Abstract
Background and objectives
Mullerian adenosarcoma is an uncommon variant of mixed mesodermal tumour of the uterus. This is a case report of a 65 year old post-menopausal lady who presented with complaints of passing tissue fragments per vaginum for 2 days followed by spotting. On examination, a polypoid mass protruding through the cervix was seen which was biopsied. Following a preliminary histologic diagnosis of poorly differentiated sarcoma on the biopsy; the patient underwent total abdominal hysterectomy with bilateral salpingo-oopherectomy.
Method
The surgical specimen was formalin fixed and paraffin embedded. Haematoxylin and eosin stained sections were studied.
Result and conclusion
Histopathological examination of the polypoid mass revealed a tumour comprising of an admixture of benign endometrial glandular component with overgrowth of sarcomatous stromal component and heterologous elements. This may pose a problem in diagnosis due to its rarity, and hence its distinctive morphological features merit attention as described here. In view of the rarity of this tumor, it is mandatory to do extensive histologic sampling to identify areas of sarcomatous overgrowth before arriving at a diagnosis of mullerian adenosarcoma as the clinical course and management vary.
Keywords: adenosarcoma, uterus, heterologous elements
Introduction
A 65 year old lady, postmenopausal for more than 20 yrs presented to the gynaecology department with complaints of severe back pain for 1 month before which she was symptom free. She presented with a history of fever for 4 days and passing tissue fragments per vagina during micturition for 2 days followed by spotting. She had no history of previous surgery or radiotherapy. General physical examination was unremarkable. Per vaginal examination revealed a large polypoid mass protruding through the cervix non separable from the uterus. Routine laboratory tests were within normal limits except for an increase in fasting and post-prandial blood sugar levels. A biopsy from the protruding polypoid mass was reported as poorly differentiated sarcoma, following which a total abdominal hysterectomy with bilateral salpingo-oopherectomy was done and the specimen sent for histopathological examination.
Method
The surgical specimen was formalin fixed and paraffin embedded. Gross examination of hysterectomy specimen with bilateral adnexa weighed 180 gm, sectioning revealed a polypoid mass measuring 7 × 6 × 4.5 cm arising from the posterior wall of uterine corpus distending the uterine cavity and protruding through the cervix (Fig 1). On sectioning the polypoid mass was firm grey-white, haemorrhagic with glistening/myxoid areas and foci of necrosis. The myometrium showed invasion upto 1 cm. The left uterine wall showed an intramural fibroid (<1 cm) with a firm whorled white cut surface. The cervix and bilateral adnexa appeared unremarkable. Haematoxylin and eosin stained sections were studied. For light microscopy, representative blocks embedded in paraffin were cut at 6 micron thickness.
Figure 1.
Gross photograph of the specimen showing a polypoid tumour arising from the uterine corpus and protruding through the os with glistening foci.
Result
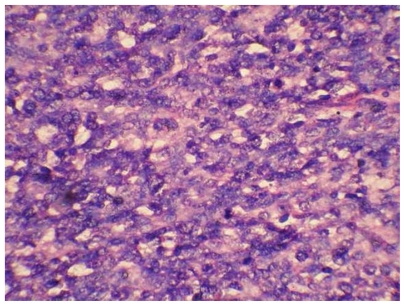
Histopathological examination of the polypoid mass revealed a tumour with a predominantly stromal component (comprising more than 25% of the tumour) which was frankly sarcomatous composed of fascicles of poorly differentiated spindle shaped cells exhibiting mild to moderate nuclear atypia (Fig 2). Brisk mitosis, tumour giant cells and heterologous elements represented by rhabdomyosarcomatous differentiation were seen. (Fig 3) Extensive sampling revealed focal benign to atypical glands including occasional cystically dilated glands.
Figure 2.
Haematoxylin and eosin staining (X 400) showing sarcomatous component.
Figure 3.
Haematoxylin and eosin staining (X 200) showing an occasional malignant gland and scattered rhabdomyoblasts (indicated by arrow).
