Abstract
The systemic inflammatory response syndrome (SIRS) often accompanies critical illnesses and can be an important cause of morbidity and mortality. Marked abnormalities in cardiovascular function accompany acute illnesses manifested as sustained tachyarrhythmias which are but one component of systemic dysregulation. The realization that cardiac pacemaker activity is under control of the autonomic nervous system has promoted the analysis of heart rate variation for assessing autonomic activities. In acute illnesses, autonomic imbalance manifesting in part as parasympathetic attenuation is associated with increased morbidity in patients who manifest SIRS phenotype. Driven by the premise that biological phenotypes emerge as the outcome of the coordinated action of network elements across the host, a multiscale model of human endotoxemia, as a prototype model of systemic inflammation in humans, is developed that quantifies critical aspects of the complex relationship between inflammation and autonomic heart rate regulation. In the present study, changes in heart rate response to acute injury, phenotypically expressed as tachycardia, are simulated as a result of autonomic imbalance that reflects sympathetic activity excess and parasympathetic attenuation. The proposed model assesses both the anti-inflammatory and cardiovascular effects of antecedent stresses upon the systemic inflammatory manifestations of human endotoxemia as well as a series of non-linear inflammatory relevant scenarios. Such a modeling approach provides a comprehensive conceptual framework linking inflammation and physiological complexity via a multiscale model that may advance the translational potential of systems modeling in clinical research.
Keywords: mathematical modeling, infection, humans, inflammation, autonomic nervous system, heart
Introduction
Inflammation is the body’s initial response to acute biological stress such as bacterial infection or tissue trauma that involves both a simultaneous local reaction and a systemic response (1). This response, often termed the systemic inflammatory response syndrome (SIRS), involves a complex network of amplifying and down-regulating signals mediated by a large array of cells and molecules that sense and control homeostatic perturbations. However, when this balance between pro- and anti-inflammatory “forces” is lost, inflammation becomes prolonged and sequelae of such immunologic dissonance include various chronic disease conditions and adverse outcomes (2). It is therefore a dysregulation of the inflammatory resolution that, in many cases, can turn what is normally a beneficial reparative process into a harmful state for the host.
Mechanisms regulating the inflammatory process involve an extensive network of multiscale interactions between the immune and the central nervous system (CNS) (3). The neuro-immune crosstalk is comprised of a descending pathway that links CNS to peripheral immune tissues and a parallel afferent arm linking the immune system with the CNS. The integrity of this loop allows for communication between the CNS and the peripheral immune system, integrating neuronal and immune signals in the periphery as well as in the CNS. In particular, the hypothalamic-pituitary adrenal axis (HPA) and the sympathetic nervous system (SNS) are the primary stress response pathways by which CNS regulates the immune response. Further, the parasympathetic division of the autonomic nervous system (ANS) also monitors and regulates inflammation (4). Such bidirectional communication pathways between the nervous, the endocrine and the immune system are essential components of the integrated homeostatic network of the primary host response and, as such, a dysregulation of autonomic function may predispose a host to excessive inflammatory responses (5).
The concept that sinus node pacemaker activity is under control of the autonomic nervous system has promoted the use of heart rate variability to quantify the cardiac autonomic input and evaluate autonomic modulation of the sinus node (6). Normal heartbeats originate from the sinoatrial (SA) node which is innervated by vagal (parasympathetic) and sympathetic fibers and thus ANS plays a pivotal role in heart rate regulation. Analysis of heart rate variation (HRV) has been widely used for assessing the activities of the autonomic nervous system and as a marker of severity of illness across several clinical disease conditions (7). HRV is measured by assessing several expressions of electrocardiogram (R-R) interval variability, including both time-domain and various frequency-domain spectral analyses that may specifically reflect changes of sympathetic and vagal activities to the heart. For instance, time-domain parameters such as the percentage of adjacent normal heart beats that differ by > 50 ms (PNN50) reflect parasympathetic influences on the heart. Such a statistical measure is strongly correlated to the high frequency component of HRV (~0.15–0.4 Hz) which is also considered to be an index of vagal tone as well as to the standard deviation of normal interbeat intervals (SDNN) that reflects overall heart rate variability (6). On the other hand, the low frequency range of HRV (0.04~0.15 Hz) tends to reflect the combined effects of sympathetic and vagal controls.
These clinical measures of heart rate variability are non-invasive assessments that may reflect real-time alterations of physiologic status. In general, autonomic dysregulation occurring in acute illnesses, is manifested as parasympathetic attenuation and diminished physiologic variability, as revealed by reduced PNN50 and SDNN indices, respectively, indicating a striking relationship of very early diminution in parameters of HRV to the later adverse outcome of critically ill patients (8). In addition to this, cardiovascular abnormalities in association with a hyperdynamic state characterized by increased cardiac index and heart rate (HR) have been also documented during the early flow phase of injury and infection.
The acute systemic inflammatory condition mediated by endotoxin (lipopolysaccharide [LPS]) administration in healthy volunteers also elicits a hemodynamic response associated with increases in heart rate and a transient decrease in cardiac vagal tone (9). Endotoxin is one of the principal components of the outer membrane of gram-negative bacteria, and the inflammation caused by the activation of the innate immune system by this moiety alters many systemic physiologic processes in a qualitatively similar manner with acute illnesses (10). The response following intravenous endotoxin administration in human subjects include core temperature, cardiac, vasomotor, hematologic, metabolic, hormonal, acute phase reactant, and cytokine components.
In an effort to establish quantifiable relationships among these various components, we have previously proposed in silico models of human endotoxemia as prototype models of acute inflammation in humans, of increasing complexity (11–13). Mathematical modeling is increasingly being used to address biological complexity and we thereby developed semi-mechanistic based host response models that integrate essential regulatory processes across the host linking the initiating signal (LPS) with transcriptional dynamics, signaling cascades, and physiological (hormonal) components. We are driven by the premise that such models can yield significant insights into how macroscopic phenotypic observations of a system emerge as the outcome of orchestrated interactions of critical network modules; thus advancing the translation of knowledge from basic research into an integrated framework sufficient to predict system behavior in the form of disturbances across an intricate web of interacting elements.
The present study describes a continuation of this effort, with the attempt to quantify essential aspects of the autonomic control of heart rate regulation. During the progression of endotoxin induced inflammation, disruptions in autonomic cardiac control are reflected by alterations in the joint activity of sympathetic and parasympathetic arms of ANS which thereby influence the intrinsic pacemaker activity (heart rate). Predicated upon this, the proposed model intends to describe changes in heart rate response to the prototypical inflammatory stimulus (endotoxin) as a result of altered autonomic activity, incorporating explicitly the relative contribution of efferent branches of the autonomic nervous system (sympathetic/parasympathetic outflow). Specifically, we expanded our prior modeling work to include physicochemical interactions related to the release, binding and degradation of cardiac neurotransmitters occurring at the systemic level of the sinus node of the heart. Of particular relevance to this study are human data associated with a constrained cardiovascular response to the endotoxin paradigm including vital signs such as heart rate measurements and parameters of heart rate variability, namely PNN50. Such response is phenotypically expressed as tachycardia which resolves within 24hours and is simulated as a result of increased efferent sympathetic activity and reduced parasympathetic response.
The dynamics of the system are characterized by twenty-six (26) variables and the validity of the proposed physiology-based human inflammation model is demonstrated through its potential to reproduce biologically relevant situations described as the following scenarios: (i) a self-limited inflammatory response evoked by low dose endotoxin corresponding to successful resolution of all clinical manifestations within 24hr after LPS exposure; (ii) cardiovascular implications of antecedent stress (i.e. acute epinephrine infusion) upon the systemic inflammatory manifestations of human endotoxemia. Prior experimental studies demonstrate that catecholamine excess, as produced by 3hr infusion before LPS challenge attenuates not only the pro-inflammatory manifestations of human endotoxemia but also vagus nerve activity followed by tachycardia (14); (iii) dose-dependent effects of acute endotoxin injury on the neuroendocrine immune axis and finally (iv) scenarios associated with systematic perturbations that modulate the host dynamics towards either an irreversible response or in favor of a balanced immune response depending on the anti-inflammatory “reservoir” of the host relative to the intense (pro) inflammatory response elicited under high doses of LPS. Such scenarios explore the importance of dynamic anti-inflammation during the course of unremitting inflammation in balancing neuro-immunologic dissonance promoting inflammatory resolution manifested by cardiovascular homeostasis. Hence, we opt to assess the qualitative behavior of our model by simulating a series of non-linear host responses to endotoxin injury that could potentially provide invaluable insights into the complex relationship between injury and cardiac dysfunction within the context of antecedent stresses occurring during critical illnesses.
It is the goal of this study to demonstrate the feasibility of a semi-mechanistic and physiology-based model of human inflammation that captures essential features of the multiscale nature of the response. Such a modeling approach integrates central influences between autonomic control systems and physiological inflammatory components making it a critical enabler for clarifying how cellular events and inflammatory processes mediate the links between patterns of autonomic activities and adverse clinical outcomes; thereby advancing the translational potential of systems modeling in clinical research.
