Abstract
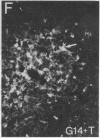
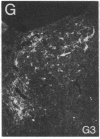
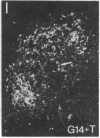
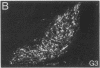
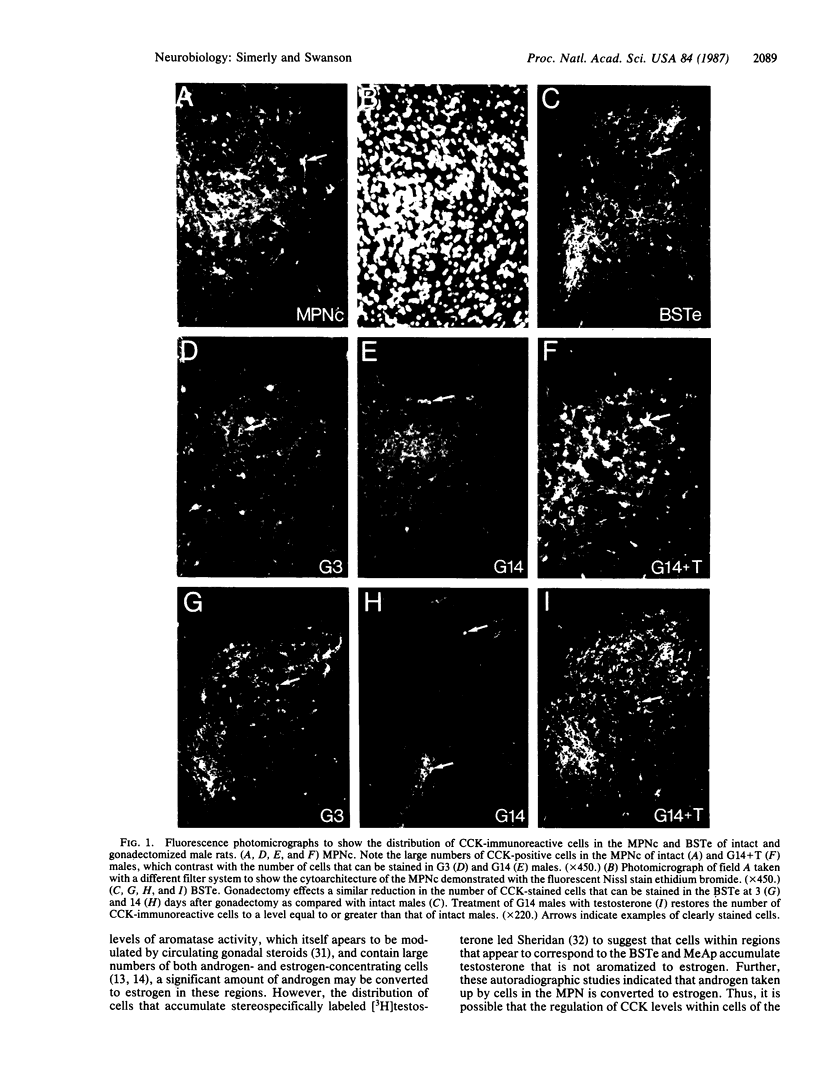
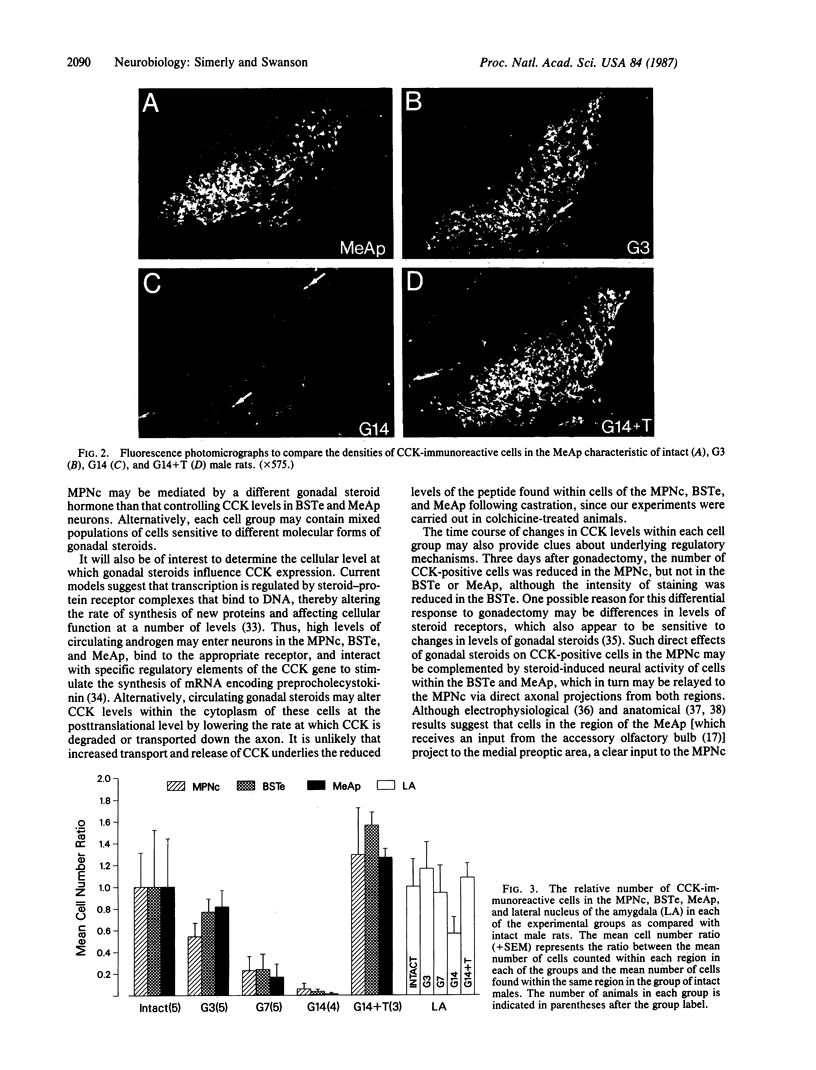
Three sexually dimorphic cell groups in the forebrain of the rat--the central part of the medial preoptic nucleus, the encapsulated part of the bed nucleus of the stria terminalis, and the posterodorsal part of the medial nucleus of the amygdala--are larger in males, contain a high density of gonadal-steroid-concentrating cells, and are thought to play important roles in the control of reproductive behavior and physiology. Since each of these regions contains a large number of cholecystokinin-immunoreactive cells, we used an indirect immunohistochemical method to examine the possibility that levels of this peptide are modulated by circulating gonadal steroids in adult male rats. Rats were castrated at 60 days of age, and one group each was pretreated with colchicine and then killed 3, 7, and 14 days after gonadectomy. Castration clearly decreased CCK immunoreactivity within cells of each region, with the most dramatic effects occurring 7 and 14 days after gonadectomy, and these effects were reversed by treatment with testosterone over a 14-day period. The results suggest that CCK levels within individual cells in each of the interconnected sexually dimorphic nuclei examined here are regulated by circulating gonadal steroids and may be related to the hormonal modulation of reproductive functions thought to be mediated by these cell groups.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beltramino C., Taleisnik S. Dual action of electrochemical stimulation of the bed nucleus of the stria terminalis on the release of LH. Neuroendocrinology. 1980 Apr;30(4):238–242. doi: 10.1159/000123007. [DOI] [PubMed] [Google Scholar]
- Chambon P., Dierich A., Gaub M. P., Jakowlev S., Jongstra J., Krust A., LePennec J. P., Oudet P., Reudelhuber T. Promoter elements of genes coding for proteins and modulation of transcription by estrogens and progesterone. Recent Prog Horm Res. 1984;40:1–42. doi: 10.1016/b978-0-12-571140-1.50005-0. [DOI] [PubMed] [Google Scholar]
- De Vries G. J., Buijs R. M., Van Leeuwen F. W. Sex differences in vasopressin and other neurotransmitter systems in the brain. Prog Brain Res. 1984;61:185–203. doi: 10.1016/S0079-6123(08)64435-0. [DOI] [PubMed] [Google Scholar]
- DeVries G. J., Buijs R. M., Van Leeuwen F. W., Caffé A. R., Swaab D. F. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985 Mar 8;233(2):236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- Dees W. L., Kozlowski G. P. Effects of castration and ethanol on amygdaloid substance P immunoreactivity. Neuroendocrinology. 1984 Sep;39(3):231–235. doi: 10.1159/000123984. [DOI] [PubMed] [Google Scholar]
