Abstract
We studied the relation between cortical oscillatory rhythms and the structural integrity of the corpus callosum in 21 children with spina bifida and hydrocephalus. Participants underwent resting state neuromagnetic recordings and diffusion tensor imaging. Areas of three segments of the corpus callosum (genu, body, splenium) were derived through diffusion tensor imaging-based morphometrics. Children with spina bifida showed reduced values of spectral power in the θ, α and β bands when compared with age-matched controls, but only in the posterior and temporal regions. Reduced spectral power in posterior regions correlated with decreased area of the posterior segments of the corpus callosum. Atypical cortical oscillatory activity is associated with reduced transcallosal connectivity in children with spina bifida.
Keywords: corpus callosum, diffusion tensor imaging, magnetoencephalography, spina bifida
Introduction
Synchronized oscillatory activity of cortical cell assemblies plays a crucial role in supporting sensory, motor and higher functions [1]. Thalamo-cortical and cortico-cortical connections through white matter tracts contribute to the synchronous activation of functionally related cortical neurons, and to the corresponding spectral responses that can be characterized through neurophysiological recordings (i.e. electroencephalography, EEG; and magnetoencephalography, MEG). Although the impact of thalamo-cortical network lesions on brain oscillations has been widely reported [2,3], little is known about the effects of reduced cortico-cortical connectivity on neuronal oscillations [4], in general, and the effect of atypical, or reduced transcallosal connectivity, in particular.
In children with spina bifida and hydrocephalus, the partial agenesis and hypoplasia of the corpus callosum is one of the most distinctive radiological findings [4]. Although a variety of strengths and weaknesses in sensorimotor and cognitive performance have been identified [5–7], little is known about the cortical signs of this congenital neurodevelopmental disorder, which represents incomplete closure of the neural tube during gestation [8]. Recent studies have shown that along with a variety of deficits in the sensorimotor and cognitive areas [6,7] cognitive operations requiring interhemispheric integration are significantly affected in children with spina bifida and hydrocephalus, especially if the child has partial agenesis of the corpus callosum [9–12]. Although it is generally accepted that the disruption of white matter tracts should modify connectivity between distant cortical areas, there is no neurophysiological evidence that interhemispheric connectivity is reduced in children with spina bifida and hydrocephalus.
The purpose of this study was to explore the relation between cortical function (i.e. oscillatory cortical rhythms) and the structural integrity of transcallosal tracts in children with spina bifida and hydrocephalus. To investigate this relation, we combined functional and structural imaging techniques. Previous work has shown the utility of MEG and EEG as powerful neurophysiologic recording techniques for characterizing spontaneous oscillatory activity to differentiate between normal individuals and patients with various neurological conditions [13–15]. Unlike EEG, where signals are distorted by the variable conductivity of the different compartments of the head and brain, MEG is not affected by such nonhomogenous conductivity, rendering it an ideal method to record and localize the cortical origin of these rhythms. For this reason, we utilized MEG-derived measurements of oscillatory activity and combined them with morphometric measurements of the corpus callosum using diffusion tensor imaging (DTI). DTI is a novel emerging technique that allows noninvasive visualization and quantification of changes in the structural integrity of the white matter tracts. The combination of imaging techniques has the potential of providing relevant information on the anatomical-functional relationship between white matter tracts and cortical functionality. We hypothesized that cortical oscillatory activity is distorted in children with spina bifida and relates to the poor integrity of the corpus callosum.
