Abstract
A receptor mediated model of endotoxin-induced human inflammation is proposed. The activation of the innate immune system in response to the endotoxin stimulus involves the interaction between the extracellular signal and critical receptors driving downstream signal transduction cascades leading to transcriptional changes. We explore the development of an in silico model that aims at coupling extracellular signals with essential transcriptional responses through a receptor mediated indirect response model. The model consists of eight (8) variables and is evaluated in a series of biologically relevant scenarios indicative of the non–linear behavior of inflammation. Such scenarios involve a self-limited response where the inflammatory stimulus is cleared successfully; a persistent infectious response where the inflammatory instigator is not eliminated, leading to an aberrant inflammatory response, and finally, a persistent non-infectious inflammatory response that can be elicited under an overload of the pathogen-derived product; as such high dose of the inflammatory insult can disturb the dynamics of the host response leading to an unconstrained inflammatory response. Finally, the potential of the model is demonstrated by analyzing scenarios associated with endotoxin tolerance and potentiation effects.
Introduction
Although systemic inflammation is but one component of the sepsis syndrome, inflammation can be studied in the absence of complex pathophysiology and co-morbidities of human sepsis using surrogate models. Human endotoxin challenge is one well-accepted surrogate model for studying the acute inflammatory response as it captures many of the clinically observed features of systemic inflammatory phenotype [1; 2; 3; 4; 5]. Endotoxin is a major component of the outer .membrane of gram-negative bacteria, and the inflammation caused by the activation of the innate immune system by this moiety can be a complicating factor in a variety of situations including trauma, burns, invasive surgery and organ-specific illnesses. The prototypical examples of endotoxin are lipopolysaccharides (LPS).
The response following endotoxin administration in human subjects include core temperature, cardiac, vasomotor, hematologic, metabolic, hormonal, acute phase reactant, and cytokine components that have been well described [4; 6; 7; 8]. Innate immune cell activation leads to production and release of pro-inflammatory cytokines, which are proximal mediators of the systemic inflammatory response. Although the bulk of this pro-inflammatory mediator release likely originates in cells of the reticuloendothelial system [9], the leukocytes present in peripheral blood are also activated, and importantly, are available for sampling with minimal invasiveness.
In order to study the underlying complexity of the dynamics of inflammation and to establish quantifiable relationships among the various components of the inflammatory response, model-based approaches have been proposed [10; 11; 12]. A number of excellent prior studies [11; 13; 14; 15; 16; 17] have placed significant emphasis on simulating inflammation based on the kinetics of well-defined features of the overall response. One of the key characteristics of these models is the a priori postulation of certain components that are consistent with prior biological knowledge and are known to play a major role in triggering the inflammatory response. The computational integration of such components can provide us with significant insight of how such elements behave over time empowering their translational application as predictive controls in clinical settings.
However, one of the big challenges is the systematic identification of such representative biological features that can adequately represent the complex dynamics of a host undergoing an inflammatory response. This requires the decomposition of the non–linear dynamics of the response into an elementary set that can serve as a surrogate for predicting the collective behavior of the system. A possible answer to this can be identified through the analysis of gene expression data aimed at monitoring the dynamics of the host response to an inflammatory agent, exploring the idea that cellular responses correspond to dynamically converging high-dimensional transcriptional trajectories [18].
Given the transcriptional profiling analysis of human blood leukocytes we are driven by the premise that genes that are most responsive to an external perturbation (endotoxin, LPS) are governed by a definite mechanism and have concerted changes in their expression profile. Thus, we test the hypothesis that there exists a representative set of ensuing responses that emerge from the dynamic evolution of the inflammatory response after exposure to endotoxin (LPS). Such responses include the pro-inflammatory response that consists of the early increased expression of cytokines and chemokines; the anti-inflammatory response which serves as the immunoregulatory arm of the host defense system and ultimately the energetic response that involves the decreased expression of genes that participate in cellular bio-energetic processes. In this paper we explore the development of a semi-mechanistic model of endotoxin-induced human inflammation though the integration of transcriptional profiling and indirect response models. Indirect response models have been widely used in pharmacokinetic/pharmacodynamic models simulating the physiological response of a system exposed to an external signal or perturbation [19; 20; 21]. The activation of the innate immune system in response to the endotoxin stimulus involves the interaction between the extracellular signal and critical receptors driving downstream signal transduction cascades that lead to transcriptional changes. Our inability to precisely model the complex signaling events that characterize system’s adaptation process to environmental changes makes IDR modeling appealing. We opt therefore to explore the development of an in silico representation that aims at coupling extracellular signals with the essential transcriptional responses through a receptor mediates indirect response model.
The dynamics of the system are described by eight (8) variables and the proposed model is evaluated in a series of biological relevant scenarios indicative of the non–linear behavior of inflammation. Such scenarios involve a self-limited response where the inflammatory stimulus (endotoxin) is cleared successfully; a persistent response where the inflammatory instigator is not eliminated leading to an aberrant inflammatory response, and finally, a persistent non-infectious inflammatory response that can be elicited under an overload of the LPS; as such high dose of the inflammatory insult can disturb the dynamics of the host response leading to an unconstrained inflammatory response. In this study the terms endotoxin and inflammatory stimulus are used interchangeably.
Finally, we demonstrate the potential of our model by analyzing scenarios associated with endotoxin tolerance and potentiation effects. The pre-exposure of the host to controlled levels of inflammatory agents affects the eventual fate of the response. These controlled perturbations aim at further deciphering the kinetics and dynamics of the host response. It is therefore believed that the dynamic response defining the fate of the host response is critically affected by the appropriate priming. We loosely connect such responses with the emergence of memory effects in the sense that preconditioning the system with low levels of endotoxin modulates the host response dynamics causing either a tolerance or a potentiation effect. It has been observed that repeated doses of endotoxin insult might lead to a less vigorous innate immune response [22]. Such an effect can reverse the lethal outcome of a high dose of the inflammatory stimulus. That is to say, in spite of the potent efficacy of LPS, if the system is pre-exposed to lower sub– lethal doses of LPS then this induces an acquired state of resistance to a subsequent endotoxin challenge [23]. Importantly, endotoxin tolerance or hypo-responsiveness is a multifactorial problem that can be associated with receptor desensitization as well as with the modulated activity of negative regulators that target intracellular or transcriptional events. On the other hand, the successive administration of sublethal doses of endotoxin can potentiate the system in that, because of the lack of an acquired state in the dynamics of the system, such an insult may dysregulate the host response dynamics leading to an exacerbated inflammation that cannot resolve. Thus, based on our model we further explore the behavior of the system when it is either pre-exposed to lower levels of endotoxin for “adequate” time as well as when the system has not manifested its “dynamic memory” to tolerate the second endotoxin challenge.
Materials and Methods
Human Endotoxin Model and data collection
The data analyzed in this study were generated by the Inflammation and Host Response to Injury Large Scale Collaborative Project funded by the USPHS, U54 GM621119 [1; 24]. In brief, human subjects (n = 8) were injected intravenously with either endotoxin (CC-RE, lot 2) at a dose of 2-ng/kg body weight or 0.9% sodium chloride (placebo treated subjects). Blood samples were collected before endotoxin infusion (0hr) and 2, 4, 6, 9 and 24 hours after injection of endotoxin or saline. Cellular RNA was isolated from the leukocyte pellets and a total of 44,924 probe sets on the Hu133A and Hu133B microarrays (Affymetrix) were hybridized and analyzed thus generating the expression measurements of thousands of genes that are activated/or repressed in response to endotoxin. More details about the experimental design are presented in the original analysis [1]. 5,093 probe sets, characterized by 0.1% false discovery rate (FDR) were considered statistically significant in the context of showing meaningful variation during the time course of the experiment using the SAM framework[25]. This set of differentially expressed genes defines the basis for our analysis. Based on this subset we are interested in extracting a critical subset of distinct and coherent transcriptional profiles, which describe the intrinsic dynamic progression of the perturbed biological system. From a disease modeling point of view, the question will, of course, be whether such a subset exists and whether it can be used to define the basic responses of dynamic model. The data are publicly available through the GEO Omnibus Database (http://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE3284. The data have been appropriately de-identified, and appropriate IRB approval and informed, written consent were obtained by the glue grant investigators [26]
Identification of Essential Transcriptional Responses
The administration of a low dose of endotoxin (LPS) to human subjects elicits the complex dynamics of a transcriptional response altering the expression level of numerous genes. We are interested in unraveling a critical set of “informative” temporal responses that are characterized as the “blueprints” of the orchestrated dynamics of the perturbed biological system. In doing so we hypothesize that there is a definite underlying mechanism that describes the emerging transcriptional dynamics of the inflammatory response [26].
Based on our prior work, we first apply a micro-clustering approach which transforms symbolically the expression time series data and assigns a unique integer identifier (hash value) to each expression motif [27]. Probe sets that are very similar in temporal shape “hash” to the same value forming an expression motifs. Having assigned the temporal expression profiles to distinct motifs, the next task is to select expression motifs that would appear to be highly non-random. Thus, the data are further processed by calculating a p-value based on the cluster size and filtering out those expression motifs that are highly likely to be generated by a random model.
Having identified the statistically significant expression motifs from the initial large set of micro-clusters we identify a discriminating set of critical temporal shapes that best characterizes the intrinsic transcriptional dynamic response of the system. In doing so, we explore the concept of Transcriptional State (TS) previously introduced in [27]. We define the TS of the system as the overall distribution of expression values at a specific time point by quantifying the deviation of the system at each time point versus a baseline distribution (t=0hr) applying a Kolmogorov-Smirnov test [28]. Therefore, we are interested in selecting a set of expression motifs whose corresponding transcriptional state deviates maximally from the baseline (distribution of expression values at time t = 0hr). This selection is a combinatorial optimization problem for which we apply a stochastic optimization algorithm, based on simulated annealing (SA) [29]. We run simulated annealing parametrically with respect to the number of expression motifs in order to identify the minimum number of informative motifs.
The basic assumption is that due to an external disturbance, i.e., LPS administration, the system is perturbed from homeostasis and eventually, once LPS is cleared and the inflammatory reaction is eliminated, the host returns to the original state. Due to global nature of the transcriptional measurements and the fact that we do not a priori select a limited set of responsive genes, the entirety of the transcriptional response is expected to exhibited a rather Gaussian type of response with no clear defining responses [30]. We have, however, previously demonstrated that through the use of the concept of TS it is possible to “tease out” the essential components of the cellular response in response to an external disturbance.
Functional Characterization of Essential Responses
Having de-convoluted the inflammatory signal into its essential components, it is hypothesized that genes whose transcriptional signatures are highly correlated with the essential responses account for the maximum deviation of the system from its baseline (homeostasis) and thus play a major role in the dynamic evolution of the inflammatory process. The biological relevance of the intrinsic responses is identified by evaluating the enrichment of the corresponding subsets in inflammation-specific pathways using ARRAYTRACK [31].
An Indirect Response Model of Inflammation
In our injury model, the inflammatory response is activated when endotoxin is recognized by pathogen recognition receptors [32]. The LPS-induced stimulation initiates a complex signaling cascade that ultimately targets the transcription initiation of pro-inflammatory cytokines [33]. During the recognition process LPS binds to the LPS-binding protein in plasma and is delivered to the surface receptor CD14. Subsequently, LPS is transferred to the signaling receptor toll-like receptor 4 (TLR4) with the recruitment of its essential accessory protein MD2 [34; 35; 36]. Downstream of the receptor-ligand complex there are intermediate secondary messages that involve both the amplification of the signaling and its diversification in the cytoplasmic region so that ultimately such a signal transduction cascade will activate critical signaling modules that will subsequently lead to the translocation (activation) of pro-inflammatory transcription factors (e.g. NF-kB) responsible for the transcription initiation of inflammatory genes. Therefore LPS interacts with its signaling receptor (TLR4) in order to induce the signal transduction cascade that triggers essential signaling modules for the activation of pro–inflammatory transcription factors. This cascade of events is eventually manifested through the coordinated transcriptional changes measured via high-throughput microarray analyses [26]. Due to our inability to precisely model such a cascade of events using elementary kinetic steps we will assume that the effect of the extracellular inflammatory signal (LPS) in initiating the transcriptional machinery is indirect. Thus we propose to model such a transcriptional event using the basic principles of an Indirect Response Model (IDR) widely used in developing pharmacodynamic and pharmacogenomic models [37; 38]. Despite the fact that an IDR model does not take explicitly cross-talk interactions into account, the principles of IDR approach can be extended to describe transduction processes and time dependent disease processes. This would lay the foundation for gaining a more “mechanistic” insight. The details of the components and structure of the model are discussed extensively in the Results section.
