Abstract
A 68 year-old Japanese man, who had been suffering from immobilization and disuse syndrome, was admitted to our hospital for evaluation of polyuria with polyposia, hyponatremia and low blood pressure. His plasma osmolality was greater than that of his urine. His endocrinological examination revealed low levels of plasma adrenocorticotropic hormone (ACTH) and cortisol, and a normal response of ACTH to the corticotrophin-releasing hormone (CRH) challenge. Plasma ACTH did not increase with insulin loading. A low plasma vasopressin (AVP) level and no response of AVP to a 5% saline administration were observed. We diagnosed central adrenal insufficiency with central diabetes insipidus. Six months after starting administration of hydrocortisone and 1-deamino-8D-arginine vasopressin, his psychological symptoms had improved, and 1.5 years after starting treatment, he was able to walk. In conclusion, it is not particularly rare for adrenal insufficiency to be misdiagnosed as depression. However, a correct early diagnosis is necessary, because, if adrenal insufficiency is not definitively diagnosed, the patient’s quality of life diminishes markedly.
Keywords: tertiary adrenal failure, diabetes insipidus, depression
Introduction
Adrenal insufficiency is a relatively rare disease. Primary adrenal insufficiency has a prevalence of 90–140 per million, and the estimated prevalence of secondary and tertiary (central) adrenal insufficiency is 150–280 per million.1 Any process that involves the hypothalamus and interferes with corticotrophin-releasing hormone (CRH) secretion, such as hypothalamic tumors, surgery and irradiation, and autoimmune hypothalamic disease, will result in tertiary adrenal failure.2 Weakness, fatigue, myalgia and psychiatric symptoms are known to be both common and non-specific in patients with central adrenal insufficiency. Thus, central adrenal insufficiency is often misdiagnosed as depression and/or chronic anxiety disorder.3 We present herein a patient with central adrenal insufficiency and diabetes insipidus who was misdiagnosed as having severe depression.
Case Presentation
A 68 year-old Japanese man, who had been suffering from immobilization and disuse syndrome, was admitted to our hospital for evaluation of polyuria with polyposia, hyponatremia and low blood pressure. He had been diagnosed with depression at another hospital, based on loss of interest in almost all activities, decreased appetite and social withdrawal, and had been treated with anti-depressants and antipsychotics, such as clomipramine, tiapride, milnacipran, zolpidem, risperidone and levomepromazine (methotrimeprazine), for the previous two years. However, his general condition had not improved. Another psychiatrist recognized an increase in urinary volume, low blood pressure and hyponatremia.
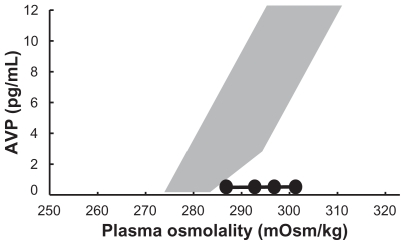
On physical examination, a depressive effect, hypotension and arthrogryposis were observed. His urinary volume was approximately 3000 mL/day. Hematological data showed mild anemia and bio-chemical analysis detected hyponatremia (133 mM) on admission. Serum levels of aspartate aminotransferase, urea nitrogen, creatinine and C-reactive peptide were elevated (40 IU/L, 38.7 mg/dL, 1.52 mg/dL and 0.8 mg/dL, respectively). His plasma osmolality (pOsm) was greater than that of his urine (uOsm) (355 mOsm/kg and 288 mOsm/kg, respectively). Endocrinological examinations showed plasma adrenocorticotropic hormone (ACTH) and cortisol levels of 23.5 pg/mL (normal range: 7.4–55.7 pg/mL) and 3.6 μg/dL (normal range: 4.0–18.3 μg/dL), respectively, at 7:30 a.m. with loss of circadian rhythm. Mean 24 hour urinary 17-hydroxycorticosteroid and 17-ketosterol levels were below normal at 1.5 mg/day (normal range: 4–12 mg/day) and 2.2 mg/day (normal range: 5–20 mg/day), respectively. The responses of plasma ACTH and cortisol to intravenous injection of CRH were normal. Plasma ACTH and cortisol levels did not increase in response to intravenous injection of insulin. The cortisol response to a single administration of 1–24 ACTH was normal. Levels of free triiodothyronine and free thyroxin were normal, and thyroid-stimulating hormone (TSH) showed a normal response to thyrotropin-releasing hormone injection. Levels of plasma prolactin, growth hormone and insulin like growth factor-1 were 9.8 ng/mL (normal range: 3.58–12.78 ng/mL), 0.1 ng/mL (normal range: <0.17 ng/mL) and 159 ng/mL (normal range: 42–250 ng/mL), respectively. The plasma vasopressin (AVP) level was relatively low, at 0.4 pg/mL (normal range: 0.3–3.5 ng/mL). AVP showed no response to a 5% saline loading test. T1-weighted magnetic resonance imaging of the pituitary gland showed an empty sella and disappearance of high intensity at the posterior lobe (Fig. 1).