Materials and methods
Participants
Twenty-one children with spina bifida and hydrocephalus and 11 age-matched healthy volunteers (control group) completed the same evaluation, including MEG and MRI. Written informed consent was obtained from the guardians and assent from the children participating in this study. Children were born with spina bifida (verified by medical record review of pathology and neurosurgical operative reports). Twenty had meningomyelocele with the characteristic Chiari II malformation and one a lipomeningomyelocele with a normal-appearing cerebellum at the time of the study, but who was shunted for hydrocephalus and for whom previous imaging studies had suggested a Chiari malformation. With the exception of one child with shunt implantation on the left side, all others had functioning shunts on the right side for hydrocephalus. All children in this group underwent spinal lesion repair at birth and ventriculoperitoneal shunt insertion during the early neonatal period and were medically stable at the time of the assessments. No child had a history of shunt infection or seizures. The shunts were revised for growth or obstructions less than four times in all but two cases that had six and 14 revisions, respectively. Spinal lesion location included two sacral, six thoracic and 13 lumbar level lesions. Coding of the corpus callosum by an experienced radiologist revealed abnormalities in all 21 cases, with the most common abnormalities involving thinning of the posterior body (n = 19) and splenium (n = 18) that was moderate to severe, although two cases had normal-appearing splenia. Thus, there was variability in the integrity of the corpus callosum. None of the control children had a previous history of neurological and/or psychiatric disorders, including learning disability or an attention disorder. Clinical and demographic information is summarized in Table 1.
Table 1.
Demographic information for the participants
| SBH | Controls | |
|---|---|---|
| Age (years, months) | Mean = 12.2 SD = 2.9 |
Mean = 12.7 SD = 3.2 |
| Handednessa | 17/21 right | 19/21 right |
| IQb | Mean = 89.7 SD = 17.4 |
– |
IQ, intelligence quotient; SBH, spina bifida with hydrocephalus.
Evaluated using the Edinburgh handedness Inventory [16]. Scores below − 40=left-handed, between −40 and +40=ambidextrous and above +40=right-handed.