Materials and Methods
Experimental models of human endotoxemia and data collection
A great deal about the initial human inflammatory response to infectious challenges has been learned from the elective administration of endotoxin (9). Using the human endotoxin challenge model (CC-RE, Lot #2 at a dose of 2-ng/kg body weight [BW]), vital signs including heart rate and parameters of heart rate variability (HRV), namely pNN50, were recorded (14, 15). Specifically, heart rate was recorded every 30 minutes from the arterial monitoring system for the first 6 hours following endotoxin challenge and at 24 hours after LPS administration. The determination of HRV indices was obtained using a continuous electrocardiography technique (ECG).
In a continuous ECG record, each QRS complex (resulting from sinus node depolarization) was detected and one of the time-domain measures, which analyzed in this study, included the percentage of interval differences of successive interbeat intervals greater than 50 milliseconds (pNN50). This time domain statistic reflects the occurrence of large changes between adjacent heart beats and it serves as surrogate for parasympathetic influences on the heart (vagal function) (7). In addition to time-domain analyses, spectral methods have also been employed to describe parasympathetic modulation of the sinus node. For instance, high frequency variability (HF) (0.15–0.4 Hz), the usual statistic for assessing respiratory sinus arrhythmia (RSA), correlates with parasympathetic and vagal tone. However, respiration rate, oftentimes, falls outside the high frequency range during the post-LPS administration and pNN50 can be used as an alternative method for assessing parasympathetic efferent activity. Thus, in this study among the various HRV indices, the time-domain measure pNN50 was used to assess vagus nerve activity and parasympathetic influences on the sinus node pacemaker activity.
Multiscale physicochemical models of systemic inflammation in humans
The administration of a low dose of endotoxin (LPS) to human subjects elicits significant dynamic transcriptional changes as well as hemodynamic and neuroendocrine responses that mimic those observed in acute injury and early sepsis (10). In an effort to establish quantifiable relationships among these components, a multiscale physicochemical host response model is developed (11–13) that addresses the following unique aspects: (i) identification of the essential responses characterizing the cellular (leukocyte) transcriptional dynamics in response to endotoxin administration.; (ii) reverse engineering of connectivity and interaction dynamics of these elements exploring the concept of physicochemical and (iii) indirect response (IDR) modeling that connect extracellular signals and intracellular signaling cascades leading to the emergent transcriptional dynamics and finally; (iv) multiscale, physiology-based modeling that quantifies critical aspects of the neuroendocrine-immune crosstalk and assesses systemic disruptions manifested by diminished physiologic variability (HRV). All the interacting components and the associated equations defining the dynamics of the host are succinctly presented in equations (1)–(7).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
The propagation of LPS signaling mediated by the activation of endotoxin signaling receptor and elementary pro-inflammatory pathways (i.e. NF-kB signaling module) is described by equations (1) – (4). Further, equations (5) – (6) quantify the release of endocrine stress hormones (cortisol, epinephrine) from neuroendocrine axis (HPA, SNS) coupled with their anti-inflammatory influence on the host and finally equation (7) integrates systemic level responses associated with the physiologic status of the host. In addition to endotoxin-induced reduced heart rate variability, endotoxin challenge also elicits tachycardia in association with a hyperdynamic cardiovascular response that mimics acute critical illness (16).
Although, the terms increased heart rate and reduced heart rate variability are, oftentimes, used interchangeably in the literature, several lines of evidence indicate examples in which concomitant changes in mean heart rate might complicate the interpretation of overall heart rate variability. This applies particularly to studies evaluating drugs which may have direct effects on sinus node pacemaker activity (6). For instance, experimental observations, at least, in a context of self-limited systemic inflammatory response (14), show that HRV indices, including pNN50 (vagal activity) and heart rate dynamics may be significantly modulated due to adrenergic infusion without reflecting any change in overall system’s adaptability as assessed by SDNN parameter. In the following section we will discuss the potential of a physiology-based model of autonomic control of heart rate response to endotoxin, to capture the cardiovascular effect of agents (i.e. catecholamines) that affect sinus pacemaker activity.
Developing a semi-mechanistic model for the autonomic control of heart rate in acute human inflammation
Among the many interesting correlates for human infectious pathology arising from the human models of endotoxemia is the documentation that low-dose LPS (2–4 ng/kg BW) induces an increase in cardiac index and heart rate (17). Overall variations in heart rate being largely dependent on autonomic modulation, an increased heart rate has been considered to reflect a diminished parasympathetic (vagal) tone and an increased sympathetic modulation of the sinus node. Such interpretation lies in agreement not only with experimental evidence indicating sympathetic activity excess (and/or parasympathetic attenuation) but also with the findings that reductions in implied vagal nerve activity is associated with increased morbidity in critically ill patients (10). Although, the mechanism for this systemic “decomplexification” is unknown, it is likely that altered central autonomic (ANS) activity and disruptions in efferent sympathetic and parasympathetic signaling are contributory.
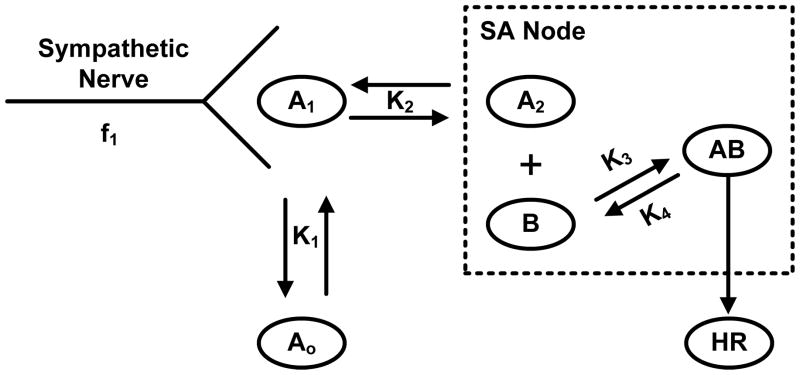
The effect on heart rate of combined modulation of sympathetic and parasympathetic (vagal) nerves has been described in a quantitative fashion since the 1960s. Warner and his colleagues developed a mathematical model (Warner model) to simulate the dynamics of sinoatrial (SA) node in response to vagal and sympathetic stimulations (18). Since this model forms the foundation of this study, we will briefly summarize the key elements and the associated interactions. The relationship, for instance, between stimulation of sympathetic nerve to the heart and the heart rate (HR) is illustrated in Figure 1.
Figure 1. Schematic illustration of the Warner model representing the relationship between stimulation of efferent sympathetic nerve activity to the heart and heart rate (HR).
A1 represents the concentration of sympathetic neurotransmitter (catecholamine) at the nerve ending and f1 represents the frequency stimulates preformed on the nerve; A0 represents the concentration of catecholamine in peripheral tissues (i.e. blood); A2 represents the concentration at the active site on sinoatrial (SA) node which must react with chemical substance B to produce a change in heart rate, adapted from (18).
Specifically, at the level of sympathetic nerve activity, the instantaneous neural activity is described by a sinusoidal function (f1) that serves as the input of the model stimulating the release of sympathetic neurotransmitters at the nerve ending (A1). However, the cardiac neurotransmitter release (A1) is not limited to centrally mediated neural traffic but may be also triggered in response to neurotransmitters from peripheral tissues such as blood (A0). On the active site (SA node), an effective concentration of catecholamine (A2) is derived from a set of first order kinetic equations that reacts with substance (B) forming the signal (AB) in order to produce a change in heart rate (HR) when only sympathetic activity is present.
In this study, the kinetic part of the sympathetic site of the Warner model will be used as a template for producing the concentrations of neurotransmitters in response to the related autonomic activities in human endotoxemia. Embedding the structure of Figure 1 in the dynamics of our human endotoxin model, we assume that the released neurotransmitters from SNS nerve are triggered by the increased circulating levels of catecholamines evoked by endotoxin, equation (8), and thereby influencing the effective neurotransmitter concentration on the sinus node of the heart, equation (9).
| (8) |
| (9) |
Specifically, the release of sympathetic neurotransmitters from the SNS nerve ending (A1) are quantified by first order kinetics (K1 and K2), equation (8). The re-uptake process of the SNS neurotransmitter from the sinus node to the nerve ending is described by the term K2(A2-A1); while the effective concentration of the neurotransmitter at the active site of the heart (A2) is influenced by the rate of sympathetic nerve traffic (A1), equation (9). We recognize that in the kinetic part of the Warner model sinusoidal functions were also used as input signals to sympathetic nerve stimulation. Since these functions represent the frequency stimulus which was experimentally performed in the nerves of anesthetized animals, such neural patterns are not considered in our model. Alternatively, for purposes of our model, plasma concentration of epinephrine serve as the primary “input” signal to the efferent sympathetic site, which in line with evidence (19) high plasma catecholamine concentrations are associated with high rate of sympathetic nerve traffic.