Figure 1.
Response of AVP to 5% saline loading test. There was no response of AVP to a 5% saline loading test in our patient (closed circle). The gray area represents the normal response of AVP to 5% saline administration.
We diagnosed central (tertiary) adrenal insufficiency with central diabetes insipidus. Replacement therapy, with 15 mg of hydrocortisone and 10 μg/day of 1-deamino-8D-arginine vasopressin, was started, and of the patient’s antidepressants and antipsychotics were canceled at the same time. A few weeks after starting the hydrocortisone treatment, his psychological symptoms, including loss of interest in most activities and decreased appetite, had improved. Six months after starting the treatment, his mobility also improved and he was able to walk.
Discussion
Our patient initially presented with psychiatric manifestations for which he had been treated with antidepressants without improvement. As a result, he suffered from immobilization and disuse syndrome for two years. However, his physical findings and data showed increased urinary volume, low blood pressure and hyponatremia, and the endocrinological findings supported a diagnosis of tertiary adrenal insufficiency with central diabetes insipidus. It is well known that depression manifests with apathy, poverty of thought and lack of initiative, as observed in patients with long-standing adrenal insufficiency. Twenty percent of patients with adrenal insufficiency are diagnosed more than five years after the onset of symptoms. More than 67% of patients consult at least three physicians, and 68% are initially given an incorrect diagnosis, usually of psychiatric disorders and gastrointestinal disease.4 Most psychiatric symptoms disappear within a few days after adequate glucocorticoid therapy is begun; however, psychosis reportedly may persist for several months.5 In our case, a few weeks after starting administration of hydrocortisone, the patient’s psychological symptoms showed improvement, and six months after starting the treatment, he was able to walk. Therefore, we suggest the necessity of blood testing for ACTH and cortisol in the field of psychiatry to ensure an early diagnosis of adrenal insufficiency.
Any process that involves the hypothalamus and interferes with CRH secretion, such as hypothalamic tumors, surgery and irradiation, and autoimmune hypothalamic disease, will result in tertiary adrenal failure.2 In this case, although low ACTH and cortisol levels and a normal ACTH response to CRH administration were observed, there was no response of ACTH to an insulin challenge. In addition, AVP was low while pOsm was high, and there was no AVP response to a 5% saline loading. Therefore, the patient was diagnosed with tertiary adrenal insufficiency with central diabetes insipidus. It has been reported that 30% of central diabetes insipidus cases are idiopathic, and that lymphocytic hypophysitis and autoimmune hypothalamic disease may cause central diabetes insipidus.6–8 The paraventricular nucleus (PVN) in the hypothalamus is the site of most CRH production,9 and AVP is also synthesized in both the supraoptic nucleus and the PVN in the hypothalamus.10,11 In our patient’s case, tertiary adrenal failure and central diabetes insipidus may have developed due to damage to the hypothalamus, including the PVN, though the cause of the damage is unclear and may thus be idiopathic.