Participants with spina bifida received the four subtest form of the Stanford-Binet Intelligence test, fourth edition [17], from which a composite was generated.
Magnetoencephalography recordings and data analysis
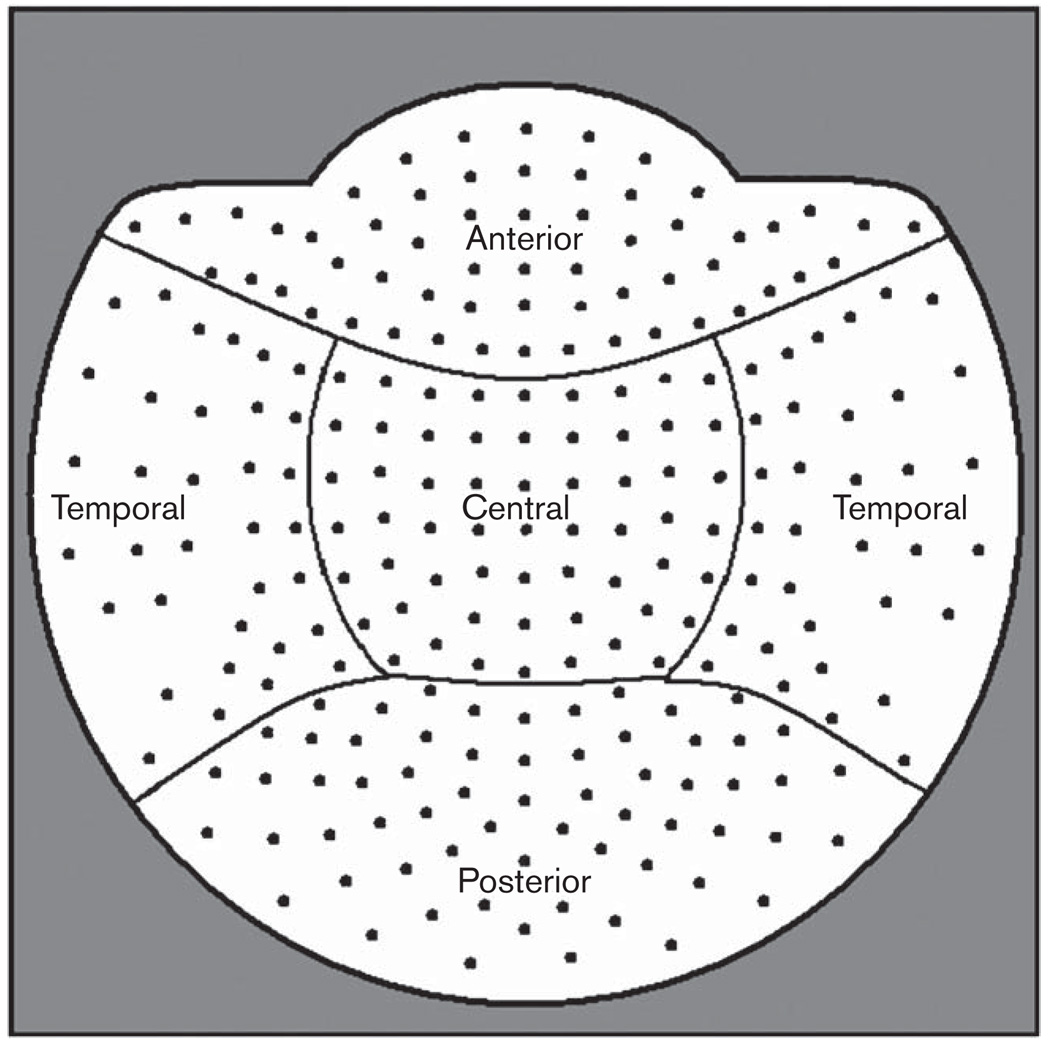
All MEG recordings were conducted using a whole-head neuromagnetometer containing an array of 248 sensors (WH 3600, 4D Neuroimaging, San Diego, California, USA) housed in a sound-damped and magnetically shielded room. Participants were asked to keep their eyes closed and avoid blinking or otherwise moving during the recordings. Five minutes of continuous MEG resting data was collected for each participant (sampling rate of 1017.25 Hz, and 0.1–200 Hz band-pass filter). Head position was monitored before and after the data acquisition by using a set of five sensors. At least three segments (mean = 5.7, SD = 2.4) of MEG data (5 s duration each segment) not affected by the presence of biogenic or environmental artifacts were selected after visual inspection for further analysis. Artifact-free segments of MEG data were noise reduced and decimated followed by a downsampling by factor of 16. Digital fast Fourier transformation-based power spectrum analysis was computed over the MEG data with a 0.19 Hz frequency resolution. Spectral power was summarized using the following standard frequency bands: δ (1.5–4 Hz), θ (4–8 Hz), α (8–12 Hz) and β (12–16 Hz). To characterize regional differences in spectral power the 248 MEG sensors were grouped as follows (Fig. 1): anterior (58 sensors), temporal (68 sensors), central (59 sensors) and posterior region (63 sensors).
Fig. 1.
Grouping of the 248 magnetoencephalographic sensors (axial gradiometers) in four regions: anterior (58 sensors), temporal (68), central (59), posterior (63).
Average spectral power estimated for each of these areas and four frequency bands was compared between the children with spina bifida with hydrocephalus and controls using three-way analysis of variance (with group as between-subject factor, and frequency band and region as within-subject factors) with age as covariate. Only significant effects (overall α < 0.05) involving group differences are reported. Bonferroni correction was used for multiple comparisons.