Discussion
Mullerian adenosarcoma is a rare malignant tumour of the uterus that was described by Clement and Scully.1 Majority of adenosarcomas, including the current case, occur in post menopausal women. These tumours can present as a pelvic mass (37%), uterine polyp (22%) as in our case or an enlarged uterus (22%).2 Risk factors that have been associated with an increased risk of uterine adenosarcoma include unopposed estrogen stimulation,3 long term oral contraceptive pills4 and prolonged use of Tamoxifen5,6 for breast carcinoma. Our patient had none of these risk factors. They are usually of low malignant potential. Few of these tumours are clinically malignant, since many of them pursue an indolent course. Zaloudek et al7 noted that the only morphological feature associated with increased risk of recurrence or metastasis is the presence of deep myometrial invasion. Mullerian adenosarcoma is characterized by an intimate admixture of benign but occasionally atypical endometrial glands and a sarcomatous, usually low grade stromal component. These glands may be lined by proliferative endometrioid (as in this case), endocervical, squamous, serous or secretory endometrioid cells and are surrounded by the sarcomatous component. The stromal cellularity is more around the glands resulting in the formation of “periglandular cuffs”. However this feature was not seen in our case. The stroma can be endometrial stromal sarcoma alone, fibrosarcoma alone or a mixture of the two. Further, stromal secondary changes including fibrosis and variable hyalinization may also be seen. In addition, foci of edema, haemorrhage, inflammation and myxoid change may be present. The term “ Mullerian adenosarcoma with sarcomatous overgrowth (MASO) was first used by Clemant and Scully8 in 1989 for those tumours in which a pure sarcoma, similar to or of a higher grade than that of the underlying adenosarcoma accounts for atleast one-quarter of the tumour after thorough histological sampling. Seidman et al9 in their study have noted that tumours with sarcomatous overgrowth occurred in younger women, were larger and were more likely to have heterologous elements as compared with those without sarcomatous overgrowth. The main differential diagnosis of MASO is typical adenosarcoma with stromal predominant areas. Hence, Clement recommends that the diagnosis of MASO be rendered only when a pure sarcoma accounts for atleast one fourth of the tumour after thorough histological sampling of the hysterectomy specimen. Correct classification of MASO is important because the limited available data suggest that the prognosis is notably worse than that for adenosarcomas without sarcomatous overgrowth.10
The management of patient with MASO is similar to that of a highly malignant uterine sarcoma. Total abdominal hysterectomy with bilateral salpingo-oopherectomy can be curative if disease is confined to the uterus with a five year survival approximately 50%.11 Adjuvant radiotherapy appears to have a role in better pelvic control and decrease in local recurrence of the tumor.12 Data regarding adjuvant chemotherapy is very controversial, especially with the paucity of studies and lack of control. Patients with MASO should therefore receive long-term postoperative surveillance in view of the likelihood of late recurrence. No pattern of difference in recurrence or survival has been documented between homologous or heterologous elements.
Conclusion
We feel that due to the rarity of this tumor, it is mandatory to do extensive histologic sampling to identify areas of sarcomatous overgrowth before arriving at a diagnosis of mullerian adenosarcoma as the clinical course and management vary.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material. Written consent was obtained from the patient or relative for publication of this study.
References
- 1.Clement PB, Scully RE. Mullerian Adenosarcoma of the uterus-A clinicopathological analysis of Ten cases of a Distinctive type of Mullerian Mixed Tumour. Cancer. 1974;34:1138–49. doi: 10.1002/1097-0142(197410)34:4<1138::aid-cncr2820340425>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Krivak TC, Seidman JD, McBroom JW, MacKoul PJ, Aye LM, Rose GS. Uterine adenosarcoma with sarcomatous overgrowth versus uterine carcinosarcoma: comparison of treatment and survival. Gynecol Oncol. 2001;83:89–94. doi: 10.1006/gyno.2001.6334. [DOI] [PubMed] [Google Scholar]
- 3.Nomura K, Aizawa S, Ushigome S. Adenosarcoma of the uterine corpus associated with ovarian thecoma. Pathol Int. 2001;51:735–8. doi: 10.1046/j.1440-1827.2001.01259.x. [DOI] [PubMed] [Google Scholar]
- 4.Tjalma WA, Michener CM. Mullerian adenosarcoma of the uterus associated with long-term oral contraceptive use. Eur J Obstet Gynecol Reprod Biol. 2005;119:253–4. doi: 10.1016/j.ejogrb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Wysowski DK, Honig SF, Beitz J. Uterine sarcoma associated with tamoxifen use. N Engl J Med. 2002;346:1832–3. doi: 10.1056/NEJM200206063462319. [DOI] [PubMed] [Google Scholar]
- 6.Varras M, Akrivis CH. Large endometrial polyp with sarcomatous stromal components following long-term tamoxifen treatment for breast cancer: a case report and review of the literature. Eur J Gynaecol Oncol. 2003;24(6):565–8. [PubMed] [Google Scholar]
- 7.Zaloudek CJ, Norris HJ. Adenofibroma and Adenosarcoma of the uterus: A clinicopathological study of 35 cases. Cancer. 1981;48:354–66. doi: 10.1002/1097-0142(19810715)48:2<354::aid-cncr2820480222>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.Clement PB. Mullerian Adenosarcoma of the uterus with sarcomatous overgrowth-A clinicopathological Analysis of 10 cases. Am J Surg Pathol. 1989;13(1):28–38. doi: 10.1097/00000478-198901000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Seidman JD, Wasserman CS, Aye LM, Mackoul PJ, O’Leary TJ. Cluster of Uterine Mullerian Adenosarcoma in the Washington, DC metropolitan area with high incidence of Sarcomatous overgrowth. Am J Surg Pathol. 1999;23(7):809–14. doi: 10.1097/00000478-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Currie John L. Malignant tumours of the uterine corpus. In: Rock John A, Thompson John D., editors. Te Linde’s operative gynecology. 8th edition. Lippincott Williams & Wilkins A Wolters Kluwer Company; 1996. pp. 1541–9. [Google Scholar]
- 11.Kahanpaa KV, Wahlstrom T, Grohn P, Heinonen E, Nieminen U, Widholm O. Sarcomas of the uterus: a clinicopathologic study of 119 patients. Obstet Gynecol. 1986;67:417–24. [PubMed] [Google Scholar]
- 12.Hornback NB, Omura G, Major FJ. Observations on the use of adjuvant radiation therapy in patients with stage I and II uterine sarcoma. Int J Radiat Oncol Biol Phys. 1986;12:2127–30. doi: 10.1016/0360-3016(86)90011-8. [DOI] [PubMed] [Google Scholar]