DOI] [PubMed] [Google Scholar]
- Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Ann. Rev. Nutr. 2002;22:347–381. doi: 10.1146/annurev.nutr.22.010402.102745E. [DOI] [PubMed] [Google Scholar]
- Cui J, Michaille JJ, Jiang W, Zile MH. Retinoid receptors and vitamin A deficiency: differential patterns of transcription during early avian development and the rapid induction of RARs by retinoic acid. Dev. Biol. 2003;260:496–511. doi: 10.1016/s0012-1606(03)00257-4. [DOI] [PubMed] [Google Scholar]
- De The H, Vivanco-Ruiz MM, Tiollais P, Stunnenberg H, Dejean A. Identification of a retinoic acid response element in the retinoic acid receptor beta gene. Nature. 1990;343:177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- Dupe V, Lumsden A. Hindbrain patterning involves graded responses to retinoic acid signalling. Development. 2001;128:2199–2208. doi: 10.1242/dev.128.12.2199. [DOI] [PubMed] [Google Scholar]
- Dupe V, Ghyselinck NB, Wendling O, Chambon P, Mark M. Key roles of retinoic acid receptors alpha and beta in the patterning of the caudal hindbrain, pharyngeal arches and otocyst in the mouse. Development. 1999;126:5051–5059. doi: 10.1242/dev.126.22.5051. [DOI] [PubMed] [Google Scholar]
- Durston AJ, Timmermans JPM, Hage WJ, Hendriks HFJ, De Vries NJ, Heideveld M, Nieuwkoop P. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989;340:140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Escriva H, Bertrand S, Germain P, Robinson-Rechavi M, Umbhauer M, Cartry J, Duffraisse M, Holland L, Gronemeyer H, Laudet V. PLoS Genet. 2006;2:e102. doi: 10.1371/journal.pgen.0020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity DM, Childs S, Fishman MC. The heartstrings mutation in zebrafish causes heart/fin Tbx5 deficiency syndrome. Development. 2002;129:4635–4645. doi: 10.1242/dev.129.19.4635. [DOI] [PubMed] [Google Scholar]
- Gibert Y, Gajewski A, Meyer A, Begemann G. Induction and prepatterning of the zebrafish pectoral fin bud requires axial retinoic acid signaling. Development. 2006;133:2649. doi: 10.1242/dev.02438. [DOI] [PubMed] [Google Scholar]
- Glover JC, Renaud JS, Rijli FM. Retinoic acid and hindbrain patterning. J. Neurobiol. 2006;66:705–725. doi: 10.1002/neu.20272. [DOI] [PubMed] [Google Scholar]
- Grandel H, Lun K, Rauch GJ, Rhinn M, Piotrowski T, Houart C, Sordino P, Kuchler AM, Schulte-Merker S, Geisler R, Holder N, Wilson SW, Brand M. Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development. 2002;129:2851–2865. doi: 10.1242/dev.129.12.2851. [DOI] [PubMed] [Google Scholar]
- Hale LA, Tallafuss A, Yan YL, Dudley L, Eisen JS, Postlethwait JH. Characterization of the retinoic acid receptor genes raraa, rarab and rarg during zebrafish development. Gene. Expr. Patterns. 2006;6:546–555. doi: 10.1016/j.modgep.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, Lehmann JM, Zhang XK, Hermann T, Husmann M, Graupner G, Pfahl M. The retinoic acid receptor-specific element controls the retinoic acid receptor-beta promoter. Mol. Endocrinol. 1990;4:1727–1736. doi: 10.1210/mend-4-11-1727. [DOI] [PubMed] [Google Scholar]
- Javidan Y, Schilling TF. Development of cartilage and bone. Meth. Cell. Biol. 2004;76:415–436. doi: 10.1016/s0091-679x(04)76018-5. [DOI] [PubMed] [Google Scholar]
- Joore J, van der Lans GB, Lanser PH, Vervaart JM, Zivkovic D, Speksnijder JE, Kruijer W. Effects of retinoic acid on the expression of retinoic acid receptors during zebrafish embryogenesis. Mech. Dev. 1994;46:137–150. doi: 10.1016/0925-4773(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Kamei Y, Kawada T, Kazuki R, Sugimoto E. Retinoic acid receptor gamma 2 gene expression is upregulated by retinoic acid in 3T3-L1 preadipocytes. Biochem. J. 1993;293:807–812. doi: 10.1042/bj2930807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide T, Downes M, Chandraratna RA, Blumberg B, Umesono K. Active repression of RAR signaling is required for head formation. Genes Dev. 2001;15:2111–2121. doi: 10.1101/gad.908801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopinke D, Sasine J, Swift J, Stephens WZ, Piotrowski T. Retinoic acid is required for endodermal pouch morphogenesis and not for pharyngeal endoderm specification. Dev. Dyn. 2006;235:2695–2709. doi: 10.1002/dvdy.20905. [DOI] [PubMed] [Google Scholar]
- Lee GS, Kochhar DM, Collins MD. Retinoid-induced limb malformations. Curr. Pharm. Des. 2004;10:2657–2699. doi: 10.2174/1381612043383728. [DOI] [PubMed] [Google Scholar]