- Fink G. Feedback actions of target hormones on hypothalamus and pituitary with special reference to gonadal steroids. Annu Rev Physiol. 1979;41:571–585. doi: 10.1146/annurev.ph.41.030179.003035. [DOI] [PubMed] [Google Scholar]
- Frankfurt M., Siegel R. A., Sim I., Wuttke W. Estrous cycle variations in cholecystokinin and substance P concentrations in discrete areas of the rat brain. Neuroendocrinology. 1986;42(3):226–231. doi: 10.1159/000124444. [DOI] [PubMed] [Google Scholar]
- Gorski R. A. The 13th J. A. F. Stevenson memorial lecture. Sexual differentiation of the brain: possible mechanisms and implications. Can J Physiol Pharmacol. 1985 Jun;63(6):577–594. doi: 10.1139/y85-098. [DOI] [PubMed] [Google Scholar]
- Handa R. J., Reid D. L., Resko J. A. Androgen receptors in brain and pituitary of female rats: cyclic changes and comparisons with the male. Biol Reprod. 1986 Mar;34(2):293–303. doi: 10.1095/biolreprod34.2.293. [DOI] [PubMed] [Google Scholar]
- Hines M., Davis F. C., Coquelin A., Goy R. W., Gorski R. A. Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: a description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci. 1985 Jan;5(1):40–47. doi: 10.1523/JNEUROSCI.05-01-00040.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevetter G. A., Winans S. S. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the "vomeronasal amygdala". J Comp Neurol. 1981 Mar 20;197(1):81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kimura F., Hashimoto R., Kawakami M. The stimulatory effect of cholecystokinin implanted in the medial preoptic area on luteinizing hormone secretion in the ovariectomized estrogen-primed rat. Endocrinol Jpn. 1983 Jun;30(3):305–309. doi: 10.1507/endocrj1954.30.305. [DOI] [PubMed] [Google Scholar]
- Krettek J. E., Price J. L. A description of the amygdaloid complex in the rat and cat with observations on intra-amygdaloid axonal connections. J Comp Neurol. 1978 Mar 15;178(2):255–280. doi: 10.1002/cne.901780205. [DOI] [PubMed] [Google Scholar]
- Krettek J. E., Price J. L. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978 Mar 15;178(2):225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- Mendelson S. D., Gorzalka B. B. Cholecystokinin-octapeptide produces inhibition of lordosis in the female rat. Pharmacol Biochem Behav. 1984 Nov;21(5):755–759. doi: 10.1016/s0091-3057(84)80015-5. [DOI] [PubMed] [Google Scholar]
- Micevych P. E., Park S. S., Akesson T. R., Elde R. Distribution of cholecystokinin-immunoreactive cell bodies in the male and female rat: I. Hypothalamus. J Comp Neurol. 1987 Jan 1;255(1):124–136. doi: 10.1002/cne.902550110. [DOI] [PubMed] [Google Scholar]