Design of in silico Experiments
Of particular interest are computational tests performed to predict the possibility and extent of abnormal responses resulting from various levels of inherent mechanistic dysregulation. In the following we will demonstrate the ability of our model to enable such “predictions” and provide further evidence of the appropriateness of the assumptions invoked in the development of the model. We have notionally devised three levels of in silico “predictions”. Regarding these “predictions” we should underline that such a term is loosely used to demonstrate the potential of our model to reproduce clinically relevant conditions. First, we explore the implication of increasing levels of initial insult since this would probably constitute the most obvious irreversible disturbance. Then we explore possible mechanistic dysregulation which may reflect secondary effects that lead to potential malfunction of the response leading to sustained inflammation. Finally, we explore the emergence of “memory” effects and evaluate the implication of priming the host with controlled level of endotoxin stimulus as a prequel to the main inflammatory stimulus.
Implications of Increased Insult
High concentrations of the inflammatory insult can be responsible for the amplification of the host immune response [39], followed by a dysregulation in the host defense intrinsic dynamics leading to a an unconstrained inflammatory response even after the circulating levels of LPS have been cleared. In order to simulate such a scenario we increase the initial condition of LPS at various levels, i.e. LPS(t=0hr) = (1, 2, 3, 4) inferring in silico the progression of the inflammatory trajectory.
Modes of Dysregulation of the Inflammatory Response
The dynamics of the host response to an inflammatory stimulus are highly complex and non-linear, suggesting that a dysregulation in the dynamics of the inflammatory response in restoring homeostasis can have multiple causes. One such possibility is associated with a malfunction in the clearance rate of endotoxin (which corresponds to a higher exposure of the host response to the stimulus followed by persistence in the concentration of LPS). Secondly, a dysregulation in the intracellular dynamics can occur being responsible for an aberrant response, given that improper regulations of TLR4 signaling might also be involved in the inflammatory component of a disease like sepsis [40]; such a mode of dysregulation separates the host response dynamics from the insulting agent. The model was probed by appropriately manipulating parameters that include: (i) a reduction in the first order degradation rate of LPS and (ii) a reduction in the degradation rate of the active signaling complex.
“Rapid” Tolerance
Repeated doses of endotoxin stimulus in many instances is considerably characterized by a less vigorous immune system which is namely known as endotoxin tolerance [41]. Even though the phenomenon of endotoxin tolerance involves the administration of low, repeated doses of endotoxin over periods of time ranging from one day to a week [42], herein, we opt to investigate the response of the system being pre-exposed to low dose of LPS for less than a day. This is because in our proposed model all the interacting components do resolve within the first 24hr while the system has not acquired a reprogramming dynamic state. However, published studies [43; 44] report that “rapid” endotoxin tolerance can be induced when the system is pre-exposed to a low endotoxin challenge for between 3–6hr. Thus, we simulate such a scenario administering low dose of LPS i.e. LPS(t=0hr)=0.5 followed at t=4hr by the main endotoxin insult, LPS(t=4hr)=1.
“Protective” Tolerance
“Protective” tolerance is another extended paradigm for endotoxin hyporesponsiveness that involves the administration of a sub-lethal dose of endotoxin followed by a high (lethal) one. Such a pre-exposure “tolerizes” the dynamics of the host reversing the implications of a high inflammatory insult. Therefore, the magnitude of endotoxin doses plays a critical role in the underlying dynamics of the host and we reproduce such a scenario infusing low dose of LPS at t=0hr, i.e., LPS(t=0hr) = 1 followed by the administration of high endotoxin concentration, i.e., LPS(t=4hr) = 4.
Lethal Potentiation
Not only the magnitude but also the timing of repeated doses of endotoxin are key determinants for discriminating between endotoxin tolerance and potentiation. The successive administration of low doses of LPS may perturb system’s homeostasis towards the progression of an unresolved inflammatory response. From the modeling standpoint, we simulate such a case administering at t=0hr low dose of endotoxin, i.e. LPS(t=0hr) = 1 which is shortly followed at t=0.5hr by another “sub-lethal” insult, i.e. LPS(t=0.5hr) = 2.
Results and Discussion
Transcriptional Analysis and Major Response Elements
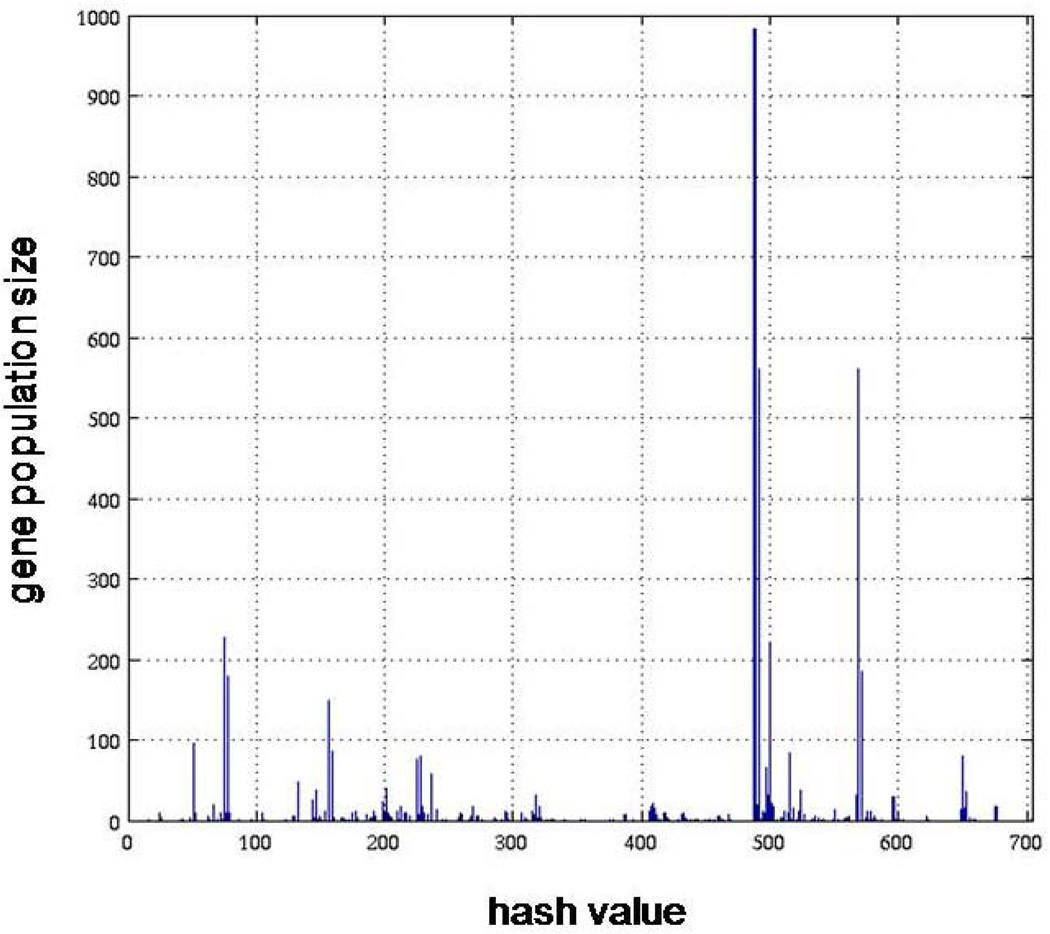
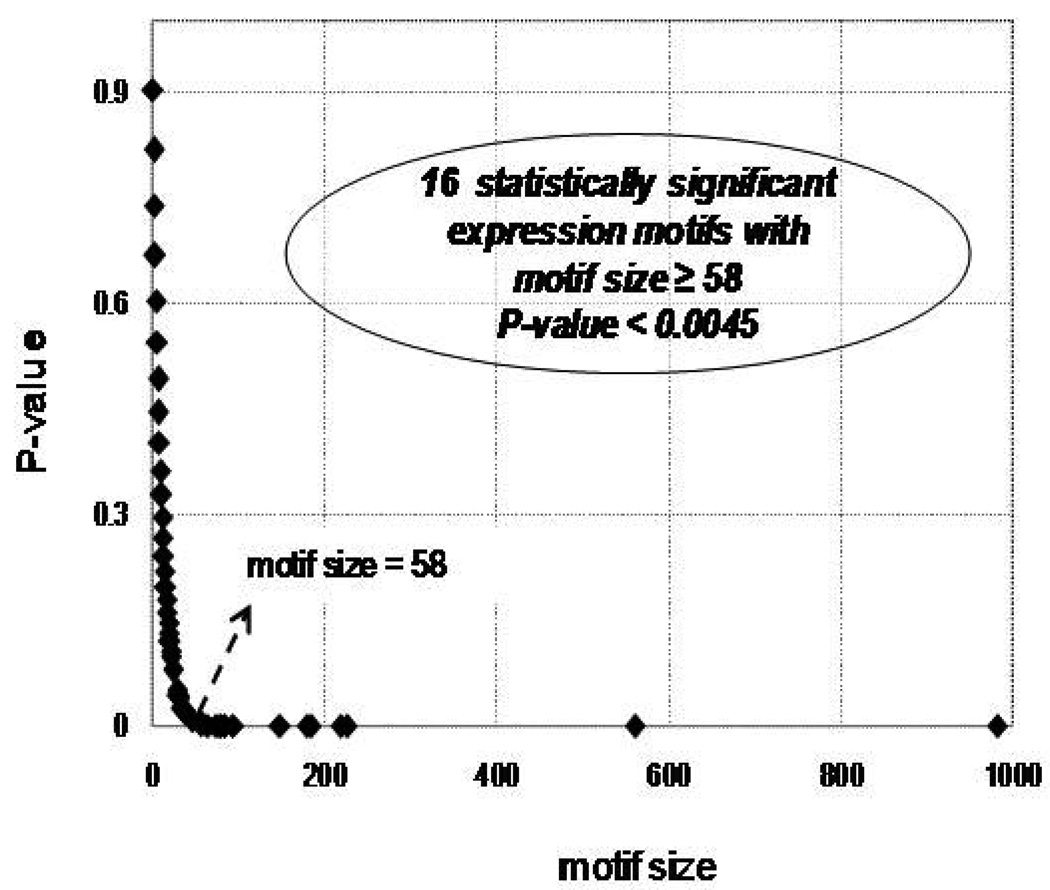
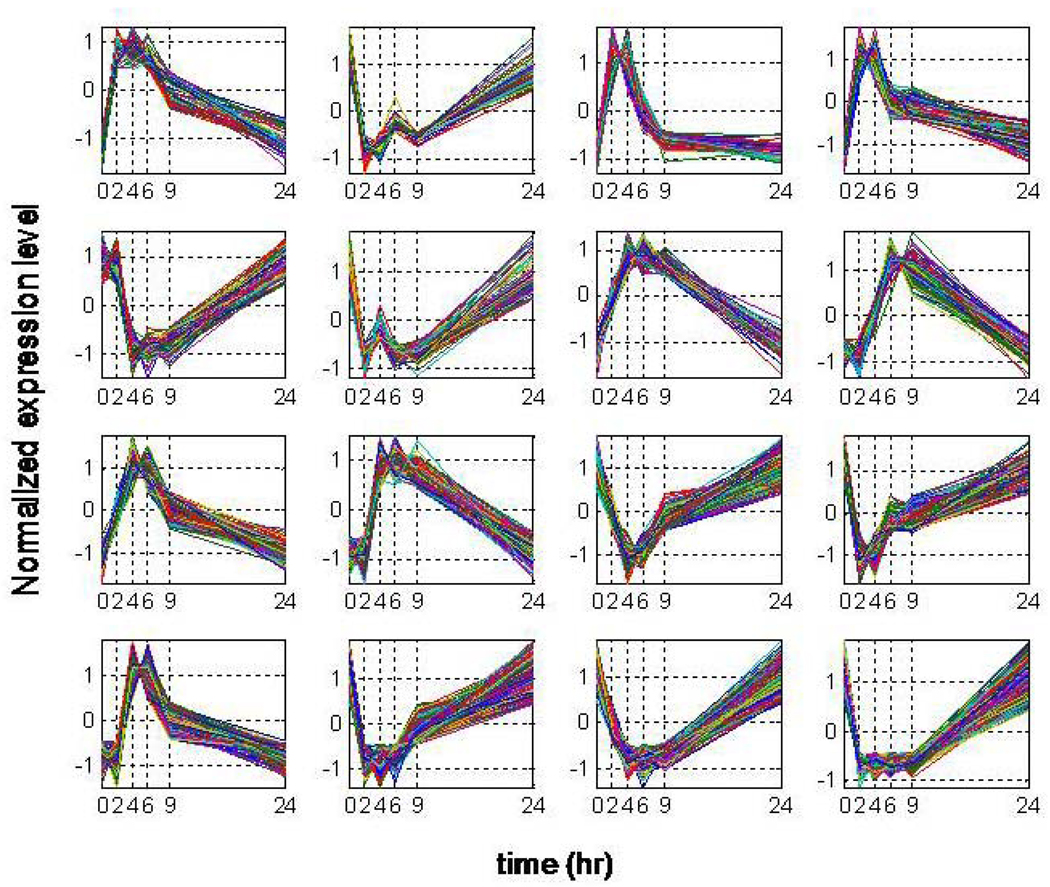
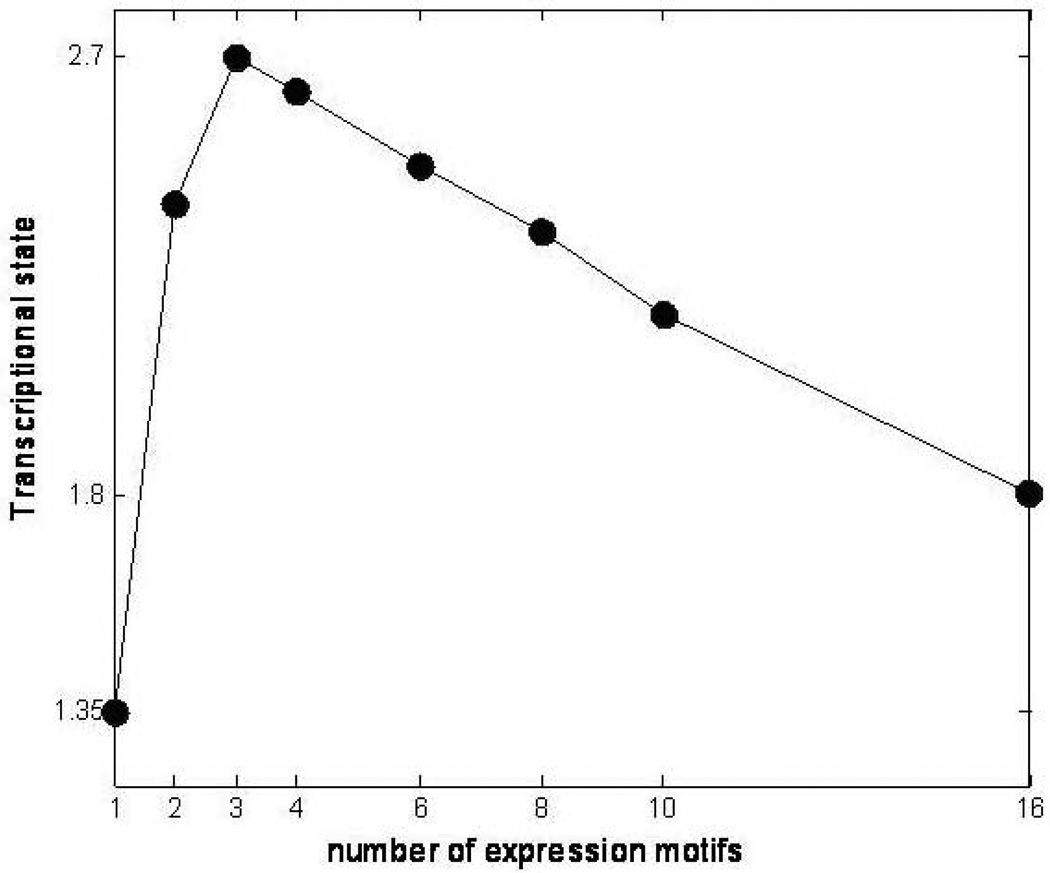
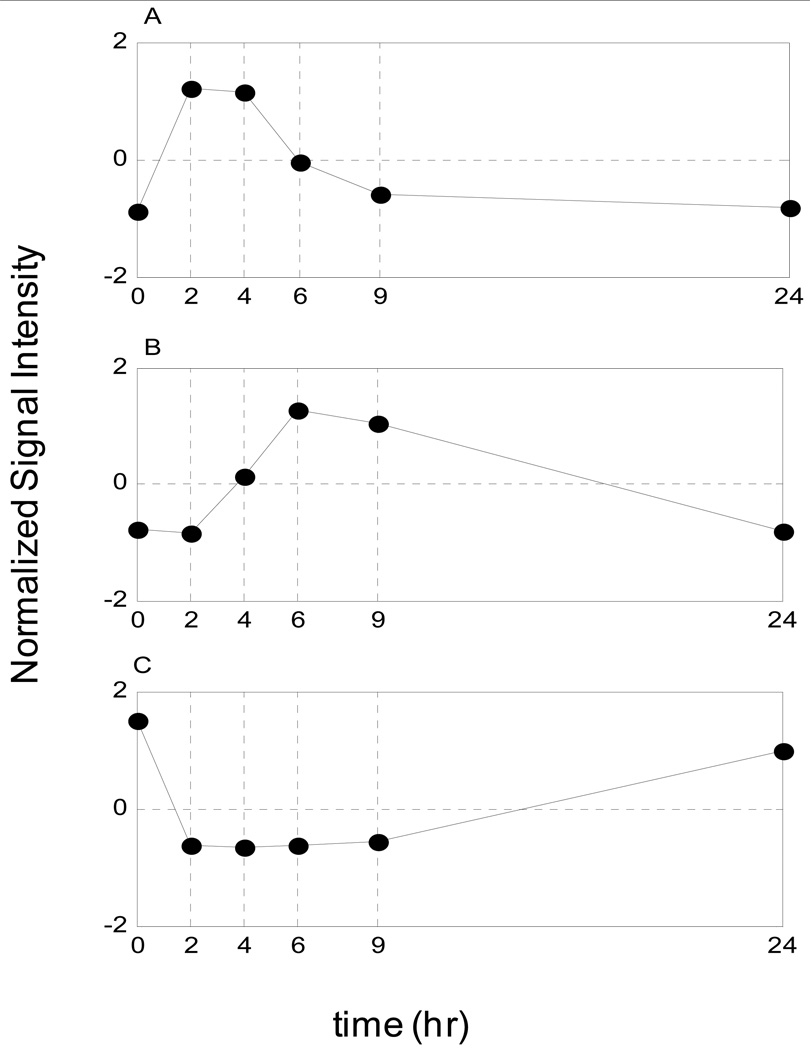