As it previously mentioned, the formed signal (AB) represents the sympathetically-mediated active signal that affects pacemaker activity and herein this mediator serves as a surrogate for the overall sympathetic response (Tsym). Regarding the dynamics of the vagal site, although the relationship between the two major autonomic divisions may be highly complex, it is believed that changes in heart rate are brought about by simultaneous reciprocal changes in the autonomic influences on the heart (20). Such mutual antagonism between the efferent sympathetic and parasympathetic branches of the (ANS) is further considered and quantified in equations (10) – (11).
| (10) |
| (11) |
Assuming that the two major autonomic control systems act as endogenous neuronal antagonists (21), such dynamic interaction is described by the kinetic parameters (kTsym,Tpar, kTpar,Tsym), equation (10). Thus, the kinetics of the Warner model are extended by incorporating the stimulatory function (1+kTsym,TparTpar) that inhibits the first order kinetic rate (K3) of sympathetic activity and represents the antagonism of parasympathetic response (Tpar). Similarly, the inhibitory effect of sympathetic response (Tsym) to vagal function is represented by the linear function (1+kTpar,TsymTsym) while the dynamics that define the substance (B) are the same as in the original Warner model, equation (11).
In an effort to quantify the overall dynamics of the parasympathetic reflex activity (Tpar) the principles of indirect response modeling are employed as previously explored (12). The underlying assumption is that the baseline of parasympathetic tone is produced in a zero order kinetics (Kin,Tpar) and removed in a first order kinetics described by a constant rate (Kout,Tpar). In our endotoxin injury model, a dynamic change in vagal function (Tpar) is simulated due to an increase in sympathetic outflow (Tsym) which is evoked by neuroendocrine stress hormones (i.e. epinephrine) coupled with the activation of other sympathetically mediated physiological processes (i.e. blood pressure) (22) that might contribute to further modulation of vagal function and are represented by (A1) signaling mediator. The potential of the IDR modeling in simulating physiological variables including autonomic reflex activity has been effectively demonstrated in (23) and herein this modeling concept will also be explored in order to quantify heart rate dynamics as follows, equation (12).
| (12) |
The basal heart rate response is assumed to be maintained by the balance of relevant neurotransmitters that are given by a constant rate of synthesis (Kin,HR) and a first order degradation rate (Kout,HR) (see Appendix, Table 1). It is well recognized that the heart rate (HR) increases when sympathetic stimulation increases and that it decelerates upon increased parasympathetic response (20). Hence, the effect of sympathetic and parasympathetic activities on the heart rate is quantified by (kHR,Tsym) and (kHR,Tpar), respectively. Specifically, in this model the vagus nerve mediates deceleration of heart rate by stimulating the degradation rate of heart rate response (Kout,HR). Taken together, in our endotoxin injury model, cardiac acceleration is induced by the antagonistic interplay of autonomic activities on the heart manifested as prevalence (increase) of efferent sympathetic activity (Tsym) and attenuation of parasympathetic nervous system function (Tpar).
Results and Discussion
Elements of the physiology-based model of human endotoxin-induced inflammation
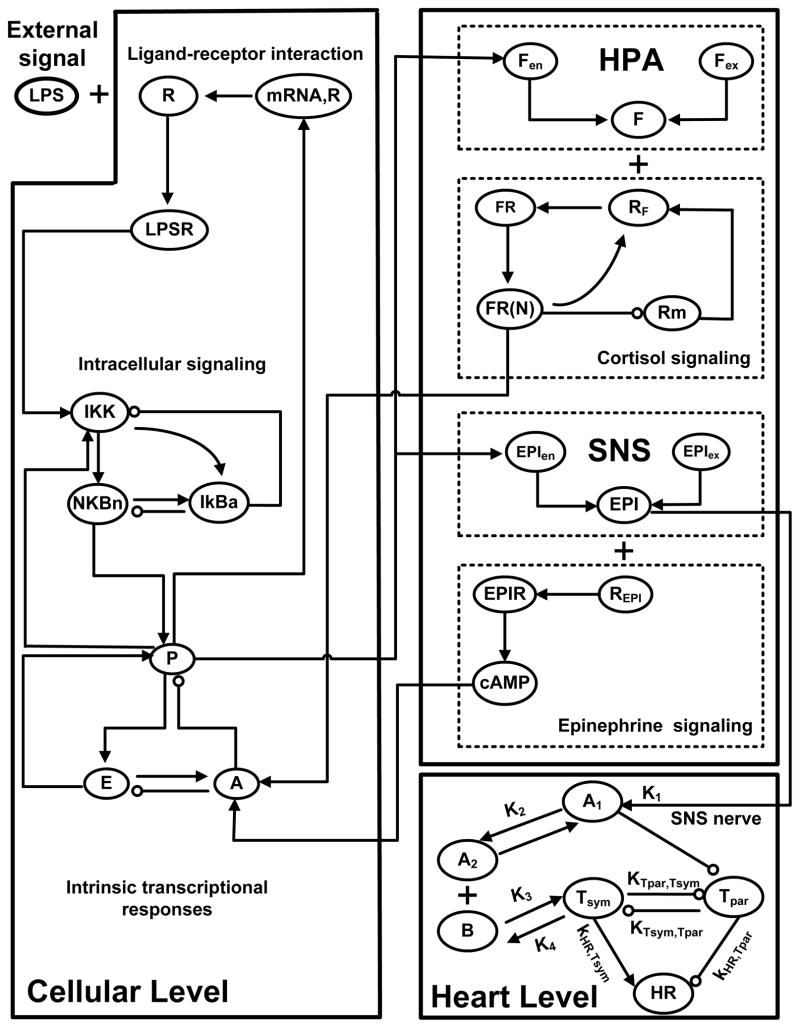
We have previously demonstrated the feasibility of a multiscale physicochemical host response model that integrates essential regulatory processes across the host linking the initiating signal (LPS) with transcriptional (cellular) dynamics, signaling cascades and hormonal (physiological) components. Specifically, elementary pro-inflammatory pathways (i.e. NF-kB signaling module) triggered by the recognition process of LPS form its signaling receptor (i.e. R, TLR4) propagate the acute inflammatory reaction at the transcriptional response level (P, A, E). Essential aspects associated with the neuroendocrine immune crosstalk and systemic “decomplexification” are also considered. In an effort to assess autonomic modulation of the sinus node of the heart, we attempted to describe the effect on heart rate of simultaneous sympathetic and vagal controls as illustrated in Figure 2. Specifically, at the systemic level of the sinus node, physicochemical interactions related to the release, binding and degradation of cardiac (SNS) neurotransmitters (A1, A2) are incorporated. Such interactions are stimulated by the neuroendocrine axis and particularly by circulating levels of epinephrine (EPI) released upon endotoxin from activation of SNS pathway. During the progression of endotoxin induced inflammation, disruptions in autonomic cardiac control are evaluated by alterations in the joint activity of sympathetic (Tsym) and parasympathetic (Tpar) arms of the autonomic nervous system which thereby influence the intrinsic pacemaker activity (heart rate). Thus, the proposed model intends to associate disordered neuroendocrine function with concomitant dysfunctional adrenergic modulation at the sinoatrial node (cardiac pacemaker).
Figure 2. Network topology of the multiscale model of human endotoxemia for the assessment of autonomic heart rate regulation.
Elementary pro-inflammatory pathways (i.e. NF-kB signaling module) triggered by the recognition process of endotoxin (LPS) from its signaling receptor (TLR4, R) propagate the effect of LPS signaling on the transcriptional (cellular) response level (P, A, E). Essential modules associated with the release of stress hormones (cortisol (F), epinephrine (EPI)) from neuroendocrine axis (HPA, SNS) coupled with their anti-inflammatory influence on the host are further considered. Finally, at the systemic level, biochemical reactions associated with the release, binding and degradation of cardiac (sympathetic) neurotransmitters (A1, A2) on the SA node are also incorporated. Efferent autonomic outflow is represented by sympathetic (Tsym) and parasympathetic activities (Tpar) that act antagonistically giving rise to changes in heart rate (HR) response assessed by clinical monitoring of vital signs.
Model calibration and validation
The appropriateness of the assumptions invoked in the construction of the proposed model will be demonstrated in the following three stages including: (i) calibration of the model using human experimental data associated with heart rate measurements and time-domain measures of heart rate variability, namely pNN50 after exposure to low-dose of LPS; (ii) model verification using experimental data that have not been used as a training data set. These data refer to human subjects who received either low dose (2ng/kg BW) LPS or an infusion of epinephrine for 3hr before LPS administration and continued until 6 hours after endotoxin administration. Hence, we opt to assess the validity of our model by assessing the cardiovascular implications of acute epinephrine infusion on the host, and finally (iii) further qualitative model validation simulating a series of biological implications of the host response to endotoxin that can be equated with the (complex) non-linear dynamics of severe inflammation in the critical care setting. Such scenarios refer to systematic perturbations that modulate the dynamics towards either an irreversible response or in favor of a balanced immune response depending on the anti-inflammatory “reservoir” of the host relative to the intense (pro) inflammatory response.