Diffusion tensor imaging-guided segmentation of the corpus callosum
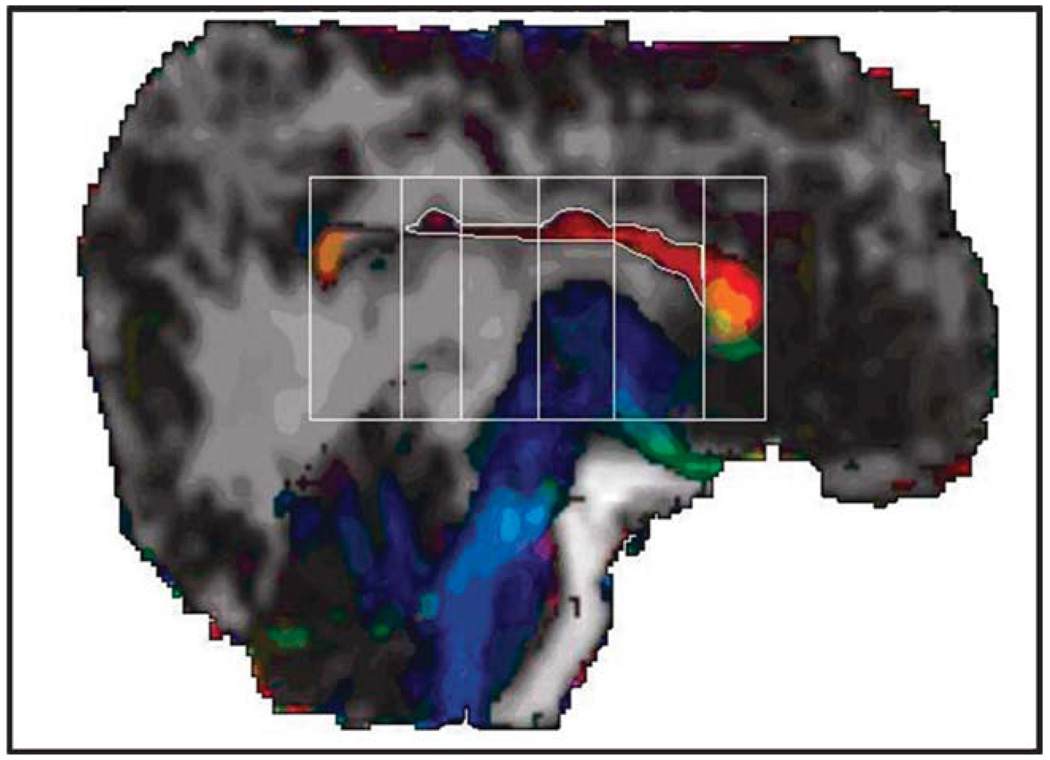
High-resolution brain MR images were acquired on a Philips 3T scanner (Koninklijke Philips Electronics NV; Amsterdam, The Netherlands) with parallel imaging technology. After conventional sagittal scout and coronal T2-weighted sequences, a three-dimensional T1-weighted sequence was performed to obtain whole-brain coverage. Acquisition parameters of the three-dimensional turbo fast spin echo sequence were as follows: repetition time/echo time = (6.5–6.7)/(3.04–3.14); flip angle = 8 degrees; field of view = 240 × 240 mm; matrix = 256 × 256; slice thickness = 1.5 mm; in-plane pixel dimensions (x, y) = 0.94, 0.94 mm; number of excitations = 2. Diffusion-weighted data covering the entire brain were acquired [18] from the same field-of-view in axial sections of 3 mm and interpolated to gain an isotropic voxel of 0.94 × 0.94 × 0.94 as described elsewhere [19]. The area of the corpus callosum was measured on a DTI midsagittal slice. The corpus callosum was subdivided into seven regions by drawing lines perpendicular to its antero-posterior length using the Witelson’s technique (Fig. 2). External boundaries of the corpus callosum were delimited by manual tracing. The area for each of the seven segments was calculated and compiled as follows: genu (two anterior segments) body (four middle segments) and splenium (posterior segment). All the measurements were repeated three times by the same specialist and intra-rater reliability was tested.
Fig. 2.
Midsagittal section of the brain (12-year-old boy) showing the directional diffusion tensor imaging color maps of the corpus callosum (C-shaped central structure). Using the Witelson method, the corpus callosum was initially subdivided into seven segments and the area corresponding to the genu (two anterior segments), body (four middle segments) and splenium (one segment posterior). Note that the rostral anterior segment was not evident in this case.
To assess the association between spectral power and integrity of the corpus callosum within the spina bifida group, Spearman’s correlation coefficient was calculated between the power in the different MEG regions and the area in the three segments of the corpus callosum for all separate frequency bands.