- Mizukami S., Nishizuka M., Arai Y. Sexual difference in nuclear volume and its ontogeny in the rat amygdala. Exp Neurol. 1983 Feb;79(2):569–575. doi: 10.1016/0014-4886(83)90235-2. [DOI] [PubMed] [Google Scholar]
- Pfaff D., Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol. 1973 Sep 15;151(2):121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Post S., Mai J. K. Contribution to the amygdaloid projection field in the rat. A quantitative autoradiographic study. J Hirnforsch. 1980;21(2):199–225. [PubMed] [Google Scholar]
- Roselli C. E., Horton L. E., Resko J. A. Distribution and regulation of aromatase activity in the rat hypothalamus and limbic system. Endocrinology. 1985 Dec;117(6):2471–2477. doi: 10.1210/endo-117-6-2471. [DOI] [PubMed] [Google Scholar]
- Scalia F., Winans S. S. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975 May 1;161(1):31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Sheridan P. J. The nucleus interstitialis striae terminalis and the nucleus amygdaloideus medialis: prime targets for androgen in the rat forebrain. Endocrinology. 1979 Jan;104(1):130–136. doi: 10.1210/endo-104-1-130. [DOI] [PubMed] [Google Scholar]
- Simerly R. B., Gorski R. A., Swanson L. W. Neurotransmitter specificity of cells and fibers in the medial preoptic nucleus: an immunohistochemical study in the rat. J Comp Neurol. 1986 Apr 15;246(3):343–363. doi: 10.1002/cne.902460305. [DOI] [PubMed] [Google Scholar]
- Simerly R. B., Swanson L. W., Gorski R. A. Demonstration of a sexual dimorphism in the distribution of serotonin-immunoreactive fibers in the medial preoptic nucleus of the rat. J Comp Neurol. 1984 May 10;225(2):151–166. doi: 10.1002/cne.902250202. [DOI] [PubMed] [Google Scholar]
- Simerly R. B., Swanson L. W., Gorski R. A. Reversal of the sexually dimorphic distribution of serotonin-immunoreactive fibers in the medial preoptic nucleus by treatment with perinatal androgen. Brain Res. 1985 Aug 5;340(1):91–98. doi: 10.1016/0006-8993(85)90777-2. [DOI] [PubMed] [Google Scholar]
- Simerly R. B., Swanson L. W., Handa R. J., Gorski R. A. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985 Jun;40(6):501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- Simerly R. B., Swanson L. W. The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol. 1986 Apr 15;246(3):312–342. doi: 10.1002/cne.902460304. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Cowan W. M. The connections of the septal region in the rat. J Comp Neurol. 1979 Aug 15;186(4):621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- Vijayan E., Samson W. K., McCann S. M. In vivo and in vitro effects of cholecystokinin on gonadotropin, prolactin, growth hormone and thyrotropin release in the rat. Brain Res. 1979 Aug 24;172(2):295–302. doi: 10.1016/0006-8993(79)90539-0. [DOI] [PubMed] [Google Scholar]