Parameter Estimation
Model kinetic parameters involved in the autonomic control of heart rate are estimated by minimizing the discrepancy (error) between model predictions and the experimental data as depicted in Table 1. Relevant experimental data are normalized by taking the ratio of the measured response at each time point of the study period with respect to the control time point (t=0hr). Thus, the associated model variables represent dimensionless entities and are considered to quantify the response of the immune function. The parameter estimation (optimization) problem consists of a nonlinear performance criterion (sum of square of errors) and is solved using MATLAB (R2008b) nonlinear optimization solvers such as fmincon (24). All the other parameters related to the propagation of LPS signaling on the transcriptional level and to the neuroendocrine immune system interactions are maintained to agree with those presented in (13) (see Appendix, Table 3). The differential equations are solved using MATLAB’s solver ode15s, which is a variable-order, variable-step solver for stiff ordinary differential equations.
Table 1.
Estimated values of parameters related to the autonomic heart rate regulation
| Parameter | Description | Value |
|---|---|---|
| K1 | Signaling rate | 3.654 |
| K2 | Uptake rate | 0.055 |
| K3 | Binding reaction rate | 2.927 |
| C | Constant | 11.286 |
| kTsym,Tpar | Parasympathetic dampening factor | 7.764 |
| K4 | Sympathetic degradation rate | 3.435 |
| Kin,Tpar | Apparent (zero-order) production rate of parasympathetic activity | 45.181 |
| kTpar,Tsym | Sympathetic dampening factor | 9.756 |
| Kout,Tpar | Parasympathetic loss of activity | 4.201 |
| Kin,HR | Apparent production rate of heart rate | 23.279 |
| kHR,Tsym | Acceleration rate due to sympathetic activity | 0.055 |
| kHR,Tpar | Deceleration rate due to parasympathetic stimulation | 0.297 |
| Kout,HR | Degradation rate of heart rate response | 18.942 |
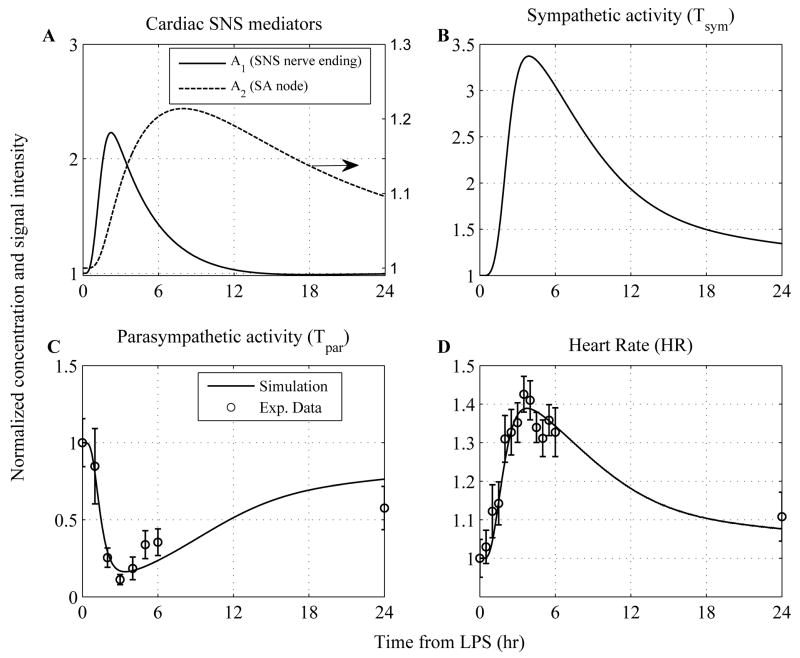
The performance of the model in reconstructing the clinical manifestations of human endotoxemia is presented in Figure 3. In essence, a self-limited inflammatory response, as previously simulated (11–13), involves the successful elimination of the inflammatory stimulus (endotoxin) within 2hr post-endotoxin administration followed by subsequent resolution of all inflammatory manifestations (i.e. transcriptional responses, hormonal concentrations) within 24hr. Herein, at the level of autonomic cardiovascular control, intravenous administration of endotoxin elicits tachycardia (elevated heart rate, HR) as a result of cardiac autonomic imbalance, reflected by increased sympathetic activity (Tsym) and reduced parasympathetic response (Tpar). An increase in sympathetic activity followed by reductions in implied vagal nerve activity have now been noted during inflammatory conditions associated with human endotoxemia (10). In our computational model, such dysregulation is mediated by an acute neuroendocrine stress response evoked by endotoxin and specifically by increased circulating levels of epinephrine which give rise to a high rate of efferent sympathetic nerve traffic. Such stimulation is manifested as up-regulation in the concentration of cardiac sympathetic neurotransmitters (A1, A2) that participate in the sympathetic control of heart rate (HR).
Figure 3. Dynamic profiles of the elements that constitute the autonomic heart rate regulation signaling module in human endotoxemia.
(A) Simulated concentrations of cardiac (SNS) neurotransmitters at the level of sympathetic nerve ending (A1) and at the active site of sinus node (SA) of the heart (A2); (B) Efferent sympathetic activity during the progression of the acute inflammatory reaction; (C) Simulated efferent parasympathetic (vagal) activity and (D) Heart rate (HR) response to endotoxin induced inflammation. Human experimental data (○ circles) associated with vagal measurements (time domain HRV measure, pNN50) and vital signs (heart rate measurements) are used to calibrate the model. Solid lines (—) represent model predictions under conditions of low-dose endotoxin while ○ circles refer to experimental data expressed as mean ± SEM. The initial condition of the inflammatory stimulus (LPS(t=0hr) = 1) reflects LPS concentration relative to 2-ng/kg body weight.
Implications of acute epinephrine infusion on the host response to endotoxin
Catecholamines, the main neurotransmitters of the sympathetic nervous system pathway, exert anti-inflammatory and vasoactive properties affecting both immune cell activation and cardiovascular function (25). At the cellular (immune) level, we have previously simulated the effect of epinephrine on attenuating the pro-inflammatory manifestations of human endotoxemia via a cAMP dependent mechanism (13). Although the immunosuppressive effects of antecedent periods of catecholamine excess following the systemic inflammatory manifestations of human endotoxemia have been well described, their effect on heart rate parameters induced by endotoxin is not well understood.
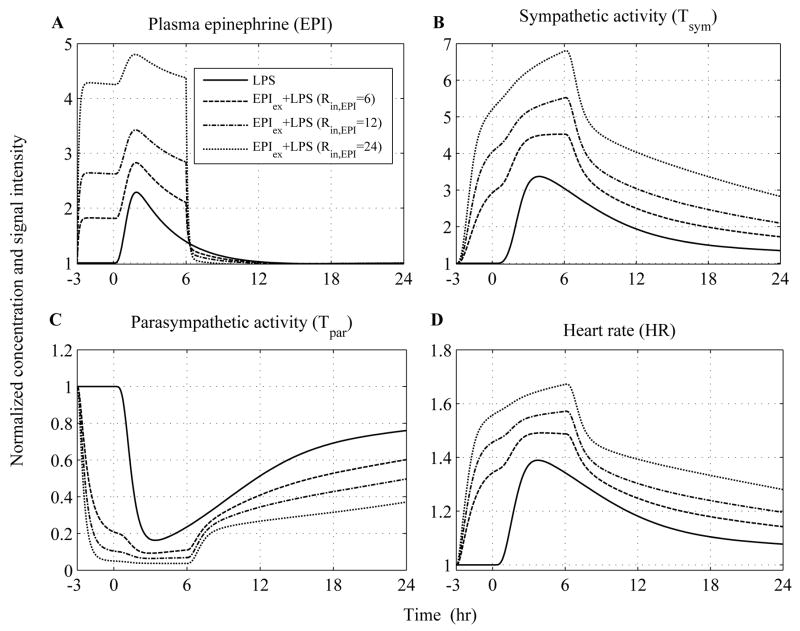
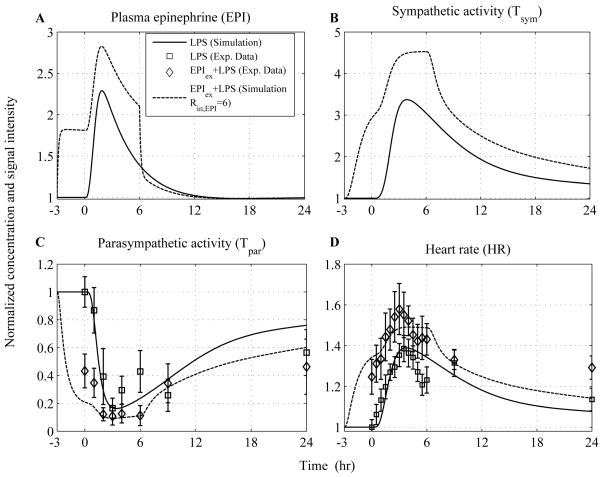
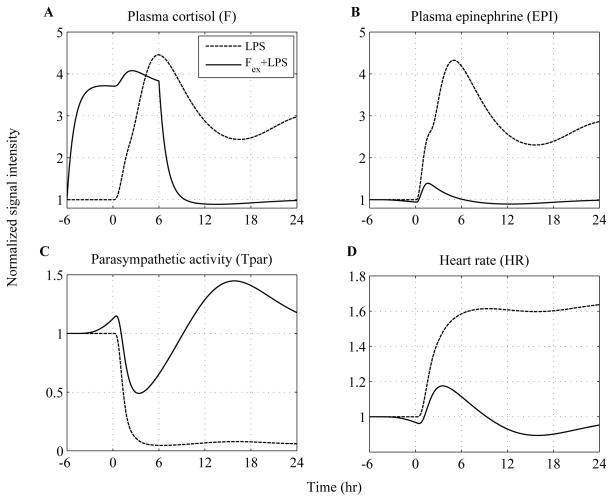
Predicated upon this, the influence of epinephrine (EPI) infusion initiated 3hr before the intravenous administration of endotoxin and continued until 6hr after LPS exposure is simulated at various doses in Figure 4. We specifically sought to simulate whether there exists a particular dose of exogenously-induced catecholamine excess (defined in our model by the parameter, Rin,EPI) that describes significantly the relevant experimental data. Thus, increasing the parameter (Rin,EPI) at various values the total concentration of EPI represented by dashed and dotted lines (Figure 4A) increases in a dose-dependent manner which subsequently potentiates the cardiac sympathetic activity (Tsym, Figure 4B) relative to the response invoked by the administration of the inflammatory stimulus (— lines). Such increase in efferent sympathetic outflow further diminishes the parasympathetic (vagal) function (Tpar) and thereby affects the intrinsic pacemaker activity as assessed by increased heart rate (HR). Experimentally, such modulation in parasympathetic and heart rate response to endotoxin under conditions of prior epinephrine infusions is demonstrated in (14). Regarding the experimental study, antecedent EPI infusion mediated a decrease in parasympathetic function which was significantly different from the effect induced by LPS (Figure 5C) and a significantly higher heart rate response (HR) (Figure 5D). It is important to emphasize that the aforementioned experimental data represent the dynamics of the host under conditions of a particular dose of epinephrine and are used to test the validity of our model in predicting inflammatory relevant responses in situations on which it has not been trained. Although plasma concentrations of epinephrine are not available under conditions of pior EPI infusion, our simulations indicate that there exists a value of the model parameter (Rin,EPI) that captures the vagolytic influence of exogenously-induced catecholamine excess as assessed by an average correlation coefficient that approximates the value of 0.8 between relevant experimental data and model output (see Appendix, Table 4).