Results
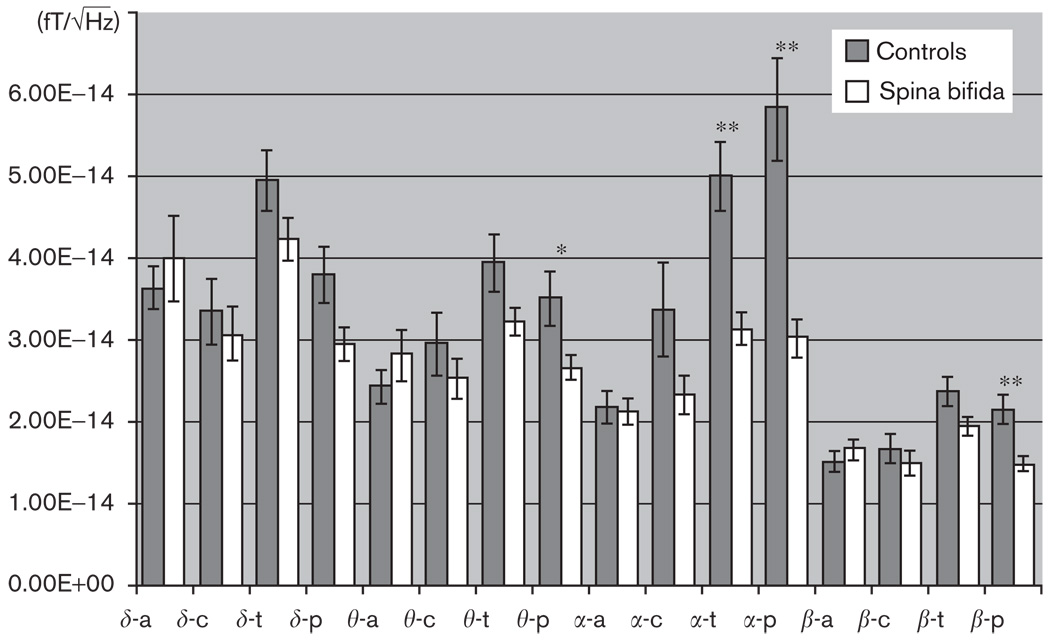
The repeated measurements analysis of variance with group as between-subjects factor and region and frequency band as within-subject variables yielded a main effect of group [F(1,30) = 2.09, P < 0.01], frequency [F(3,30) = 3.86, P < 0.05] and region [F(3,30) = 3.86, P < 0.05]. Overall, patients with spina bifida showed lower values of spectral power compared with controls (Fig. 3). There was a significant interaction effect of group × region [F(3,30) = 6.29, P = 0.05], and no other effects or interactions. The analysis did not reveal significant differences between the groups in anterior and central areas for any of the frequency bands.
Fig. 3.
Absolute spectral power (and standard error) derived from magnetoencephalography recordings in children with spina bifida (N = 21) and age-matched controls (N = 11). Four areas corresponding to anterior (a), central (c), temporal (t) and posterior (p) sensors were used to calculate the spectral power for each frequency band (δ: 1.5–4 Hz; θ: 4–8 Hz: α: 8–12 Hz and β: 12–16 Hz). The histogram shows a significant reduction in the spectral power in the SB group for posterior regions (θ, α and β) and temporal regions (θ and α). , absolute spectral power. *P<0.05; **P<0.01.