In this case, the volume of urine was relatively low for diabetes insipidus, initially being 3000 ml. However, the urinary volume increased markedly after steroid replacement therapy. Central diabetes insipidus is known to be masked by the presence of glucocorticoid deficiency, so-called masked diabetes insipidus,10,12 Glucocorticoid deficiency causes AVP-dependent as well as independent impairment of water diuresis due to renal factors. It was recently reported that the production of aquaporin 2, a vasopressin-dependent water channel in the kidney, is enhanced in the absence of glucocorticoids.13 As a result, the antidiuretic action of vasopressin is exaggerated, such that the masked diabetes insipidus in this patient surfaced once treatment had begun.
In conclusion, misdiagnosis of adrenal insufficiency is not particularly rare. However, a correct early diagnosis is necessary, because, if adrenal insufficiency is not definitively diagnosed, the patient’s quality of life is markedly reduced. We have described this patient with tertiary adrenal failure associated with diabetes insipidus, misdiagnosed as severe depression, to raise clinicians’ awareness of this disorder.
Figure 2.
Coronal T1-weighted magnetic resonance imaging of the pituitary gland. The arrow indicates an empty sella.
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material. Written consent was obtained from the patient for publication of this study.
References
- 1.Arlt W, Allolio B. Adrenal insufficiency. Lancet. 2003;361:1881–93. doi: 10.1016/S0140-6736(03)13492-7. [DOI] [PubMed] [Google Scholar]
- 2.Endert E, Ouwehand A, Fliers E, Prummel MF, Wiersinga WM. Establishment of reference values for endocrine tests. Part IV: adrenal insufficiency. Neth J Med. 2005;63:435–43. [PubMed] [Google Scholar]
- 3.Kaushik ML, Sharma RC. Addison’s disease presenting as depression. Indian J Med Sci. 2003;57:249–51. [PubMed] [Google Scholar]
- 4.Bleicken B, Hahner S, Ventz M, Quinkler M. Delayed diagnosis of adrenal insufficiency is common: a cross-sectional study in 216 patients. Am J Med Sci. 2010 Jun;339(6:):525–31. doi: 10.1097/MAJ.0b013e3181db6b7a. [DOI] [PubMed] [Google Scholar]
- 5.Orth DN, Kovacs WJ. Disease of the adrenal cortex. The Adrenal Cortex. In: Wilsom JD, Foster DW, Kronenberg HM, Larsen PR, editors. Williams Text Book of Endocrinology. 9th ed. Philadelphia, PA: Sounders; 1998. pp. 547–610. [Google Scholar]
- 6.Moses AM, Notman DD. Diabetes insipidus and syndrome of inappropriate antidiuretic hormone secretion (SIADH) Adv Intern Med. 1982;27:73–100. [PubMed] [Google Scholar]
- 7.Imura H, Nakao K, Shimatsu A, et al. Lymphocytic infundibuloneurohypophysitis as a cause of central diabetes insipidus. N Engl J Med. 1993;329:683–9. doi: 10.1056/NEJM199309023291002. [DOI] [PubMed] [Google Scholar]
- 8.Abe T, Matsumoto K, Sanno N, Osamura Y. Lymphocytic hypophysitis: case report. Neurosurgery. 1995;36:1016–9. doi: 10.1227/00006123-199505000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43:425–73. [PubMed] [Google Scholar]
- 10.Zimmerman EA, Robinson AG. Hypothalamic neurons secreting vasopressin and neurophysin. Kidney Int. 1976;10:12–24. doi: 10.1038/ki.1976.75. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman EA, Nilaver G, Hou-Yu A, Silverman AJ. Vasopressinergic and oxytocinergic pathways in the central nervous system. Fed Proc. 1984;43:91–6. [PubMed] [Google Scholar]
- 12.Huang CH, Chou KJ, Lee PT, Chen CL, Chung HM, Fang HC. A case of lymphocytic hypophysitis with masked diabetes insipidus unveiled by glucocorticoid replacement. Am J Kidney Dis. 2005;45:197–200. doi: 10.1053/j.ajkd.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 13.Saito T, Ishikawa SE, Ando F, Higashiyama M, Wagasaka S, Sasaki S. Vasopressin-dependent upregulation of aquaporin-2 gene expression in glucocorticoid-deficient rats. Am J Physiol Renal Physiol. 2000;279:F502–8. doi: 10.1152/ajprenal.2000.279.3.F502. [DOI] [PubMed] [Google Scholar]