Figure 4. In silico simulation of the cardiovascular effects of acute epinephrine infusion on the host initiated 3hr prior to endotoxin challenge (t = 0hr) and continued for another 6hr after LPS.
Solid lines simulate the host dynamics under conditions of low-dose endotoxin (LPS) while dashed and dotted lines reflect the dynamics of the host pre-exposed to epinephrine infusion at various doses. The acute pre-exposure of the host into epinephrine (wEPI,ex = 1) at increasing values of the parameter Rin,EPI = 6, 12, 24 potentiates (A) circulating levels of epinephrine and (B) the overall efferent sympathetic outflow (Tsym) relative to the responses induced by endotoxin administration while (C) vagal activity is significantly attenuated compared to the effect induced only by LPS and finally (D) heart rate response to endotoxin is further increased due to prior epinephrine infusion.
Figure 5. In silico assessment of the cardiovascular implications associated with acute epinephrine infusion on the host.
Human experimental data depicted by □ and ◇ represent mean ± SEM refer to human subjects that received either low dose (2ng/kg BW) LPS or an infusion of epinephrine for 3hr before LPS administration and continued until 6 hours after LPS, respectively. These data are specifically used to validate qualitatively the structure of the proposed human inflammation model employed from (14) and not to train the model. Descriptive statistics in the original experimental study show that there was a significant change in the parasympathetic activity (Tpar) and heart rate response (HR) across the two experimental conditions (□ vs. ◇) from 0hr until 24 hours after LPS exposure. Computationally such situation is captured by the differential predicted responses between dashed and solid lines. We specifically observe that there exists a simulated trajectory of exogenously induced catecholamine excess (represented by dashed line - Rin,EPI = 6) that lies in general agreement with the relevant human experimental data. More details about the statistical assessment of the model in comparison to the data are provided in Appendix (Table 4).
Dose-dependent effects of acute endotoxin injury on the neuroendocrine-immune axis
The dose-dependence LPS inflammatory effects on immune-endocrine host responses is reported by relevant human studies (26). Specifically, in this study increasing the concentration of LPS leads to differential peak responses of the human host response as assessed by immune-neuroendocrine parameters including cytokines, stress hormones (i.e. cortisol) and physiological responses (i.e. heart rate) after the administration of low doses of endotoxin (i.e. 0.4 ng/kg) in healthy human subjects. On the other hand, high doses of endotoxin can be responsible for a dysregulation in the host defense intrinsic dynamics, even though this bacterial byproduct does not proliferate as a Gram-negative bacteria (27). Regarding endotoxin administration and mortality, it is generally accepted that the maximum dose of LPS that can be safely administered to humans is 4ng/kg BW. In the following we will demonstrate the ability of our model to enable such “predictions” providing further evidence of the validity of the assumptions invoked in the development of our model.
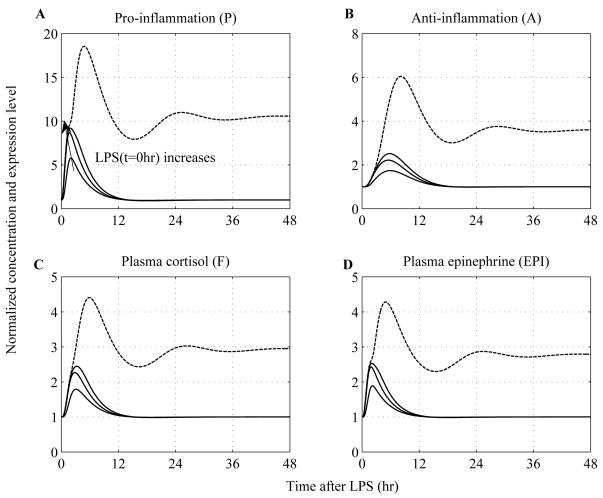
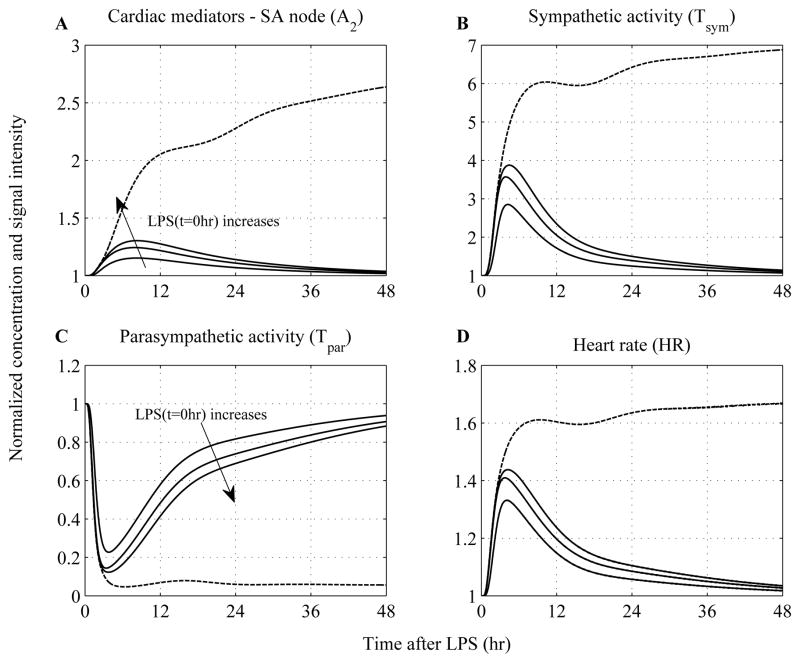
In an effort to simulate proper responses to survivable and lethal endotoxin doses, we simply vary the concentration of LPS at time zero (LPS(t=0hr)) carrying out simulations with low (i.e. LPS(t=0hr)=0.4 ng/kg) and high LPS doses (i.e. 8ng/kg - four times greater than the nominal value (2ng/kg BW) used to calibrate the model), (Figure 6 and Figure 7). We observe that when the concentration of LPS exceeds a critical threshold, the inflammatory response does not abate as was seen in solid lines where lower doses of LPS were simulated. This response is characterized by the uncontrolled secretion of endocrine hormones (cortisol, epinephrine) that are not adequate to balance (control) the overall immune response, thereby giving rise to a cytokine “burst”, Figure 6. Such dysregulation is further accompanied by impaired autonomic function and cardiac instability manifested as sustained elevations in heart rate response, Figure 7. Prolonged heart rate elevations are particularly simulated due to a persistent diminished vagus nerve activity (Tpar) and/or sympathetic (Tsym) overshooting that occur under conditions of severe endotoxin injury. In acute critical illness, comparable to the overwhelming immune response, adrenergic stress may also be uncontrolled and cause adverse effects.
Figure 6. Simulated dose dependent effects of LPS on neuroendocrine immune system interactions.