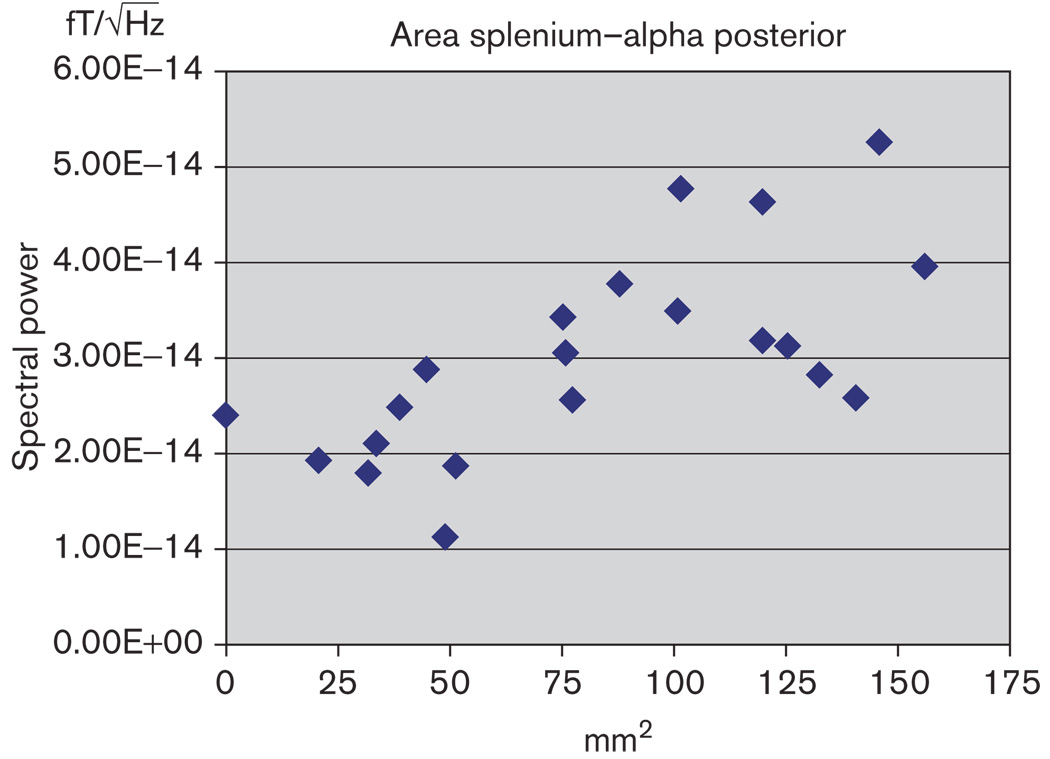
Intra-rater reliability was performed on all sub-regions of the corpus callosum and was between 0.92 and 0.97 (P < 0.001). Associations between areas of the corpus callosum and spectral power were explored within the group of children with spina bifida. Negative and significant correlations were found between the area of the splenium and the α spectral power in the posterior regions (r = 0.68; P < 0.05) (Fig. 4). Significant correlation was obtained between the cross-sectional area of the splenium and α power in temporal regions (r = 0.53; P < 0.05).
Fig. 4.
Plot representing the relation between the area of the splenium and the spectral power in the α frequency in posterior regions. Reduced area in the splenium correlates with reduced spectral power (r=0.68, P<0.05). , absolute spectral power.
Discussion
In this study, we sought to evaluate whether children with spina bifida and hydrocephalus and abnormalities of the corpus callosum present a pattern of spontaneous oscillatory activity that differs from age-matched controls. We hypothesized that this pattern of oscillatory activity is associated with the atypical anatomy of the corpus callosum in these children.
The results show that children with spina bifida present an atypical pattern of brain oscillatory activity characterized by reduced absolute spectral power of α, β and θ rhythms mainly in posterior and temporal regions. This reduced spectral power is more prominent in the α band and correlates with a reduction of the area of posterior segments of the corpus callosum (splenium). To our knowledge this is the first study to describe atypical patterns of cortical oscillations in children with spina bifida and to relate the structural integrity of transcallosal tracts with neurophysiological patterns.