The symbolic transformation of the expression motifs and the subsequent assignment of hash values to each expression profile [27] produces a distribution of motif values for all the available probes, Figure 1. In order to estimate a p-value for each expression motif we generate random data with the same dimensions as the original dataset (5,093 probe sets and 6 time points). Genes that hash to the same integer value for the random data are characterized by a distribution that approximately follows an exponential decay and subsequently we can estimate the cumulative distribution for an exponential model. Thus, an appropriate p-value = 1/(total number of expression motifs) which equals 0.0045, is used to evaluate highly non-random expression motifs (clusters) Figure 2. The analysis generates a sub-set of 16 transcriptional motifs which are considered to be statistically significant in terms of their population size, Figure 3. Therefore, this family of expression motifs, and the associated probe sets, is most characteristic of the exposure of the host to LPS. In order to evaluate the essential basis set, subsets of the 16 profiles that exhibit the largest deviation from homeostasis, we solve the combinatorial selection problem which predicts that the maximum deviation from homeostasis would require three elementary motifs. As seen in Figure 4 the maximum deviation from homeostasis is observed for 3 motifs, whereas further addition of motifs reduced the deviation indicating the addition of less critical responses. Therefore, this result illustrates the existence of distinct critical sets of temporal responses that capture the intrinsic dynamics of the host response to endotoxin administration, Figure 5. The first response is characterized by an early increase in the gene expression level during the first 2hrs after the endotoxin challenge, whereas the second essential response shows an increase at the gene expression level at a later time event (4hrs – 6hrs). The third response is characterized by a downregulation during the time course of the experiment and eventual return to baseline at 24 hrs. All responses resolve, i.e. return to base line 24 hr post-exposure, which is in agreement with the overall design of the study and the reversible nature of the elicited response. Upon identification of the probe sets composing these three essential transcriptional responses we identify significant localization in relevant biological pathways:
Figure 1.
Expression motifs of inflammatory transcriptional signatures of human blood leukocytes. 5,093 probe sets are micro-clustered to 224 expression motifs
Figure 2.
Estimated p-value vs. expression motif sizes. Expression motifs with size > 58 correspnding to p-value < 1/224 (total number of probe sets in informative motifs)
Figure 3.
Temporal Profiles of statistically significant expression motifs: Normalized Signal Intensity of expression motifs with P-value <0.0045 vs. time
Figure 4.
Quantified Transcriptional State of the System vs. number of expression motifs. The maximum perturbation in the intrinsic dynamics of the system occurs for 3 distinct expression motifs
Figure 5.
Essential Transcriptional Elements of the Inflammatory Response. A) Pro-inflammatory response (P), B) Anti-inflammatory response (A) and C) Energetic response (E)
The early up-regulation response, denoted as the pro-inflammatory component (P), is enriched in genes involved in Cytokine-Cytokine receptor interactions (C-X-C motifs and cytokines – CXCL1, CXCL2, CCL20) as well as in Toll like receptor signaling pathway (CCL4, IL1B, IL8). Moreover, we identified genes (IL1A, IL1B, IL1R1, IL1R2) which participate in MAPK signaling pathway – crucial in activating transcription factors that act synergistically with proinflammatory transcription factors such as members of NFkB/RelA family. The late up-regulation response, denoted as anti-inflammatory component (A), is enriched in genes functionally participating in the JAK – STAT cascade (IL10RB, JAK3, STAT2, STAT5B) which is essential to regulate the expression of target genes that counter - react the inflammatory response. In addition to this, it is emphasized [45] that a STAT pathway from a receptor signaling system is a major determinant of key regulatory systems including feedback loops such as SOCS induction which subsequently suppresses the early induced cytokine signaling. Moreover, in this class we capture the increased expression of the gene (SP1) that encodes a protein which acts as an essential activator for IL10 signaling [46]. Moreover, we identified the late increased expression of IL10RB which is assumed to be indicative of the IL10 signaling cascade. Finally, the down-regulation response, denoted as the energetic component (E), is characterized by a set of genes, which are mainly involved in the cellular bio-energetic processes. In addition we find genes essential to ribosome biogenesis and assembly (RPL/RPS family) as well as genes participating in the protein synthesis machinery, oxidative phosphorylation (ATP5A, COX11, NDUFA11) and pyruvate metabolism (PDHB, PDHX, MDH1). Endotoxin–induced inflammation causes the dysregulation of leukocyte bioenergetics and persistent decrease in mitochondrial activity can lead to reduced cellular metabolism [47]. Organ function has been associated with changes in bioenergetics and metabolic activity [48]. These transcriptional responses effectively decompose the overall dynamic and potentially define the constitutive elements of the overall response thus defining the transcriptional signatures in response to LPS administration. We will further explore the possibility of using the elementary responses as the surrogates for predicting the complex dynamic behavior of the system through an appropriately constructed mathematical model.