- Leroy P, Krust A, Zelent A, Mendelsohn C, Garnier JM, Kastner P, Dierich A, Chambon P. Multiple isoforms of the mouse retinoic acid receptor alpha are generated by alternative splicing and differential induction by retinoic acid. EMBO J. 1991a;10:59–69. doi: 10.1002/j.1460-2075.1991.tb07921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy P, Nakshatri H, Chambon P. Mouse retinoic acid receptor alpha 2 isoform is transcribed from a promoter that contains a retinoic acid response element. Proc. Natl. Acad. Sci. U.S.A. 1991b;88:1110138–1110142. doi: 10.1073/pnas.88.22.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E, Sucov HM, Lee KF, Evans RM, Jaenisch R. Normal development and growth of mice carrying a targeted disruption of the alpha 1 retinoic acid receptor gene. Proc. Natl. Acad. Sci. U. S. A. 1993;90:1590–1594. doi: 10.1073/pnas.90.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linville A, Gumusaneli E, Chandraratna RA, Schilling TF. Independent roles for retinoic acid in segmentation and neuronal differentiation in the zebrafish hindbrain. Dev. Biol. 2004;270:186–199. doi: 10.1016/j.ydbio.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Kastner P, Dierich A, Mark M, LeMeur M, Chambon P. Function of retinoic acid receptor gamma in the mouse. Cell. 1993;73:643–658. doi: 10.1016/0092-8674(93)90246-m. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- Luo J, Sucov HM, Bader JA, Evans RM, Giguere V. Compound mutants for retinoic acid receptor (RAR) beta and RAR alpha 1 reveal developmental functions for multiple RAR beta isoforms. Mech. Dev. 1996;55:33–44. doi: 10.1016/0925-4773(95)00488-2. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat. Rev. Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Maden M, Gale E, Kostetskii I, Zile M. Vitamin A-deficient quail embryos have half a hindbrain and other neural defects. Curr. Biol. 1996;6:417–426. doi: 10.1016/s0960-9822(02)00509-2. [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Retinoic acid signalling in the development of branchial arches. Curr. Opin. Genet. Dev. 2004;14:591–598. doi: 10.1016/j.gde.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu. Rev. Pharmacol. Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- Marletaz F, Holland LZ, Laudet V, Schubert M. Retinoic acid signaling and the evolution of chordates. Int. J. Biol. Sci. 2006;2:38–47. doi: 10.7150/ijbs.2.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt N, Ghyselinck NB, Wendling O, Chambon P, Mark M. Retinoic acid-induced developmental defects are mediated by RARbeta/RXR heterodimers in the pharyngeal endoderm. Development. 2003;130:2083–2093. doi: 10.1242/dev.00428. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Mark M, Dolle P, Dierich A, Gaub MP, Krust A, Lampron C, Chambon P. Retinoic acid receptor beta 2 (RAR beta 2) null mutant mice appear normal. Dev. Biol. 1994a;166:246–258. doi: 10.1006/dbio.1994.1311. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994b;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Mercader N, Fischer S, Neumann CJ. Prdm1 acts downstream of a sequential RA, Wnt and Fgf signaling cascade during zebrafish forelimb induction. Development. 2006;133:2805–2815. doi: 10.1242/dev.02455. [DOI] [PubMed] [Google Scholar]
- Moens CB, Cordes SP, Giorgianni MW, Barsh GS, Kimmel CB. Equivalence in the genetic control of hindbrain segmentation in fish and mouse. Development. 1998;125:381–391. doi: 10.1242/dev.125.3.381. [DOI] [PubMed] [Google Scholar]
- Mollard R, Viville S, Ward SJ, Decimo D, Chambon P, Dolle P. Tissue-specific expression of retinoic acid receptor isoform transcripts in the mouse embryo. Mech. Dev. 2000;94:223. doi: 10.1016/s0925-4773(00)00303-8. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development. 2000;127:75–85. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development. 2002;129:3563–3574. doi: 10.1242/dev.129.15.3563. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Le Roux I, Schuhbaur B, Chambon P, Dolle P. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development. 2003;130:2525–2534. doi: 10.1242/dev.00463. [DOI] [PubMed] [Google Scholar]
- Oxtoby E, Jowett T. Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 1993;21:1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perz-Edwards A, Hardison NL, Linney E. Retinoic acid-mediated gene expression in transgenic reporter zebrafish. Dev. Biol. 2001;229:89–101. doi: 10.1006/dbio.2000.9979. [DOI] [PubMed] [Google Scholar]