Our findings support the hypothesis of a decreased interhemispheric connectivity within the temporal and occipital lobes, diminishing the capacity of posterior regions to generate sustained rhythmic oscillations. Rhythmic cortical activity has been associated with a variety of behavioural states (i.e. exploring, learning, sleeping) and it is well known that synchronized oscillatory neuronal activity plays a crucial role in supporting higher function processes [1]. In children with spina bifida and hydrocephalus, although there are a variety of deficits in the sensorimotor and cognitive areas [5–7], it has been shown that cognitive operations requiring interhemispheric integration are especially affected [9–12]. It has been suggested that the deficits in cognitive functions requiring interhemispheric coordination described in children with spina bifida may arise from abnormalities in the corpus callosum, midbrain and posterior cortexes [20]. The ‘connectivity’ explanation concurs with recent data on white matter and grey matter in children with spina bifida and hydrocephalus [21] reporting a decrease of deep white matter, predominantly in posterior brain regions. Our study supports the hypothesis of a close relation between changes in the anatomy of the corpus callosum in children with spina bifida and modifications in cortical functionality in temporal and occipital regions. The lack of significant differences between controls and children with spina bifida in the functionality of the anterior region agrees with the anatomical observation of a relatively unaffected genu in the corpus callosum of children with spina bifida.
The results of the study support previous findings suggesting that the contribution of transcallosal fibres in facilitating synchronous cortical oscillations might be significant [22]. The role of the thalamus as pacemaker of cortical synchrony has been widely accepted and, although our results do not contradict this assertion, they do demonstrate that abnormal cortico-cortical connectivity can have a substantial impact on cortical oscillations. Although abnormalities in midbrain and subcortical structures are frequent in spina bifida and hydrocephalus [20], changes in the thalamus have not been described.
Conclusion
Our study indicates that, in children with spina bifida and hydrocephalus, the presence of atypical cortical oscillatory activity is associated with the compromised integrity of the posterior segments of the corpus callosum.
Acknowledgment
This study is funded by NIH-NICHD grant P01-HD35946 to JMF.
References
- 1.Schnitzler A, Gross J. Functional connectivity analysis in magnetoencephalography. Int Rev Neurobiol. 2005;68:173–195. doi: 10.1016/S0074-7742(05)68007-5. [DOI] [PubMed] [Google Scholar]
- 2.Ball GJ, Gloor P, Schaul N. The cortical electromicrophysiology of pathological delta waves in the electroencephalogram of cats. Electroencephalogr Clin Neurophysiol. 1977;43:346–361. doi: 10.1016/0013-4694(77)90258-9. [DOI] [PubMed] [Google Scholar]
- 3.Villablanca J, Salinas-Zeballos ME. Sleep-wakefulness, EEG and behavioral studies of chronic cats without the thalamus: the athalamic cat. Archives Italiennes de Biologie. 1972;110:383–411. [PubMed] [Google Scholar]
- 4.Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. A developmental and genetic classification for malformations of cortical development. Neurology. 2005;65:1873–1887. doi: 10.1212/01.wnl.0000183747.05269.2d. [DOI] [PubMed] [Google Scholar]
- 5.Dennis M, Landry SH, Barnes MH, Fletcher JM. A model of neurocognitive function in spina bifida over the lifespan: a model of core and functional deficits. Int Neuropsychol Soc. 2006;12:285–296. doi: 10.1017/S1355617706060371. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher JM, Copeland K, Frederick J, Blaser SE, Kramer LA, Northrup H, et al. Spinal lesion level in spina bifida meningomyelocele: a source of neural and cognitive heterogeneity. J Neurosurg. 2005;102:268–279. doi: 10.3171/ped.2005.102.3.0268. [DOI] [PubMed] [Google Scholar]