A high concentration of LPS can cause a dysregulation in the host dynamics characterized by abnormal transcriptional and hormonal responses. Temporal responses of critical inflammatory components for various initial conditions of the inflammatory stimulus include: (A) pro-inflammatory response (P); (B) anti-inflammatory response (A) and stress hormones such as (C) cortisol (F) and (D) epinephrine (EPI). Solid lines simulate the progression of a self-limited inflammatory response at increasing LPS doses (LPS(t=0hr) = 0.4 ng/kg, LPS(t=0hr) = 4ng/kg and LPS(t=0hr) = 6ng/kg) that are less than the critical threshold (LPS(t=0hr) = 8ng/kg - four times greater than the nominal value (2ng/kg BW) used to calibrate the model) that gives rise to unresolved inflammatory responses represented by sustained inflammatory markers and hormonal responses (dashed lines).
Figure 7. Simulated dose dependence of LPS effects on systemic (heart) level responses.
A high inflammatory challenge disrupts the autonomic control systems which give rise to prolonged elevations in heart rate. (A) Dynamic responses of catecholamines at the sinus node of the heart (A2); (B) efferent sympathetic, (C) parasympathetic activities and (D) heart rate (HR) responses to increasing levels of the inflammatory stimulus (LPS). While — lines represent constrained inflammatory responses, dashed lines refer to a persistent inflammatory response simulated by high LPS concentration given that such situation can be equated to the severely stressed clinical phenotype manifested as sustained elevations in heart rate (cardiovascular instability).
We recognize that we have previously simulated the dose-dependence of LPS effects on the cellular host response level (11, 12). However, the proposed model allows us to simulate concomitant dysfunctional adrenergic modulation of the heart and peripheral blood leukocytes during the progression of severe human injury. Specifically, impaired neuroendocrine regulation during systemic inflammatory response syndrome contributes to disruptions in cardiovascular homeostasis phenotypically expressed as persistent elevations in heart rate that are, in many cases, associated with increased morbidity and mortality (28). Having established these nonlinear responses, we now consider scenarios that involve possible reversibility in the dynamics of unremitting inflammation in response to a dynamic anti-inflammatory intervention strategy.
Evaluation of hormone replacement “therapy” in modulating severe acute inflammation
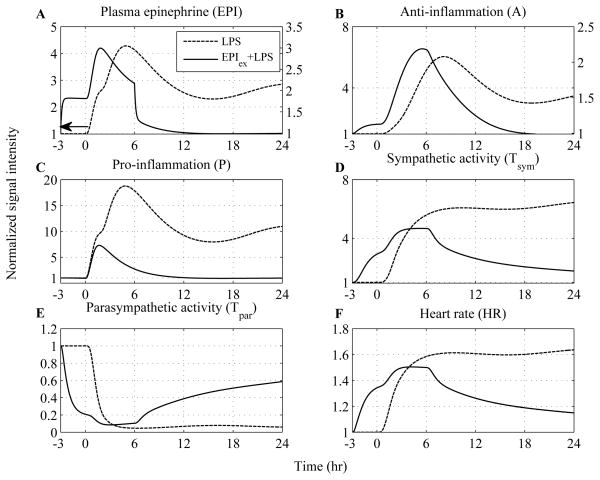
A fundamental assumption of our model is the existence of two relevant asymptotically stable steady states, which depending on the anti-inflammatory capacity of the host can represent either “recovery/self-limited” or “uncontrolled/sustained tachyarrhythmias) that might account for the transient clinical phenotype of severely stressed patients. To illustrate such scenarios, we consider the trajectory of an unconstrained response, simulated as high concentration of the initial stimulus (LPS), to serve as a surrogate for the high-risk profile of severely stressed patients. Predicated upon the fact that antecedent stress hormone excess abrogates several features of human endotoxemia (29), anti-inflammatory intervention strategies will involve pre-exposure of the host into either exogenously-induced catecholamine excess (Figure 8) or hypercortisolemia (Figure 9).
Figure 8. Dynamic inflammatory cellular and physiological responses as a function of time after the administration of high inflammatory challenge (dashed lines) while solid lines represent “virtual” human subjects that receive an infusion of epinephrine.
The acute pre-exposure of the host into epinephrine (initiated 3hr before LPS and continued until 6hr after endotoxin, i.e. Rin,EPI = 6) attenuates the pro-inflammatory response (P), relative to the effect mediated by high concentration of LPS, via potentiation of the anti-inflammatory component of the host (A). In addition to the anti-inflammatory role of epinephrine, exogenous up-regulation in circulating levels of epinephrine increase the efferent sympathetic activity (Tsym) which is followed by further reduction in vagal function (Tpar) and these autonomic changes mediate early tachycardia (HR) which is eventually restored within 24 hours.
Figure 9. Modulation of the progression of unresolved inflammatory response due to high LPS concentration under conditions of hydrocortisone infusion.
The effect of low-dose steroid administration initiated 6hr before high LPS concentration and continued until 6hr after LPS is simulated in — lines. Such exogenously-induced hypercortisolemia (wFex=1, Rin,F=2.922 – parameters taken from our prior work (13)) potentiates total plasma concentration of cortisol ashown in panel (A) and modulates cytokine and hormonal responses. Circulating levels of epinephrine are attenuated in response to antecedent periods of hypercortisolemia relative to the excessive adrenergic response which is illustrated in panel (B). At the autonomic level such attenuation is expected to reduce efferent sympathetic activity mediating (C) an increase in implied vagal function (Tpar) and finally (D) controlling heart rate as shown by reversibility in the progression of the inflammatory reaction towards homeostasis (baseline) – “recovery phase” (solid lines).
Catecholamines, as potent anti-inflammatory and vasoactive agents, have received increased recognition as part of “replacement” therapy in the critical care setting (30). In order to capture such situation, antecedent periods of epinephrine (EPI) infusion following high inflammatory challenge (t=0hr) are simulated in Figure 8. We observe that acute pre-exposure of the host to catecholamine excess reverses the dynamics of the intense inflammatory reaction towards homeostasis - “recovery phase” - manifested as autonomic restoration and control of cardiovascular instability. Such reversibility in the transient inflammatory phenotype of severe injury annotates the impact of dynamic anti-inflammation on compromising outcome. It is worth mentioning that the sympathomimetic properties of epinephrine prevail, during the first hours of stress hormone (EPI) infusion, i.e. 3 hours before LPS, against its anti-inflammatory effect. This prevalence is illustrated by diminished vagal function (Tpar) and/or increased sympathetic control of heart rate (HR) as shown by solid lines relative to the effect invoked by LPS administration (dashed representation). However, in our model as the inflammatory response evolves the dynamic anti-inflammatory mechanism (A) mediated by epinephrine signaling becomes activated and attenuates the build-up of pro-inflammation (P) mitigating the subsequent protracted stimulation of neuroendocrine axis and the heart rate response. Such dynamics indicate the careful use of catecholamine vasopressors in the critically ill to an extent where beneficial effects still prevail without putting an excessive adrenergic stress on the heart (31).
During the progression of sustained tachycardia (dashed lines – Figure 8F) attained by an irreversible disturbance (i.e. high LPS concentration), the heart rate response (HR) settles to an “unhealthy” steady state which approximates the value of 1.68 or else 107bpm. Such simulations associate adverse stress (adrenergic) outcomes with severe cardiovascular complications manifested as persistent tachycardia at a high rate. As reviewed by Dunser et al (32), among the several hemodynamic parameters, heart rate >106 bpm was linked to mortality in patients with septic shock. Such strong association between increased heart rate and cardiovascular mortality has resurged interest in treatments that compromise heart rate control and reduce excessive adrenergic stress including hydrocortisone infusion as simulated in Figure 9.
Prior studies evaluating human responses to infectious challenge (endotoxin) within the context of antecedent stress hormone excess have shown that glucocorticoid excess, as produced by 6hr infusion before LPS challenge, abrogates much of the clinical responses to endotoxin including heart rate (29). It also attenuates the production of circulating pro-inflammatory cytokines through an increase in plasma IL-10 concentrations. In our prior models, we simulated the immunosuppressive effects of low-dose hydrocortisone infusion upon the pro-inflammatory manifestations of human endotoxemia (13). The aim of the present study, however, is to associate the anti-inflammatory effects of glucocorticoids, during the progression of uncontrolled inflammation, with the abrogation of prolonged and intense adrenergic stress that mitigates the subsequent amplified inflammatory response.