In order to reproduce the experimental data we select the transcriptional signature of specific genes representative of each essential response. IL1B is selected to serve as the representative “biomarker” of the pro-inflammatory response. The gene transcript of IL10RB is considered to be indicative of the immune-regulatory signal of the anti-inflammatory response. Finally, a subunit of NADH ubiquinone dehydrogenase complex (mitochondrial component) NDUFC2 is considered as the proxy for the energetic component. These essential transcriptional signatures are normalized by taking the ratio of the measured mRNA level at each time point with respect to the control time point (t = 0hr). Selecting any other gene that belongs to the aforementioned essential inflammatory responses can very well be used as a surrogate for a representative of the response and will not alter the qualitative characteristic of our semi–mechanistic mathematical model to be described in the following section.
Elements of an indirect response model of human endotoxin-induced inflammation
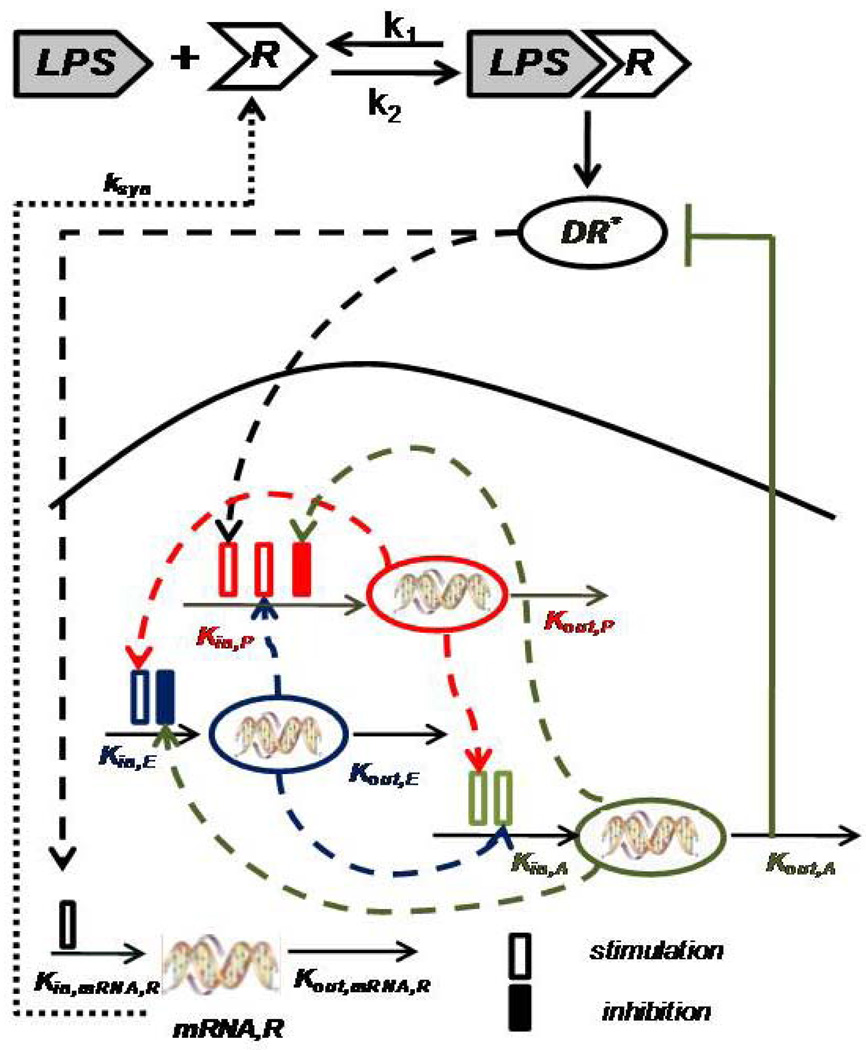
We consider each previously identified transcriptional motif to be the manifestation of a process involving a synthesis and a degradation term. In the most general case it is assumed that the synthesis, or production, follows 0th order kinetics, whereas the degradation follows 1st order kinetics [49]. The underlying assumption of IDR models is that external signals affect indirectly the synthesis and/or degradation term of the response of interest, in our case the transcriptional dynamics. As a result, the existence of such signals can either stimulate or inhibit the production and degradation rate of the response. Therefore, we assume that the upstream activated ligand-receptor signaling complex serves as the intracellular signal that indirectly will stimulate the production rate of transcriptional effects associated with the pro-inflammatory response as well as with the transcriptional activation of the gene transcript of the receptor (mRNA,R).
The binding interaction between the endotoxin (LPS) and the receptor (R) is assumed to be a standard ligand-receptor interaction [50]. The activated ligand-receptor complex triggers an intracellular signal (DR*) which indirectly stimulates the production rate of the pro-inflammatory response (P). This pro–inflammatory response can be characterized as the “first-line” transcriptional response that is triggered upon the recognition of the extracellular ligand (LPS) by the pattern recognition receptors (PRR e.g. TLR4) [51]. Pro-inflammation will serve as the signal that will further stimulate the downregulation of genes that are associated with the cellular energetic processes [52]. We hypothesize that the pro-inflammatory response acts as the stimulatory factor for the energetic response whilst a dysregulation in the cellular bio-energetics can serve as a positive feedback danger signal to the pro-inflammatory response.
The anti-inflammatory response serves as the essential immunoregulatory signal that aims at restoring homeostasis in the host defense system. Thus, it will be stimulated by the activation of the inflammatory components which are the pro–inflammation and the energetic response. Thus, it will serve as the inhibitory signal on the production rate of the pro–inflammation and the energetic response. We are assuming that it will negatively regulate the TLR pathway [51] modeling it as inhibition of the activated intracellular signal DR*. The elements of the network of interactions defining the indirect response model are shown in Figure 6.
Figure 6.
Qualitative Structure of the Indirect Response Model: LPS binds to the receptor (R) and forms the complex (LPSR) while it activates the signaling complex (DR*) which indirectly stimulates the production rate Kin, P of the pro-inflammatory (P) response. The pro-inflammatory response indirectly stimulates the production rate of the energetic (E) response (Kin, E) and the production rate of anti-inflammatory (A) response (Kin, A). The energetic response will stimulate both pro-inflammation and anti-inflammation whilst anti-inflammation will serve as the immunoregulatory component of the system restoring homeostasis intracellularly.
Developing an indirect response model1
The dynamics of the inflammatory stimulus (LPS) is described in [a] as a convolution of two terms: a first order elimination with rate klps, 2 and a logistic-type function with growth rate klps, 1. Effectively despite the presence of various mediators that are activated in response to LPS (e.g. LPS binds to LBP plasma protein during its recognition from the host) we model a single compartment pharmacodynamics model for LPS assuming a homogeneous circulating blood compartment. The logistic function is usually used to model a variety of physical situations in which a quantity’s growth is “self-limited” which means that the initial growth is approximately exponential and as saturation begins the growth stops [53]. Therefore, depending on the relative magnitude of the two rate parameters of the clearance of LPS we can simulate situations where the bacterial concentration is not fully eliminated. In human subjects endotoxin is cleared within the first 2 hrs of post-LPS administration with an approximate average half time τ1/2 ~ 8–15 min [54]. The two parameters klps, 1 and klps, 2 have been independently estimated so that the LPS profile decays within two hrs in the absence of any complications.
The dynamics of the TLR4 receptor (R), [b], depend on the association/dissociation parameters of the ligand–receptor interaction [50] whose the corresponding parameters k1 and k2, are based on literature values [55] and the translation of its mRNA,R to surface protein (ksyn). The rate of translation ksyn of the mRNA, R to the corresponding surface protein describes the dynamic evolution of synthesis of new receptors; hence this parameter is estimated so that the dynamic profile of the surface free receptor is down-regulated based on the premise that under the inflammatory stimulus the surface free receptors are occupied.
The dynamics of the gene transcript of the receptor, [c], is characterized by a production rate (Kin,mRNA,R) and a degradation rate (Kout,mRNA,R) but is assumed to be indirectly stimulated by the convoluted activated DR* signal as shown in Figure 6. Experimentally, the transcript of the receptor is characterized by an up-regulation for the first 4 hrs post-LPS administration and then returns to baseline [56].
The dynamics of the equilibrium complex (LPSR) is characterized by the binding parameters k1, k2 and the parameter k3 which characterizes the rate of formation of the activated signaling complex, DR*, [d]. The formation of the activated signaling complex (DR*) is proportional to the equilibrium complex with a rate constant k3 and it decays with rate k4 [e]. However, we assume that the essential anti-inflammatory component will indirectly regulate the activated intracellular signaling complex. Such a negative feedback serves the purpose of incorporating a regulatory effect on the intracellular activated signaling complex once the transcriptional response has been initiated. In addition to this, the non-linear Hill type function serves the purpose of modeling a bistable behavior of the system [57]. Such a bistability is an essential characteristic of the non-linear dynamics of inflammation as suggested from various animal studies [58; 59; 60; 61; 62]. An increase in the dose of the inflammatory stimulus can be responsible for an overwhelming inflammatory response. We should emphasize that the exponent coefficient of the non-linear function in [e] does not quantitatively correspond to a Hill coefficient; instead it can be characterized as an ultrasensitive parameter which is associated with the bistable behavior of the system. What is more, the functional form of the activated signaling complex (DR*) in [e] allows us to model an improper (uncontrolled) TLR4 signaling even though the inflammatory stimulus (LPS) has been completely eliminated from the system. Given the role of TLRs in inducing strong inflammation improper regulation of this signaling pathway may also be involved in inflammatory diseases [40].
At the transcriptional response level the convoluted activated signal complex (DR*) indirectly stimulates the production rate of the essential pro-inflammatory response (P) which quantitatively is expressed by the linear function (HP,DR*), [f]. We also assume that the energetic response variable will be responsible for more pronounced inflammation and thus stimulates the pro-inflammatory response (HP, E). The anti-inflammatory signaling component is assumed to inhibit the production rate of the pro-inflammatory transcriptional signature.
The anti-inflammatory signal (A) is stimulated by the activated pro-inflammatory response (HA,P) as well as by the energetic response (HA, E) and it decays with rate Kout,A., [g]. Finally, the energetic response (E) is indirectly stimulated by the proinflammatory response (P) and the anti-inflammatory component (A) indirectly counter-regulates both inflammatory components, i.e., the pro-inflammation and the energetic response of the system, [h]. The parameters associated with the production and degradation rate of each essential transcriptional signature are estimated in order to best predict the essential responses with their experimental measurements. Equations [i–m] denote the functional forms of the indirect response. It is important to realize that special effort was placed in order to avoid high non-linearities in the system.