- 7.Yeates KO, Fletcher JM, Dennis M. Spina bifida and hydrocephalus. In: Morgan JE, Ricker JH, editors. Handbook of neuropsychology. New York: Taylor & Francis; 2008. pp. 128–148. [Google Scholar]
- 8.Charney F. Neural tube defects: spina Bifida and myelomeningocele. In: Dans M, Batshaw Y, editors. Children with disabilities: a Medical Primer. 3rd ed. Baltimore: Brookes; 1992. pp. 471–488. [Google Scholar]
- 9.Hannay HJ. Functioning of the corpus callosum in children with early hydrocephalus. J Int Neuropsychol Soc. 2000;6:351–361. doi: 10.1017/s1355617700633106. [DOI] [PubMed] [Google Scholar]
- 10.Hannay HJ, Boudousquie A, Dennis M, Kramer L, Blaser S, Fletcher JM. Auditory interhemispheric transfer in children with spina bifida and corpus callosum anomalies: MRI correlates and compensatory mechanisms. J Int Neuropsychol Soc. 2008;14:771–781. doi: 10.1017/S1355617708080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber-Okrainec J, Dennis M. Idiom comprehension deficits in relation to corpus callosum agenesis and hypoplasia in children with spina bifida myelomeningocele. Brain Lang. 2005;93:349–368. doi: 10.1016/j.bandl.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Klaas P, Hannay HJ, Caroselli J, Fletcher JM. Interhemispheric transfer of information in children with partial agenesis of the corpus callosum. J Clin Exp Neuropsychol. 1999;21:837–850. doi: 10.1076/jcen.21.6.837.851. [DOI] [PubMed] [Google Scholar]
- 13.Cañive JM, Lewine JD, Edgar JC, Davis JT, Miller GA, Torres F, et al. Spontaneous brain magnetic activity in schizophrenia patients treated with aripiprazole. Psychopharmacol Bull. 1998;34:101–105. [PubMed] [Google Scholar]
- 14.Fernández A, Hornero R, Mayo A, Poza J, Maestú F, Ortiz Alonso T. Quantitative magnetoencephalography of spontaneous brain activity in Alzheimer disease: an exhaustive frequency analysis. Alzheimer Dis Assoc Disord. 2006;20:153–159. doi: 10.1097/00002093-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Tecchio F, Zappasodi F, Pasqualetti P, Tombini M, Salustri C, Oliviero A, et al. Rhythmic brain activity at rest from rolandic areas in acute mono-hemispheric stroke: a magnetoencephalographic study. Neuroimage. 2005;28:72–83. doi: 10.1016/j.neuroimage.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 16.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 17.Thorndike R, Hagen E, Sattler J. The Stanford–Binet Intelligence Scale. 4th ed. Itasca, IL: Riverside; 1986. [Google Scholar]
- 18.Hasan KM, Eluvathingal TJ, Kramer LA, Ewing-Cobbs L, Dennis M, Fletcher JM. White matter microstructural abnormalities in children with spina bifida myelomeningocele and hydrocephalus: a diffusion tensor tractography study of the association pathways. J Magn Reson Imaging. 2008;27:700–709. doi: 10.1002/jmri.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasan KM, Gupta RK, Santos RM, Wolinsky JS, Narayana PA. Diffusion tensor fractional anisotropy of the normal-appearing seven segments of the corpus callosum in healthy adults and relapsing-remitting multiple sclerosis patients. J Magn Reson Imaging. 2005;21:735–743. doi: 10.1002/jmri.20296. [DOI] [PubMed] [Google Scholar]
- 20.Dennis M, Edelstein K, Frederick J, Copeland K, Francis D, Blaser SE, et al. Peripersonal spatial attention in children with spina bifida: associations between horizontal and vertical line bisection and congenital malformations of the corpus callosum, midbrain, and posterior cortex. Neuropsychologia. 2005;43:2000–2010. doi: 10.1016/j.neuropsychologia.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Juranek J, Fletcher JM, Hasan KM, Breier JI, Cirino PT, Pazo-Alvarez P, et al. Neocortical reorganization in spina bifida. Neuroimage. 2008;40:1516–1522. doi: 10.1016/j.neuroimage.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stancák A, Svoboda J, Rachmanová R, Vrána J, Králík J, Tintera J. Desynchronization of cortical rhythms following cutaneous stimulation: effects of stimulus repetition and intensity, and of the size of corpus callosum. Clin Neurophysiol. 2003;114:1936–1947. doi: 10.1016/s1388-2457(03)00201-3. [DOI] [PubMed] [Google Scholar]