Exogenously-induced hypercortisolemia initiated 6hr before the LPS challenge potentiates total cortisol levels (solid lines - Figure 9A) which accounts for alterations in the observed adrenergic stress signaling (Figure 9B). Such dynamic changes are simulated by reductions in circulating levels of epinephrine (EPI) that mediate further attenuation in the efferent sympathetic activity; whilst the latter gives rise to increased parasympathetic function (Tpar) when compared to the dynamics elicited upon the manifestation of severe endotoxin injury. Such altered adaptability in neuroendocrine and autonomic function under conditions of acute hypercortisolemia results in improved autonomic heart rate regulation as assessed by reversibility in sustained tachyarrhythmias towards homeostasis. Qualitatively, such dynamics might reflect the transient clinical improvement (i.e. “survivors”) noted to critically ill patients that respond to a treatment. For example, in the observational study (33) the impact of low-dose hydrocortisone infusion on modulating the course of the systemic inflammatory response syndrome is manifested by reduced heart rate, inflammatory markers and eventual recovery from stress-induced implications. However, we would like to emphasize that it is not the purpose of this study to make direct comparisons between our model predictions and clinical observations. Instead, the overall goal of this study is to develop a semi-mechanistic model of human endotoxemia as a prototype model of acute human inflammation that would potentially allow us to evaluate antecedent stresses upon the systemic inflammatory manifestations of acute infectious illnesses.
A key assumption of the present study is the association between increased circulating levels of epinephrine and a high rate of sympathetic nerve traffic (outflow to the sinus node). From a modeling standpoint, it is expected any modulation in the plasma concentration of epinephrine to drive subsequent changes in the cardiac sympathetic nerve activity accompanied by further changes in the autonomic heart rate dynamics. We note, however, that such relationship is not simple given that a modulation in the plasma concentration of catecholamines does not necessarily indicate a change in the rate of sympathetic nerve traffic (34). Recently, exogenously-induced hypercortisolemia within the context of human endotoxemia modulated inflammatory responses to low-dose endotoxin without affecting any autonomic relevant parameter including heart rate (15). In this experimental study, the implied discordance between acute hypercortisolemia and no modulation of adrenergic stress response to endotoxin as previously shown (29) raises questions related to a possible non-linear relationship between glucocorticoid activity and these inflammatory parameters. As new mechanisms become established and their role demonstrated reproducibly, these other mechanisms can be integrated leading to more complete in silico representations.
In summary, a semi-mechanistic, physiology-based model of human endotoxemia is developed, as a prototype model of acute inflammation in humans that quantifies essential aspects of the autonomic heart rate regulation. We expanded our prior mathematical modeling work to include systemic level interactions associated with the dynamic interplay of sympathetic and parasympathetic nerves to the heart. Such physicochemical interactions are related to the release, binding and degradation of cardiac neurotransmitters that allow us to associate endogenous neuroendocrine stress responses with centrally altered autonomic activities that give rise to heart rate changes. Kinetic parameters are estimated by reconstructing human relevant experimental data associated with a constrained hyperdynamic cardiovascular response to the endotoxin paradigm. Such response is phenotypically expressed as tachycardia which resolves within 24 hours and is simulated as a result of increased efferent sympathetic activity and reduced parasympathetic response.
Despite suffering from various limitations (i.e. calibration to limited data) the proposed model can simulate the cardiovascular implications of acute epinephrine infusion on the host as well as a series of systematic perturbations that qualitatively can be equated with the (complex) non-linear dynamics of severe acute inflammation. Such scenarios explore the importance of dynamic anti-inflammation during the course of unremitting inflammation in balancing neuro-immunologic dissonance promoting inflammatory resolution and thereby cardiovascular homeostasis. Although, the present manuscript describes a continuation of our prior work, we plan in future studies to concatenate processes involved in the autonomic regulation of heart rate with diminished physiologic variability (HRV). It is therefore our future goal to further describe changes in HRV by taking the mean heart rate into account assessing the contribution of efferent branches of the autonomic nervous system to changes in overall system adaptability. Developing more mechanistic-based and physiological relevant in silico models of inflammation can yield significant insights into the complex relationship between injury and cardiac dysfunction in severely stressed patients; thereby advancing the translational potential of systems modeling in clinical research.
Acknowledgments
PTF and IPA acknowledge support from NIH GM082974, NSF 0519563, EPA GAD R 832721-010-RRA and a Busch Biomedical Research Grant. The human volunteer experimental endotoxin studies, and SEC and SFL are supported, in part, from NIGMS Grant GM34695.
List of abbreviations
- LPS
Lipopolysaccharide, endotoxin
- R
Endotoxin signaling receptor (TLR4)
- LPSR
Endotoxin-TLR4 complex
- mRNA,R
Gene transcript of endotoxin receptor (TLR4)
- IKK
Kinase activity
- NFkBn
Nuclear concentration of NF-kB
- mRNAIkBa
Gene transcript of NF-kB inhibitor (IkBa)
- IkBa
Protein inhibitor IkBa
- P
Transcriptional pro-inflammatory response
- A
Transcriptional anti-inflammatory response
- E
Transcriptional energetic response
- F
Cortisol
- Rm
Gene transcript of glucocorticoid receptor
- RF
Free cytosolic glucocorticoid receptor
- FR
Cytosolic steroid-receptor complex
- FR(N)
Nucler steroid-receptor complex
- EPI
Epinephrine
- REPI
β adrenergic receptor
- EPIR
epinephrine-adrenergic receptor complex
- cAMP
cyclic adenosine monophosphate
- fP
efferent (cardiac) nerve activity
- Sf
cardiac active signal
- HRV
heart rate variability
- A1
Catecholamines at the SNS nerve ending
- A2
Catecholamines at the sinus node (SA)
- B
Chemical substance at the SA of the heart
- Tsym
Efferent sympathetic activity
- Tpar
Efferent parasympathetic activity
- HR
Heart rate
Appendix
Steady-State Equations
The baseline responses were defined as shown in Table 1:
Table 1.
Steady-state baseline equations
|
|
where A10, A20, B0 = C-Tsym0, Tpar0 and Tsym0 represent the baseline values of catecholamine concentration at the SNS nerve ending, catecholamine concentration at the SA node, chemical substance (B) and autonomic outflow respectively and were fixed based on the initial conditions (values at control time point, t=0h) as follows:
Table 2.
Initial conditions of relevant model components
| LPS(0) = 1 | NFkBn(0) = 0 | E(0) = 1 | FR(N)(0) = 0 | A1(0) = 1 |
| R(0) = 1 | mRNAIkBa(0) = 1 | F(0) = 1 | EPI(0) = 1 | A2(0) = 1 |
| LPSR(0) = 0 | IkBa(0) = 0 | Rm(0) = 25.8 | REPI(0) = 1 | Tsym(0) = 1 |
| mRNA,R(0) = 1 | P(0) = 1 | RF(0) = 540.7 | EPIR(0) = 0 | Tpar(0) = 1 |
| IKK(0) = 0 | A(0) = 1 | FR(0) = 0 | cAMP(0) = 1 | HR(0) = 1 |
Table 3.
Parameter values involved in the neuro-endocrine immune axis
| kLPS,1 = 4.500 | kNFkB,1 = 16.294 | Kout,A = 0.809 | kE,P = 2.216 | n = 5.509 |
| kLPS,2 = 6.790 | kNFkB,2 = 1.186 | kA,cAMP = 0.145 | kFen,P = 0.256 | k1,REPI = 3.005 |
| ksyn = 0.020 | Kin,IKBa = 0.463 | kA,E = 0.534 | k2,REPI = 5.465 | Kin,Fen = 0.842 |
| k1 = 3.000 | kIkBa,1 = 13.273 | kA,FRN = 0.401 | k0REPI = 11.011 | Rin,F(wFex=1) = 2.922 |
| k2 = 0.040 | kI,1 = 1.400 | Kin,E = 0.080 | KEPI,P = 0.231 | τ = 0.053 |
| k3 = 5.000 | kI,2 = 0.870 | Kout,E = 0.257 | Kout,EPI = 7.286 | Kin,EPI = 5.921 |
| k4 = 2.240 | Kin,P = 0.033 | kmRNAR,P = 1.740 | Rin,F(wFex=0) = 0 | kR,EPI = 0.845 |
| kin,mRNA,R = 0.090 | Kout,P = 0.332 | kP,1 = 29.741 | Kout,F = 1.058 | |
| kout,mRNA,R = 0.250 | Kin,A = 0.461 | kP,2 = 9.050 | k3,REPI = 5.546 |
Statistical Complement
The performance of the model in capturing the characteristics of actual measurements is assessed by measures of goodness of fit including estimation of a correlation coefficient between the data and the model as previously proposed (35). We test the null hypothesis that the correlation coefficient between experimental data and calculated values is zero, versus the alternative that it is greater than zero yielding a p-value quantifying the likelihood of getting a correlation as large as the one calculated by chance. If the p-value is small, for example less than 0.05 (a commonly used threshold for significance level), then the correlation is significant at 95% confidence interval and the model accurately captures the characteristics of the data. The correlation coefficients between experimental data not used to calibrate the model and the simulated Tpar and HR values represented by dashed lines (Figure 5) were calculated across the two experimental scenarios (LPS, EPIex+LPS) as illustrated in Table 4. This analysis yields a significant strong correlation between actual measurements and the model output.