Estimation of Relevant Model Parameters
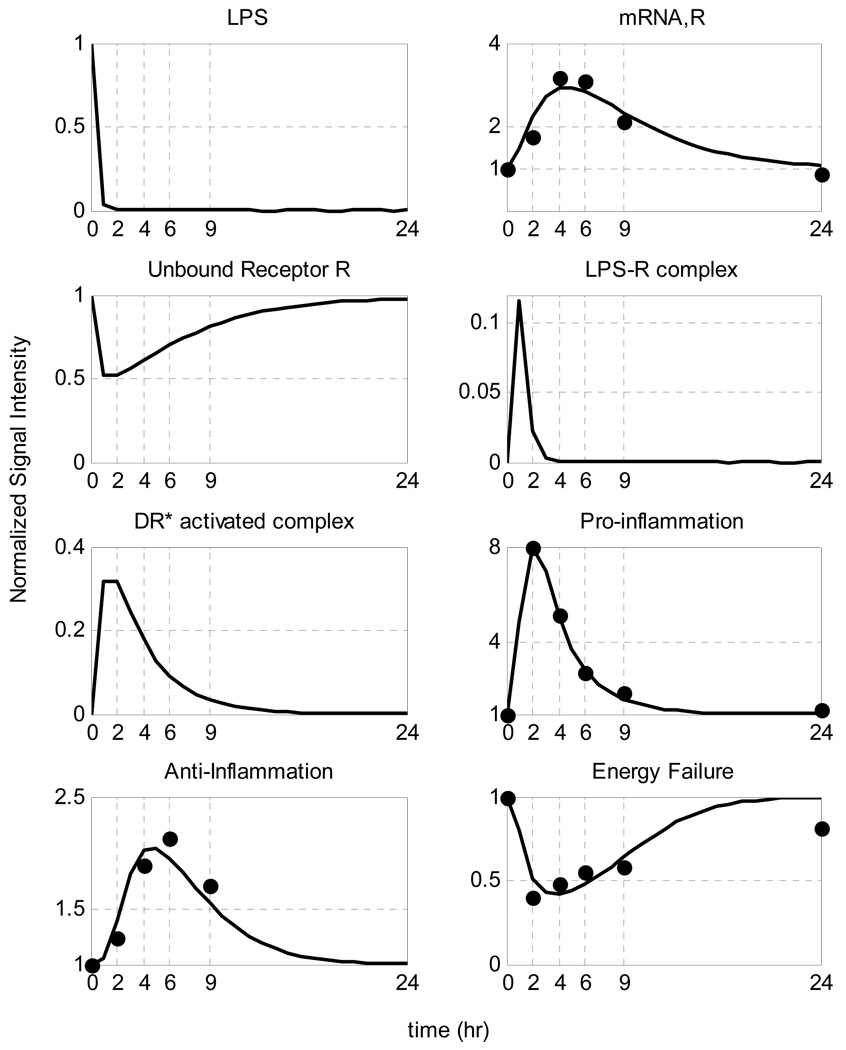
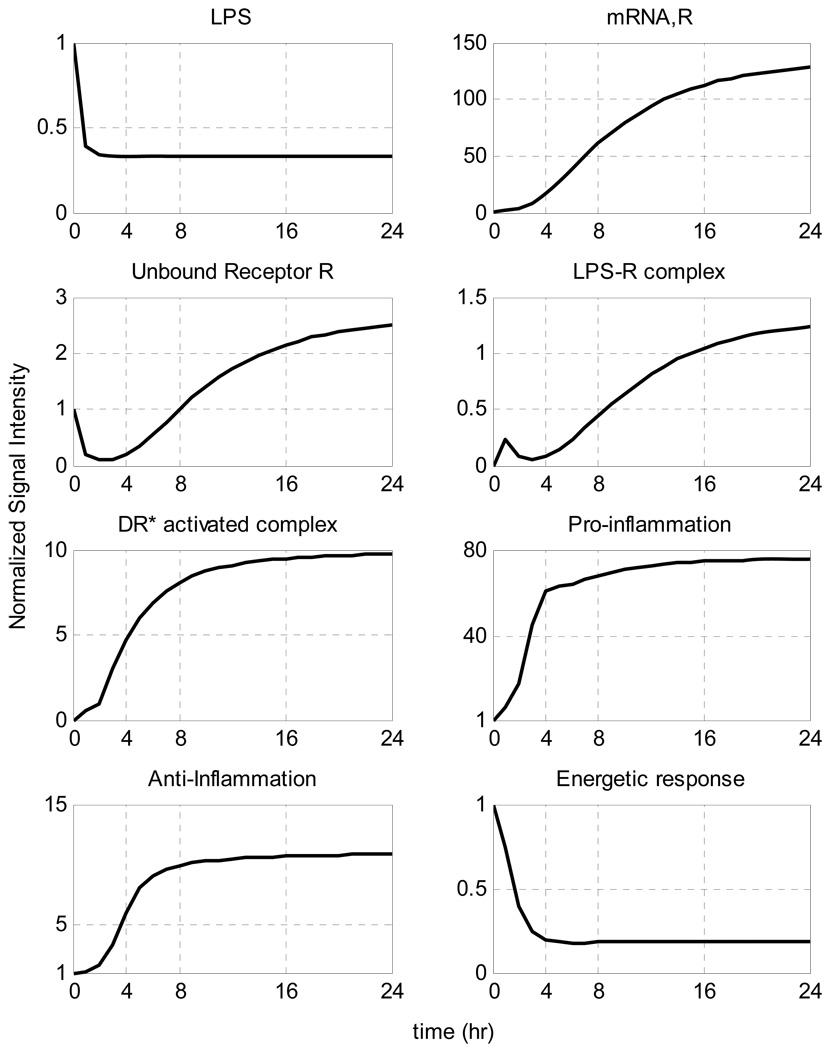
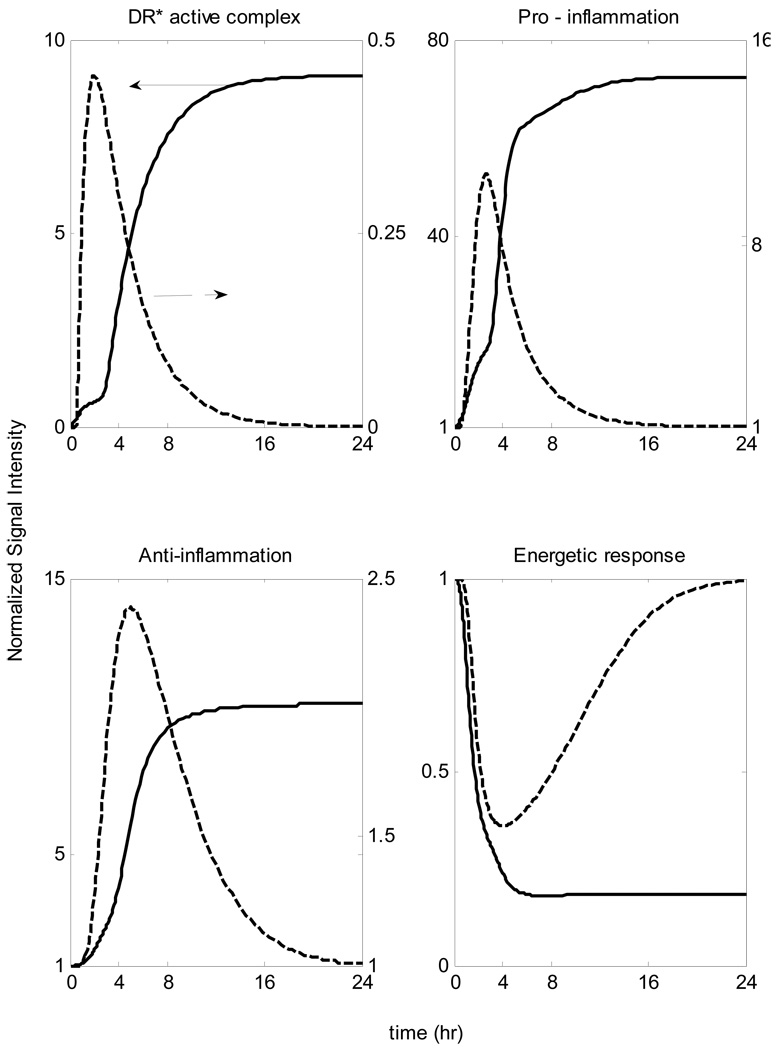
A self-limited inflammatory response to the endotoxin stimulus corresponds to resolved dynamic profiles for all the elements that constitute our model. In our computational model the host restores homeostasis without any external intervention. We speculate that the initial normalized concentration of the inflammatory stimulus (LPS) quickly decays so that it completely clears within the first 2 hours whereas the other essential components should return to their baseline within the first 24 hrs after the endotoxin administration (homeostasis) based on the design of the experiment. Given the available experimental data [1] we can, in principle, evaluate appropriate model parameters using standard parameter estimation techniques. The relevant kinetic parameters are depicted in Table 2, whereas the performance of the model in reproducing the self-limited responses is shown in Figure 7.
Table 2.
Estimated values of the parameters based on self-limited response data. The values for k1 and k2 are taken from [55]
| Parameter | Value | Parameter | Value | Parameter | Value | Parameter | Value |
|---|---|---|---|---|---|---|---|
| klps,1 | 4.500 | k2 | 0.040 | k4 | 0.330 | Kin,E | 0.050 |
| klps,2 | 6.790 | k3 | 2.000 | kc | 3.000 | Kout,E | 0.234 |
| ksyn | 0.020 | Kin,mRNA,R | 13.467 | Kin,P | 0.093 | Kin,A | 0.256 |
| k1 | 3.000 | Kout,mRNA,R | 0.211 | Kout,P | 2.428 | Kout,A | 0.860 |
| Kp,DR* | 15.717 | Kp,E | 25.191 | KA,P | 0.022 | KA,E | 2.291 |
| KE,P | 3.644 |
Figure 7.
Model Building Results: Dynamic profiles of the elements that constitute the mechanistic model of endotoxin-induced inflammation. Experimentally measured normalized mRNA transcript levels are denoted by symbols (•), solid lines (-) are the model predictions.
Performing in silico experiments
Building a mathematical model that can predict relevant biological implications to the host response to endotoxin allows us to identify ways of both controlling and modulating such a complex phenomenon. Thus, the correctness of the model will be tested based on its ability to not only reproduce available data, but rather to qualitatively predict uncontrolled responses to be discussed as it follow.
