Abstract
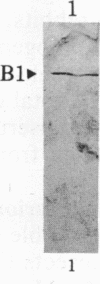
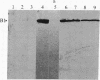
Antisera raised against the denatured polypeptide of two organophosphate-detoxifying esterases (B1 and A1) of Culex mosquitoes were used in an immunoblot method to quantify esterase production in resistant versus susceptible strains and to detect the presence of immunologically related proteins in other insects. It was demonstrated that esterase B1 of Culex quinquefasciatus and esterase A1 of Culex pipiens are overproduced in resistant strains by factors of at least 500-fold and 70-fold, respectively, as compared with the corresponding susceptible strains. These factors approximate the levels of resistance to the organophosphate chlorpyrifos determined by bioassay--i.e., about 800-fold and 100-fold, respectively. Antiesterase B1 antiserum was found to react with other type B esterases (B2 of C. quinquefasciatus and B3 of Culex tarsalis) but not with type A esterases (A1 of C. pipiens, A2 of C. quinquefasciatus, or A3 of C. tarsalis); similarly, antiesterase A1 antiserum was found to react with other type A esterases (A2 and A3) but not with type B esterases (B1, B2, and B3). Proteins immunologically related to esterase B1 were detected in Aedes aegypti L., Myzus persicae Sultzer, and Musca domestica L., although they were not overproduced in the organophosphate-resistant strains of these species. In none of these species were proteins immunologically related to esterase A1 found.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Georghiou G. P., Pasteur N. Electrophoretic esterase patterns in insecticide-resistant and susceptible mosquitoes. J Econ Entomol. 1978 Apr 17;71(2):201–205. doi: 10.1093/jee/71.2.201. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Pasteur N., Iseki A., Georghiou G. P. Genetic and biochemical studies of the highly active esterases A' and B associated with organophosphate resistance in mosquitoes of the Culex pipiens complex. Biochem Genet. 1981 Oct;19(9-10):909–919. doi: 10.1007/BF00504256. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]