Table 4.
| Experimental scenario | Measure of goodness of fit (correlation coefficient) | p-value |
|---|---|---|
| LPS (Error! Reference source not found. Error! Reference source not found. C) | 0.92 | 0.0012 |
| LPS (Error! Reference source not found. Error! Reference source not found. D) | 0.89 | 1.e-4 |
| EPIex+LPS (Error! Reference source not found. Error! Reference source not found. C) | 0.77 | 0.023 |
| EPIex+LPS (Error! Reference source not found. Error! Reference source not found. D) | 0.76 | 9.e-3 |
It is important to note that the data displayed in Figure 5C and Figure 5D (□ and ⋄ markers), albeit well described by our model yielding a correlation coefficient ~ 0.8 (Table 4), are not used to calibrate our model but rather to validate the intended structure of our model in predicting the sympathomimetic properties of acute epinephrine infusion upon the inflammatory manifestations of human endotoxemia. Relevant quantitative data (Tpar and HR) are employed from the experimental study (15) (depicted in Figure 3C and Figure 3D) and the measure of goodness of fit yields a correlation coefficient of 0.95 for Tpar dynamics (p-value 3.e-4) and 0.97 for HR (p-value 1.e-4) indicating strong correlation between the experimental measurements and the predicted (estimated) values of the proposed model.
Footnotes
Financial Disclosure: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. PTF and IPA acknowledge support from NIH GM082974, NSF 0519563, EPA GAD R 832721-010-RRA and a Busch Biomedical Research Grant. The human volunteer experimental endotoxin studies, and SEC and SFL are supported, in part, from NIGMS Grant GM34695.
References
- 1.Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res. 2006;12:151–70. doi: 10.1179/096805106X102246. [DOI] [PubMed] [Google Scholar]
- 2.Bone RC. Immunologic dissonance: a continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann Intern Med. 1996;125:680–7. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Sharshar T, Hopkinson NS, Orlikowski D, Annane D. Science review: The brain in sepsis--culprit and victim. Crit Care. 2005;9:37–44. doi: 10.1186/cc2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–9. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 5.Taub DD. Neuroendocrine interactions in the immune system. Cell Immunol. 2008;252:1–6. doi: 10.1016/j.cellimm.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaza A, Lombardi F. Autonomic indexes based on the analysis of heart rate variability: a view from the sinus node. Cardiovasc Res. 2001;50:434–42. doi: 10.1016/s0008-6363(01)00240-1. [DOI] [PubMed] [Google Scholar]
- 7.Lombardi F. Clinical implications of present physiological understanding of HRV components. Card Electrophysiol Rev. 2002;6:245–9. doi: 10.1023/a:1016329008921. [DOI] [PubMed] [Google Scholar]
- 8.Lowry SF, Calvano SE. Challenges for modeling and interpreting the complex biology of severe injury and inflammation. J Leukoc Biol. 2008;83:553–7. doi: 10.1189/jlb.0607377. [DOI] [PubMed] [Google Scholar]
- 9.Lowry SF. Human endotoxemia: a model for mechanistic insight and therapeutic targeting. Shock. 2005;24(Suppl 1):94–100. doi: 10.1097/01.shk.0000191340.23907.a1. [DOI] [PubMed] [Google Scholar]
- 10.Lowry SF. The stressed host response to infection: the disruptive signals and rhythms of systemic inflammation. Surg Clin North Am. 2009;89:311–26. doi: 10.1016/j.suc.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. In silico simulation of corticosteroids effect on an NFkB- dependent physicochemical model of systemic inflammation. PLoS One. 2009;4:e4706. doi: 10.1371/journal.pone.0004706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. Modeling endotoxin-induced systemic inflammation using an indirect response approach. Math Biosci. 2009;217:27–42. doi: 10.1016/j.mbs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. A Multi-scale Model for the Assessment of Autonomic Dysfunction in Human Endotoxemia. Physiol Genomics. 2010 doi: 10.1152/physiolgenomics.00184.2009. 0.1152/physiolgenomics.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jan BU, Coyle SM, Oikawa LO, Lu SE, Calvano SE, Lehrer PM, Lowry SF. Influence of acute epinephrine infusion on endotoxin-induced parameters of heart rate variability: a randomized controlled trial. Ann Surg. 2009;249:750–6. doi: 10.1097/SLA.0b013e3181a40193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez SM, Katsamanis Karavidas M, Coyle SM, Lu SE, Macor M, Oikawa LO, Lehrer PM, Calvano SE, Lowry SF. Low-dose steroid alters in vivo endotoxin-induced systemic inflammation but does not influence autonomic dysfunction. J Endotoxin Res. 2007;13:358–68. doi: 10.1177/0968051907086465. [DOI] [PubMed] [Google Scholar]
- 16.Santos AA, Wilmore DW. The systemic inflammatory response: perspective of human endotoxemia. Shock. 1996;6(Suppl 1):S50–6. [PubMed] [Google Scholar]
- 17.Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Moller K. Human endotoxemia as a model of systemic inflammation. Curr Med Chem. 2008;15:1697–705. doi: 10.2174/092986708784872393. [DOI] [PubMed] [Google Scholar]
- 18.Warner HR, Cox A. A mathematical model of heart rate control by sympathetic and vagus efferent information. J Appl Physiol. 1962;17:349–55. doi: 10.1152/jappl.1962.17.2.349. [DOI] [PubMed] [Google Scholar]
- 19.Grassi G, Esler M. How to assess sympathetic activity in humans. J Hypertens. 1999;17:719–34. doi: 10.1097/00004872-199917060-00001. [DOI] [PubMed] [Google Scholar]
- 20.Glick G, Braunwald E. Relative Roles of the Sympathetic and Parasympathetic Nervous Systems in the Reflex Control of Heart Rate. Circ Res. 1965;16:363–75. doi: 10.1161/01.res.16.4.363. [DOI] [PubMed] [Google Scholar]
- 21.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–87. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davos CH, Davies LC, Piepoli M. The effect of baroreceptor activity on cardiovascular regulation. Hellenic J Cardiol. 2002;43:143–155. [Google Scholar]
- 23.Perlstein I, Stepensky D, Krzyzanski W, Hoffman A. A signal transduction pharmacodynamic model of the kinetics of the parasympathomimetic activity of low-dose scopolamine and atropine in rats. J Pharm Sci. 2002;91:2500–10. doi: 10.1002/jps.10243. [DOI] [PubMed] [Google Scholar]
- 24.Contreras M, Ryan LM. Fitting nonlinear and constrained generalized estimating equations with optimization software. Biometrics. 2000;56:1268–71. doi: 10.1111/j.0006-341x.2000.01268.x. [DOI] [PubMed] [Google Scholar]
- 25.van der Poll T. Effects of Catecholamines on the Inflammatory Response. Sepsis. 2000;4:159–167. [Google Scholar]
- 26.Vedder H, Schreiber W, Yassouridis A, Gudewill S, Galanos C, Pollmacher T. Dose-dependence of bacterial lipopolysaccharide (LPS) effects on peak response and time course of the immune-endocrine host response in humans. Inflamm Res. 1999;48:67–74. doi: 10.1007/s000110050408. [DOI] [PubMed] [Google Scholar]
- 27.Munford RS. Severe Sepsis and Septic Shock: The Role of Gram - Negative Bacteremia. Annu Rev Pathol Mech Dis. 2006;1:467–496. doi: 10.1146/annurev.pathol.1.110304.100200. [DOI] [PubMed] [Google Scholar]
- 28.Cook S, Togni M, Schaub MC, Wenaweser P, Hess OM. High heart rate: a cardiovascular risk factor? Eur Heart J. 2006;27:2387–93. doi: 10.1093/eurheartj/ehl259. [DOI] [PubMed] [Google Scholar]
- 29.Barber AE, Coyle SM, Marano MA, Fischer E, Calvano SE, Fong Y, Moldawer LL, Lowry SF. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol. 1993;150:1999–2006. [PubMed] [Google Scholar]
- 30.Santman FW. Catecholamines in critical care. The commonly used catecholamines: receptor and clinical profile, indications and dosages. Pharm Weekbl Sci. 1992;14:290–6. doi: 10.1007/BF01977616. [DOI] [PubMed] [Google Scholar]
- 31.Dunser MW, Hasibeder WR. Vasopressin in vasodilatory shock: ensure organ blood flow, but take care of the heart! Crit Care. 2006;10:172. doi: 10.1186/cc5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunser MW, Hasibeder WR. Sympathetic overstimulation during critical illness: adverse effects of adrenergic stress. J Intensive Care Med. 2009;24:293–316. doi: 10.1177/0885066609340519. [DOI] [PubMed] [Google Scholar]
- 33.Briegel J, Kellermann W, Forst H, Haller M, Bittl M, Hoffmann GE, Buchler M, Uhl W, Peter K. Low-dose hydrocortisone infusion attenuates the systemic inflammatory response syndrome. The Phospholipase A2 Study Group. Clin Investig. 1994;72:782–7. doi: 10.1007/BF00180547. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther. 2003;305:800–11. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- 35.Chow CC, Clermont G, Kumar R, Lagoa C, Tawadrous Z, Gallo D, Betten B, Bartels J, Constantine G, Fink MP, Billiar TR, Vodovotz Y. The acute inflammatory response in diverse shock states. Shock. 2005;24:74–84. doi: 10.1097/01.shk.0000168526.97716.f3. [DOI] [PubMed] [Google Scholar]