Implications of Increased Insult
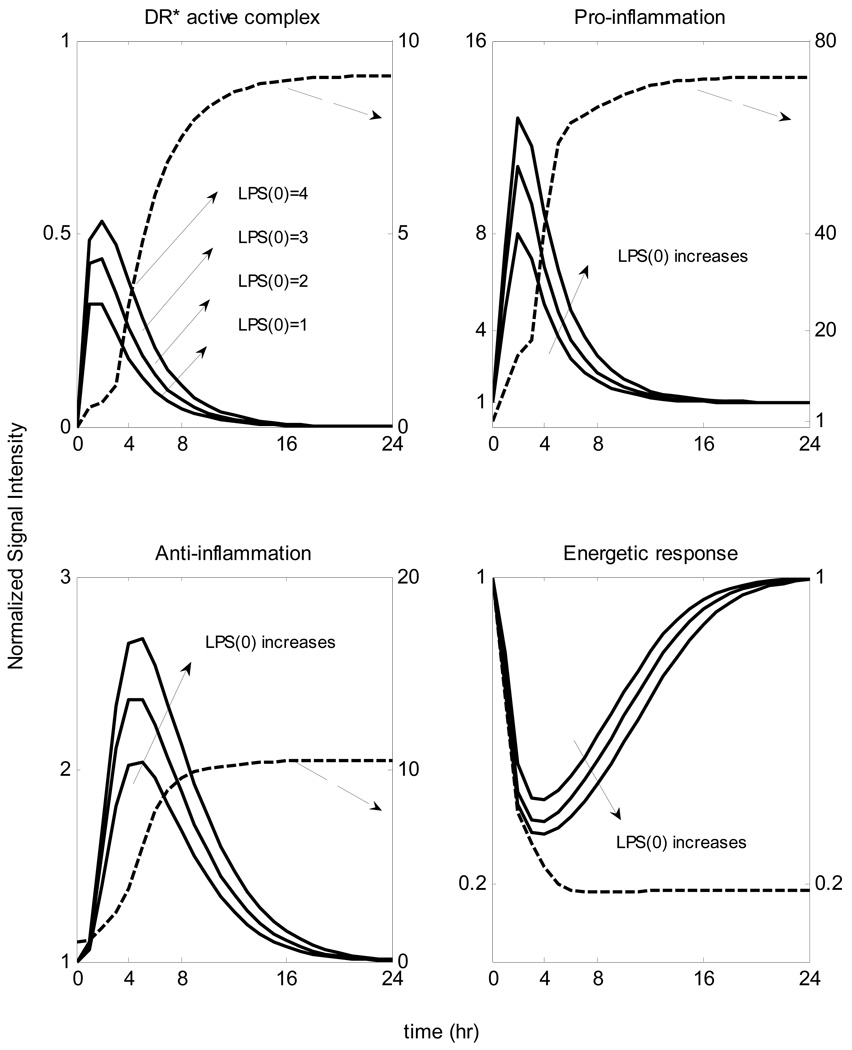
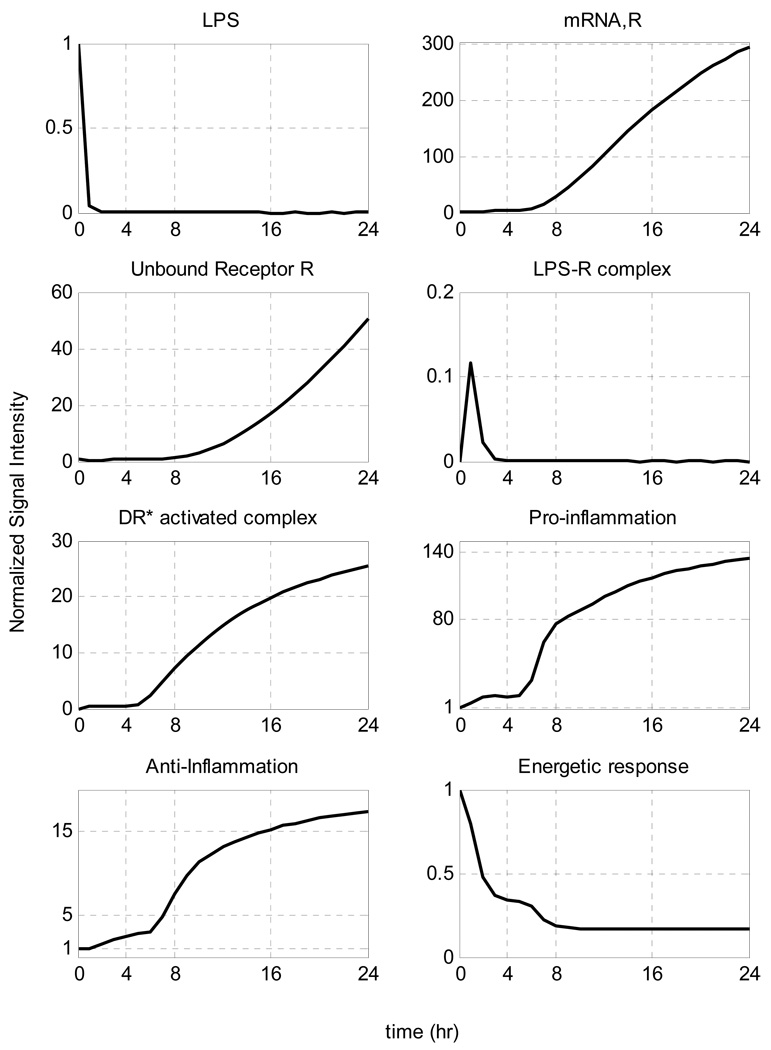
In Figure 8 we simulate the situation in which the initial levels of LPS are increased. We observe that when the concentration of the inflammatory stimulus exceeds a critical threshold, the inflammatory response does not abate. In this case it is the host response to endotoxin rather than the stimulus itself that yields the progression of a systemic inflammatory response syndrome that fails to resolve. Clinically, in a retrospective analysis of critically ill patients [63], a progression of septic shock characterized a number of patients without documented infection. Namely, the mortality rate of these patients was higher compared to infected patients.
Figure 8.
Temporal responses of critical inflammatory components for various initial conditions of the inflammatory stimulus. A high concentration of LPS can cause a malfunction in the dynamics of the host response to infection described by an exacerbated inflammatory response (dashed line). Solid lines correspond to self-limited responses, the dashed line represent a predicted unconstrained inflammatory response when the LPS concentration exceeds a critical value
Malfunction in the clearance rate of LPS
In Figure 9 we simulate the case of a persistent disease which corresponds to an increased exposure of the host response to the inflammatory stimulus. Such a case is simulated by manipulating (decreasing) the parameter associated with the degradation rate of LPS. Although decreased degradation of LPS is not associated with a distinct, defined clinical condition, it is possible that this phenomenon may exist. For example, it is known that triglyceride-rich lipoproteins bind to LPS and that these complexes are cleared by binding to lipoprotein receptors. Furthermore, these receptors are abundant in the liver which clears ~70% of lipoproteins from the circulation. Therefore, it can be postulated that patients with liver dysfunction may have impaired clearance of LPS. But, even without this speculation, another purpose for simulating “decreased LPS clearance” was to determine the response of the model to differing, but plausible, perturbations.[64]. Thus, as seen in Figure 9 decreasing the degradation rate to half of its initial value (self-limited response) we observe that even small amounts of endotoxin might account for an overwhelming inflammatory response. Such persistence in the inflammatory stimulus leads to a sustained elevation of the activated intracellular signaling complex (DR*) which subsequently accounts for an uncompensated inflammatory response. In particular, there is an overexcitation of both pro– and anti– inflammatory mediators that are coupled with the uncontrolled regulation of the energetic response which settles to an uncontrolled state (energetic depletion). Such a simulated clinical scenario lies in agreement with experimental evidence about persistent endotoxin activity in critically ill patients undergoing gram-negative sepsis [65].
Figure 9.
Temporal responses of inflammatory components in persistent infectious inflammatory response where the inflammatory stimulus cannot be eliminated responsible for the observed persistence in the dynamic profiles of the inflammatory constituents. Reducing the degradation rate of LPS to half of its initial value the inflammatory stimulus cannot be cleared
Maladaption in the active intracellular signaling DR*
It is now generally accepted that the host response plays a pivotal role in determining the outcome of an overwhelming inflammatory response. Thus, our model allows us to explore another mode of unconstrained inflammatory response that emerges from a dysregulation in the intracellular dynamics downstream of the ligand – receptor complex. In general, downstream of the activated LPSR complex, there are various kinases, second messengers that are being activated in response to LPS that are constituents of the signal transduction cascade. A dysregulation in the dynamics of the intracellular domain might hamper the homeostatic control of its domain, accounting for a persistent activated signaling that will over – excite the elementary inflammatory mediators, Figure 10. We explore such a perturbation in the dynamics of the system by decreasing the degradation rate of the active signaling complex (DR*) to a value which is about half of its initial value which corresponds to a self-limited inflammatory response. The unconstrained inflammatory effect occurs downstream of the activated signaling complex and as result the rate of the formed complexes is not affected by this perturbation given that the inflammatory stimulus is completely eliminated from the system. However, a dysregulation in the intracellular homeostasis can lead to a cytokine “storm” that is followed by a global disturbance in the inflammatory control system followed by elevated/uncompensated anti – inflammatory signaling as seen in Figure 10. If the gene transcript of the receptor is persistently elevated it will also lead to an overproduction of protein receptors; therefore, the baseline of total free receptors is dysregulated. Both cases correspond to biological scenarios exploring different modes of dysregulation in the inflammatory response which suggest that there is a critical time interval during which any therapeutic intervention may restore homeostasis. In the drug discovery area there is emphasis on discovering endogenous mediators that can modulate the inflammatory response but they are not clinically tractable in terms of a broader therapeutic time window [66]; for example, one reason for the failure of anti – TNF treatment for sepsis in clinical settings may be due to its narrow therapeutic window (very early release).
Figure 10.
Temporal profiles of persistent non – infectious inflammation. It is not the infection itself but rather the host response to infection that plays a determinant role in controlling the outcome of an overwhelming inflammatory response. Manipulating the degradation rate of the activated intracellular signaling (e.g. reducing it by half of its initial value) perturbs the homeostasis of the system
The Emergence of Memory Effects
“Rapid” Tolerance
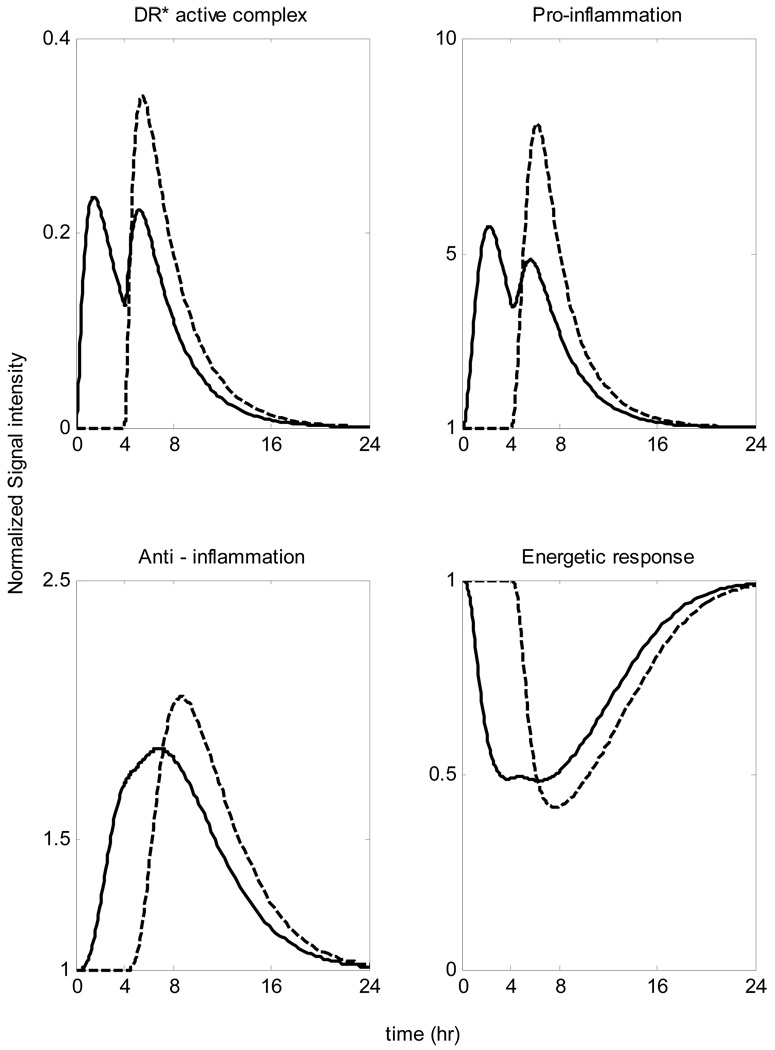
When the system is pre-exposed to a lower inflammatory stimulus for about 4 hrs before the main endotoxin challenge our model predicts a much less vigorous inflammatory response as seen in Figure 11. In particular such an event, which can be characterized either as a short - time attenuation effect or else rapid tolerance is experimentally observed by the decreased concentrations of various pro-inflammatory mediators e.g. TNF-a, IL1B in response to secondary ex vivo whole blood stimulation with LPS [43]. In addition to this, in the experimental study [44] concentrations of the particular pro – inflammatory mediator (TNF-a) were decreased profoundly ex vivo at 3hr – 6 hr after in vivo endotoxin administration. However, by 24 hrs the endotoxin tolerance had completely resolved. Such pre-conditioning results in an attenuation of the inflammatory response characterized by a less vigorous intracellular signaling coupled with the decreased peak level of the pro-inflammatory response.
Figure 11.