- Plaster N, Sonntag C, Busse CE, Hammerschmidt M. p53 deficiency rescues apoptosis and differentiation of multiple cell types in zebrafish flathead mutants deficient for zygotic DNA polymerase delta1. Cell Death Differ. 2006;13:223–235. doi: 10.1038/sj.cdd.4401747. [DOI] [PubMed] [Google Scholar]
- Prince VE, Moens CB, Kimmel CB, Ho RK. Zebrafish hox genes: expression in the hindbrain region of wild-type and mutants of the segmentation gene, valentino. Development. 1998;125:393–406. doi: 10.1242/dev.125.3.393. [DOI] [PubMed] [Google Scholar]
- Rallis C, Bruneau BG, Del Buono J, Seidman CE, Seidman JG, Nissim S, Tabin CJ, Logan MP. Tbx5 is required for forelimb bud formation and continued outgrowth. Development. 2003;130:2741–2751. doi: 10.1242/dev.00473. [DOI] [PubMed] [Google Scholar]
- Rancourt DE, Tsuzuki T, Capecchi MR. Genetic interaction between hoxb-5 and hoxb-6 is revealed by nonallelic noncomplementation. Genes Dev. 1995;9:108–122. doi: 10.1101/gad.9.1.108. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Kimmel CB. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development. 1994;120:483–494. doi: 10.1242/dev.120.3.483. [DOI] [PubMed] [Google Scholar]
- Serpente P, Tumpel S, Ghyselinck NB, Niederreither K, Wiedemann LM, Dolle P, Chambon P, Krumlauf R, Gould AP. Direct crossregulation between retinoic acid receptor beta and Hox genes during hindbrain segmentation. Development. 2005;132:503–513. doi: 10.1242/dev.01593. [DOI] [PubMed] [Google Scholar]
- Stafford D, White RJ, Kinkel MD, Linville A, Schilling TF, Prince VE. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development. 2006;133:949–956. doi: 10.1242/dev.02263. [DOI] [PubMed] [Google Scholar]
- Sucov HM, Murakami KK, Evans RM. Characterization of an autoregulated response element in the mouse retinoic acid receptor type beta gene. Proc. Natl. Acad. Sci. U.S.A. 1990;87:5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarayan V, Kastner P, Mark M, Dierich A, Gorry P, Chambon P. Limited specificity and large overlap of the functions of the mouse RAR gamma 1 and RAR gamma 2 isoforms. Mech. Dev. 1997;66:131–142. doi: 10.1016/s0925-4773(97)00098-1. [DOI] [PubMed] [Google Scholar]
- Tallafuss A, Hale LA, Yan YL, Dudley L, Eisen JS, Postlethwait JH. Characterization of retinoid-X receptor genes rxra, rxrba, rxrbb, and rxrg during zebrafish development. Gene. Expr. Patterns. 2006;6:556–565. doi: 10.1016/j.modgep.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Taneja R, Bouillet P, Boylan JF, Gaub MP, Roy B, Gudas LJ, Chambon P. Reexpression of retinoic acid receptor (RAR) gamma or overexpression of RAR alpha or RAR beta in RAR gamma-null F9 cells reveals a partial functional redundancy between the three RAR types. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7854–7858. doi: 10.1073/pnas.92.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- Trevarrow B, Marks DL, Kimmel CB. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990;4:669–679. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- Waxman JS, Yelon D. Comparison of the expression patterns of newly identified zebrafish retinoic acid and retinoid X receptors. Dev. Dyn. 2007;236:587–595. doi: 10.1002/dvdy.21049. [DOI] [PubMed] [Google Scholar]
- Wendling O, Dennefeld C, Chambon P, Mark M. Retinoid signaling is essential for patterning the endoderm of the third and fourth pharyngeal arches. Development. 2000;127:1553–1562. doi: 10.1242/dev.127.8.1553. [DOI] [PubMed] [Google Scholar]
- Wendling O, Ghyselinck NB, Chambon P, Mark M. Roles of retinoic acid receptors in early embryonic morphogenesis and hindbrain patterning. Development. 2001;128:2031–2038. doi: 10.1242/dev.128.11.2031. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. U Oregon Press; 1998. [Google Scholar]
- White R, Nie Q, Lander AD, Schilling TF. Complex regulation of Cyp26a1 creates a robust retinoic acid signaling gradient in the zebrafish embryo. PLoS. Biol. 2007;5(11):e304. doi: 10.1371/journal.pbio.0050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ES, Allende ML, Kelly CS, Abdelhamid A, Murakami T, Andermann P, Doerre OG, Grunwald DJ, Riggleman B. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development. 1996;122:271–280. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.