Rapid Endotoxin Tolerance: Pre – exposuring the system into a smaller inflammatory insult results in a reduction in the cell capacity to respond to the main endotoxin challenge which is characterized as a short-time attenuation scenario Solid line: LPS(t=0hr)=0.5 & LPS(t=4hr)=1 Dashed line: LPS(t=0hr)=0 & LPS(t=4hr)=1
“Protective” Tolerance
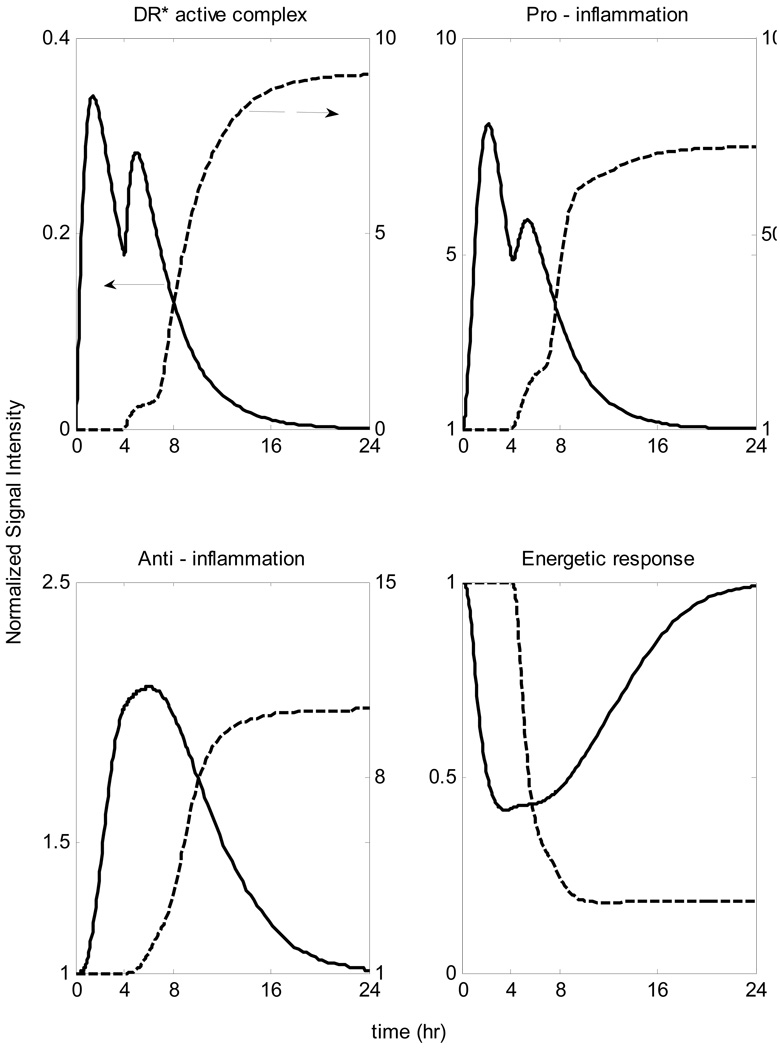
The rapid tolerance scenario observed in Figure 11 implies that the emergence of controlled memory in the system, elicited after the first dose of endotoxin is administered, plays a major role in determining the modulated dynamics of the system in response to the second dose. If the system is primarily exposed to a much higher dose of endotoxin which is responsible for an overwhelming inflammatory response then the pre-exposure to lower non - lethal dose of LPS can modulate its intracellular dynamics that reverses the lethal outcome of the main endotoxin dose, Figure 12. This is a response characteristic of endotoxin hypo-responsiveness. As a result the system shows a reduced capacity (hypo-responsiveness) in response to a high concentration of the inflammatory stimulus which may be associated with decreased TLR signaling by proteins that negatively regulate LPS-induced inflammatory responses [67].
Figure 12.
Endotoxin Hypo-responsiveness: Pre-existing infection might cause a profound hypo-responsiveness in system’s response to a lethal LPS challenge Solid line: LPS(t=0hr) =1 & LPS(t=4hr)=4 Dashed line: LPS(t=0hr)=0 & LPS(t=4hr)=4
Lethal Potentiation
Rapid tolerance and endotoxin hypo-responsiveness (or else “protective” tolerance) are associated with an emergent acquired dynamic state of the system that actually modulates the response of the system not to respond rigorously to the primary endotoxin challenge. However, such an emergence is highly time dependent which implies that if the repeated doses are characterized by a very short time interval it is possible the dynamics of the system to result in an overwhelming inflammatory response. Therefore, the successive administration of two inflammatory insults that individually account for constrained (“self-limited”) inflammatory responses might be determinant to the outcome of sepsis (unresolved inflammatory response). Such an event can occur because of the absence of a “protective” memory in the system so that the system has not elicited its regulatory mechanism to compensate for the cumulative result of two successive doses. Such an abrupt insult might dysregulate the dynamics of the host response to infection having a detrimental effect in the physiological state of the system as seen in Figure 13.
Figure 13.
Lethal Potentiation: Successive administration of small doses of endotoxin can lead to an unresolved inflammatory response (due loss of “regulatory” memory Solid line: LPS(t=0hr)=1 & LPS(t=0.5hr)=2 Dashed line: LPS(t=0hr)=0 & LPS(t=0.5hr)=2
These results indicate that the cellular response is critically affected by the mode of exposure, thus demonstrating the need for an appropriate, quantifiable, model to account for, and integrate the, various components constituting the response. Furthermore, our results clearly indicate that the dynamics of the response are definitely affected by the parameters defining the exposure to the inflammatory agent.
In conclusion, the proposed receptor mediated indirect response model of inflammation describes the sequence of inflammatory events connecting extracellular signals and transcriptional dynamics. The mechanistic-based indirect response model, allows us to identify possible critical targets either upstream of the activated signaling, such as endotoxin elimination rate, or downstream are associated with modulating the Toll- like receptor signaling pathway. The temporal profiles of the essential inflammatory components under an unresolved inflammatory state highlight the potential importance of early effective therapeutic interventions e.g. (2hr – 4hr) whilst after 4hrs the system seems to have lost any potential for attenuation. Furthermore, we explored the possible effects of systemic perturbations associated with repeated pre-exposure to endotoxin (tolerance and potentiation scenarios) emphasizing timing and dosing as the key determinants for endotoxin hypo-responsiveness or lethality. Such a modeling approach enables us to gain a better understanding of the complexities of inflammation via the development of a more mechanistically interpretable model of human inflammation.
Table 1.
Mathematical representation of an indirect response model of endotoxin-induced human inflammation
|
|
(a) | |
|
|
(b) | |
|
|
(c) | |
|
|
(d) | |
|
|
(e) | |
|
|
(f) | |
|
|
(g) | |
|
|
(h) | |
|
|
(i) | |
|
|
(j) | |
|
|
(k) | |
|
|
(l) | |
|
|
(m) |
Acknowledgements
PTF and IPA acknowledge support from NSF grant 0519563, EPA grant GAD R 832721-010-RRA and a Busch Biomedical Research Grant. SEC and SFL are supported, in part, from USPHS Grant GM34695. The authors would like to acknowledge critical input and guidance from Prof. W.J. Jusko and R.R. Almon (SUNY Buffalo). The investigators acknowledge the contribution of the Inflammation and the Host Response to Injury Large-Scale Collaborative Project Award # 2-U54-GM062119 from the National Institute of General Medical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The quantitative model of endotoxin induced inflammation is succinctly presented in Table 1. All equation numbers, denoted as [a] through [m], refer to Table 1
Disclaimer
The Inflammation and the Host Response to Injury “Glue Grant” program is supported by the National Institute of General Medical Sciences. This Manuscript was prepared using a dataset obtained from the Glue Grant program and does not necessarily reflect the opinions or views of the Inflammation and the Host Response to Injury Investigators or the NIGMS.
References
- 1.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF. A network-based analysis of systemic inflammation in humans. Nature. 2005;437:1032–1037. doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 2.Fannin RD, Auman JT, Bruno ME, Sieber SO, Ward SM, Tucker CJ, Merrick BA, Paules RS. Differential gene expression profiling in whole blood during acute systemic inflammation in lipopolysaccharide-treated rats. Physiol Genomics. 2005;21:92–104. doi: 10.1152/physiolgenomics.00190.2004. [DOI] [PubMed] [Google Scholar]
- 3.Talwar S, Munson PJ, Barb J, Fiuza C, Cintron AP, Logun C, Tropea M, Khan S, Reda D, Shelhamer JH, Danner RL, Suffredini AF. Gene expression profiles of peripheral blood leukocytes after endotoxin challenge in humans. Physiol Genomics. 2006;25:203–215. doi: 10.1152/physiolgenomics.00192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowry SF. Human endotoxemia: a model for mechanistic insight and therapeutic targeting. Shock. 2005;24 Suppl 1:94–100. doi: 10.1097/01.shk.0000191340.23907.a1. [DOI] [PubMed] [Google Scholar]
- 5.Wittebole X, Hahm S, Coyle SM, Kumar A, Calvano SE, Lowry SF. Nicotine exposure alters in vivo human responses to endotoxin. Clin Exp Immunol. 2007;147:28–34. doi: 10.1111/j.1365-2249.2006.03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12:60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Zee KJ, Coyle SM, Calvano SE, Oldenburg HS, Stiles DM, Pribble J, Catalano M, Moldawer LL, Lowry SF. Influence of IL-1 receptor blockade on the human response to endotoxemia. J Immunol. 1995;154:1499–1507. [PubMed] [Google Scholar]
- 8.van Deventer SJ, Buller HR, ten Cate JW, Aarden LA, Hack CE, Sturk A. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990;76:2520–2526. [PubMed] [Google Scholar]
- 9.Fong YM, Marano MA, Moldawer LL, Wei H, Calvano SE, Kenney JS, Allison AC, Cerami A, Shires GT, Lowry SF. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Invest. 1990;85:1896–1904. doi: 10.1172/JCI114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross AS, Opal SM. A new paradigm for the treatment of sepsis: is it time to consider combination therapy? Ann Intern Med. 2003;138:502–505. doi: 10.7326/0003-4819-138-6-200303180-00016. [DOI] [PubMed] [Google Scholar]
- 11.Chow CC, Clermont G, Kumar R, Lagoa C, Tawadrous Z, Gallo D, Betten B, Bartels J, Constantine G, Fink MP, Billiar TR, Vodovotz Y. The acute inflammatory response in diverse shock states. Shock. 2005;24:74–84. doi: 10.1097/01.shk.0000168526.97716.f3. [DOI] [PubMed] [Google Scholar]
- 12.Lagoa CE, Bartels J, Baratt A, Tseng G, Clermont G, Fink MP, Billiar TR, Vodovotz Y. The role of initial trauma in the host's response to injury and hemorrhage: insights from a correlation of mathematical simulations and hepatic transcriptomic analysis. Shock. 2006;26:592–600. doi: 10.1097/01.shk.0000232272.03602.0a. [DOI] [PubMed] [Google Scholar]
- 13.Day J, Rubin J, Vodovotz Y, Chow CC, Reynolds A, Clermont G. A reduced mathematical model of the acute inflammatory response II. Capturing scenarios of repeated endotoxin administration. J Theor Biol. 2006;242:237–256. doi: 10.1016/j.jtbi.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Kumar R, Clermont G, Vodovotz Y, Chow CC. The dynamics of acute inflammation. J Theor Biol. 2004;230:145–155. doi: 10.1016/j.jtbi.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 15.Prince JM, Levy RM, Bartels J, Baratt A, Kane JM, 3rd, Lagoa C, Rubin J, Day J, Wei J, Fink MP, Goyert SM, Clermont G, Billiar TR, Vodovotz Y. In silico and in vivo approach to elucidate the inflammatory complexity of CD14-deficient mice. Mol Med. 2006;12:88–96. doi: 10.2119/2006-00012.Prince. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds A, Rubin J, Clermont G, Day J, Vodovotz Y, Bard Ermentrout G. A reduced mathematical model of the acute inflammatory response: I. Derivation of model and analysis of anti-inflammation. J Theor Biol. 2006;242:220–236. doi: 10.1016/j.jtbi.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Vodovotz Y, Chow CC, Bartels J, Lagoa C, Prince JM, Levy RM, Kumar R, Day J, Rubin J, Constantine G, Billiar TR, Fink MP, Clermont G. In silico models of acute inflammation in animals. Shock. 2006;26:235–244. doi: 10.1097/01.shk.0000225413.13866.fo. [DOI] [PubMed] [Google Scholar]
- 18.Huang S, Eichler G, Bar-Yam Y, Ingber DE. Cell fates as high-dimensional attractor states of a complex gene regulatory network. Phys Rev Lett. 2005;94:128701. doi: 10.1103/PhysRevLett.94.128701. [DOI] [PubMed] [Google Scholar]
- 19.Krzyzanski W, Jusko WJ. Integrated functions for four basic models of indirect pharmacodynamic response. J Pharm Sci. 1998;87:67–72. doi: 10.1021/js970168r. [DOI] [PubMed] [Google Scholar]
- 20.Mager DE, Wyska E, Jusko WJ. Diversity of mechanism-based pharmacodynamic models. Drug Metab Dispos. 2003;31:510–518. doi: 10.1124/dmd.31.5.510. [DOI] [PubMed] [Google Scholar]
- 21.Sharma A, Jusko WJ. Characteristics of indirect pharmacodynamic models and applications to clinical drug responses. Br J Clin Pharmacol. 1998;45:229–239. doi: 10.1046/j.1365-2125.1998.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitting C, Dhawan S, Cavaillon JM. Compartmentalization of tolerance to endotoxin. J Infect Dis. 2004;189:1295–1303. doi: 10.1086/382657. [DOI] [PubMed] [Google Scholar]
- 23.Cook JA. Molecular basis of endotoxin tolerance. Ann N Y Acad Sci. 1998;851:426–428. doi: 10.1111/j.1749-6632.1998.tb09020.x. [DOI] [PubMed] [Google Scholar]
- 24.Cobb JP, Mindrinos MN, Miller-Graziano C, Calvano SE, Baker HV, Xiao W, Laudanski K, Brownstein BH, Elson CM, Hayden DL, Herndon DN, Lowry SF, Maier RV, Schoenfeld DA, Moldawer LL, Davis RW, Tompkins RG, Baker HV, Bankey P, Billiar T, Brownstein BH, Calvano SE, Camp D, Chaudry I, Cobb JP, Davis RW, Elson CM, Freeman B, Gamelli R, Gibran N, Harbrecht B, Hayden DL, Heagy W, Heimbach D, Herndon DN, Horton J, Hunt J, Laudanski K, Lederer J, Lowry SF, Maier RV, Mannick J, McKinley B, Miller-Graziano C, Mindrinos MN, Minei J, Moldawer LL, Moore E, Moore F, Munford R, Nathens A, O'Keefe G, Purdue G, Rahme L, Remick D, Sailors M, Schoenfeld DA, Shapiro M, Silver G, Smith R, Stephanopoulos G, Stormo G, Tompkins RG, Toner M, Warren S, West M, Wolfe S, Xiao W, Young V. Application of genome-wide expression analysis to human health and disease. Proc Natl Acad Sci U S A. 2005;102:4801–4806. doi: 10.1073/pnas.0409768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Storey JD, Xiao W, Leek JT, Tompkins RG, Davis RW. Significance analysis of time course microarray experiments. Proc Natl Acad Sci U S A. 2005;102:12837–12842. doi: 10.1073/pnas.0504609102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF I.A. Large Scale Collab Res Program. A network-based analysis of systemic inflammation in humans. Nature. 2005 doi: 10.1038/nature03985. [DOI] [PubMed] [Google Scholar]
- 27.Yang E, Maguire T, Yarmush ML, Berthiaume F, Androulakis IP. Bioinformatics analysis of the early inflammatory response in a rat thermal injury model. BMC Bioinformatics. 2007;8:10. doi: 10.1186/1471-2105-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lampariello F. On the use of the Kolmogorov-Smirnov statistical test for immunofluorescence histogram comparison. Cytometry. 2000;39:179–188. doi: 10.1002/(SICI)1097-0320(20000301)39:3<179::AID-CYTO2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 29.Kirkpatrick S. CDG, Jr, Vecchi P. Optimization by simulated annealing. Science. 1983;220 doi: 10.1126/science.220.4598.671. [DOI] [PubMed] [Google Scholar]
- 30.Vemula M, Berthiaume F, Jayaraman A, Yarmush ML. Expression profiling analysis of the metabolic and inflammatory changes following burn injury in rats. Physiol Genomics. 2004;18:87–98. doi: 10.1152/physiolgenomics.00189.2003. [DOI] [PubMed] [Google Scholar]
- 31.Tong W, Cao X, Harris S, Sun H, Fang H, Fuscoe J, Harris A, Hong H, Xie Q, Perkins R, Shi L, Casciano D. ArrayTrack--supporting toxicogenomic research at the U.S. Food and Drug Administration National Center for Toxicological Research. Environ Health Perspect. 2003;111:1819–1826. doi: 10.1289/ehp.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells CA, Ravasi T, Hume DA. Inflammation suppressor genes: please switch out all the lights. J Leukoc Biol. 2005;78:9–13. doi: 10.1189/jlb.1204710. [DOI] [PubMed] [Google Scholar]
- 33.Kishore R, McMullen MR, Cocuzzi E, Nagy LE. Lipopolysaccharide-mediated signal transduction: Stabilization of TNF-alpha mRNA contributes to increased lipopolysaccharide-stimulated TNF-alpha production by Kupffer cells after chronic ethanol feeding. Comp Hepatol. 2004;3 Suppl 1:S31. doi: 10.1186/1476-5926-2-S1-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Amersfoort ES, Van Berkel TJ, Kuiper J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin Microbiol Rev. 2003;16:379–414. doi: 10.1128/CMR.16.3.379-414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 36.Du X, Poltorak A, Silva M, Beutler B. Analysis of Tlr4-mediated LPS signal transduction in macrophages by mutational modification of the receptor. Blood Cells Mol Dis. 1999;25:328–338. doi: 10.1006/bcmd.1999.0262. [DOI] [PubMed] [Google Scholar]
- 37.Krzyzanski W, Jusko WJ. Mathematical formalism for the properties of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm. 1997;25:107–123. doi: 10.1023/a:1025723927981. [DOI] [PubMed] [Google Scholar]
- 38.Jin JY, Almon RR, DuBois DC, Jusko WJ. Modeling of corticosteroid pharmacogenomics in rat liver using gene microarrays. J Pharmacol Exp Ther. 2003;307:93–109. doi: 10.1124/jpet.103.053256. [DOI] [PubMed] [Google Scholar]
- 39.Munford RS. Severe Sepsis and Septic Shock: The Role of Gram - Negative Bacteremia. Annu.Rev.Pathol.Mech.Dis. 2006;1:467–496. doi: 10.1146/annurev.pathol.1.110304.100200. [DOI] [PubMed] [Google Scholar]
- 40.Bhattacharjee RN, Akira S. Toll - like Receptor Signaling: Emerging Opportunities in Human Diseases and Medicine. Current Immunology Reviews. 2006;1:81–90. [Google Scholar]
- 41.Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- 42.Wysocka M, Robertson S, Riemann H, Caamano J, Hunter C, Mackiewicz A, Montaner LJ, Trinchieri G, Karp CL. IL-12 suppression during experimental endotoxin tolerance: dendritic cell loss and macrophage hyporesponsiveness. J Immunol. 2001;166:7504–7513. doi: 10.4049/jimmunol.166.12.7504. [DOI] [PubMed] [Google Scholar]
- 43.McCall CE, Grosso-Wilmoth LM, LaRue K, Guzman RN, Cousart SL. Tolerance to endotoxin-induced expression of the interleukin-1 beta gene in blood neutrophils of humans with the sepsis syndrome. J Clin Invest. 1993;91:853–861. doi: 10.1172/JCI116306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poll vd. J Infect Disease. 1996;174:1356–1360. doi: 10.1093/infdis/174.6.1356. [DOI] [PubMed] [Google Scholar]
- 45.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 46.Brightbill HD, Plevy SE, Modlin RL, Smale ST. A prominent role for Sp1 during lipopolysaccharide-mediated induction of the IL-10 promoter in macrophages. J Immunol. 2000;164:1940–1951. doi: 10.4049/jimmunol.164.4.1940. [DOI] [PubMed] [Google Scholar]
- 47.Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004;364:545–548. doi: 10.1016/S0140-6736(04)16815-3. [DOI] [PubMed] [Google Scholar]
- 48.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 49.Derendorf H, Lesko LJ, Chaikin P, Colburn WA, Lee P, Miller R, Powell R, Rhodes G, Stanski D, Venitz J. Pharmacokinetic/pharmacodynamic modeling in drug research and development. J Clin Pharmacol. 2000;40:1399–1418. [PubMed] [Google Scholar]
- 50.Lauffenburger DA, Linderman JJ. Receptors. Models for Binding, Trafficking, and Signalling. The International Journal of Biochemistry and Cell Biology. 1996;28:1418–1418. [Google Scholar]
- 51.Aderem A, Smith KD. A systems approach to dissecting immunity and inflammation. Semin Immunol. 2004;16:55–67. doi: 10.1016/j.smim.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Protti A, Singer M. Strategies to modulate cellular energetic metabolism during sepsis. Novartis Found Symp. 2007;280:7–16. discussion 16-20, 160-4. [PubMed] [Google Scholar]
- 53.Zwietering MH, Jongenburger I, Rombouts FM, van 't Riet K. Modeling of the Bacterial Growth Curve. Appl Environ Microbiol. 1990;56:1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greisman SE, Hornick RB, Wagner HN, Jr, Woodward WE, Woodward TE. The role of endotoxin during typhoid fever and tularemia in man. IV. The integrity of the endotoxin tolerance mechanisms during infection. J Clin Invest. 1969;48:613–629. doi: 10.1172/JCI106020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shin HJ, Lee H, Park JD, Hyun HC, Sohn HO, Lee DW, Kim YS. Kinetics of binding of LPS to recombinant CD14, TLR4, and MD-2 proteins. Mol Cells. 2007;24:119–124. [PubMed] [Google Scholar]
- 56.Bosisio D, Polentarutti N, Sironi M, Bernasconi S, Miyake K, Webb GR, Martin MU, Mantovani A, Muzio M. Stimulation of toll-like receptor 4 expression in human mononuclear phagocytes by interferon-gamma: a molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood. 2002;99:3427–3431. doi: 10.1182/blood.v99.9.3427. [DOI] [PubMed] [Google Scholar]
- 57.Xiong W, Ferrell JE., Jr A positive-feedback-based bistable 'memory module' that governs a cell fate decision. Nature. 2003;426:460–465. doi: 10.1038/nature02089. [DOI] [PubMed] [Google Scholar]
- 58.Rifkind D. Prevention by polymyxin B of endotoxin lethality in mice. J Bacteriol. 1967;93:1463–1464. doi: 10.1128/jb.93.4.1463-1464.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lehmann V, Freudenberg MA, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-galactosamine-treated mice. J Exp Med. 1987;165:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tschaikowsky K, Schmidt J, Meisner M. Modulation of mouse endotoxin shock by inhibition of phosphatidylcholine-specific phospholipase C. J Pharmacol Exp Ther. 1998;285:800–804. [PubMed] [Google Scholar]
- 61.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 62.Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, Castellino FJ, Mackman N, Griffin JH, Weiler H. Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C. J Exp Med. 2007;204:2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reyes WJ, Brimioulle S, Vincent JL. Septic shock without documented infection: an uncommon entity with a high mortality. Intensive Care Med. 1999;25:1267–1270. doi: 10.1007/s001340051055. [DOI] [PubMed] [Google Scholar]
- 64.Lauffenburger DA, Kennedy CR. Analysis of a Lumped Model for Tissue Inflammation Dynamics. Mathematical Biosciences. 1980;53:189–221. doi: 10.1016/0025-5564(81)90018-3. [DOI] [PubMed] [Google Scholar]
- 65.Marshall JC, Walker PM, Foster DM, Harris D, Ribeiro M, Paice J, Romaschin AD, Derzko AN. Measurement of endotoxin activity in critically ill patients using whole blood neutrophil dependent chemiluminescence. Crit Care. 2002;6:342–348. doi: 10.1186/cc1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sama AE, D'Amore J, Ward MF, Chen G, Wang H. Bench to bedside: HMGB1-a novel proinflammatory cytokine and potential therapeutic target for septic patients in the emergency department. Acad Emerg Med. 2004;11:867–873. doi: 10.1197/j.aem.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 67.Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]