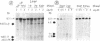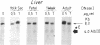Abstract
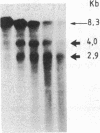
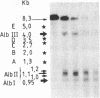
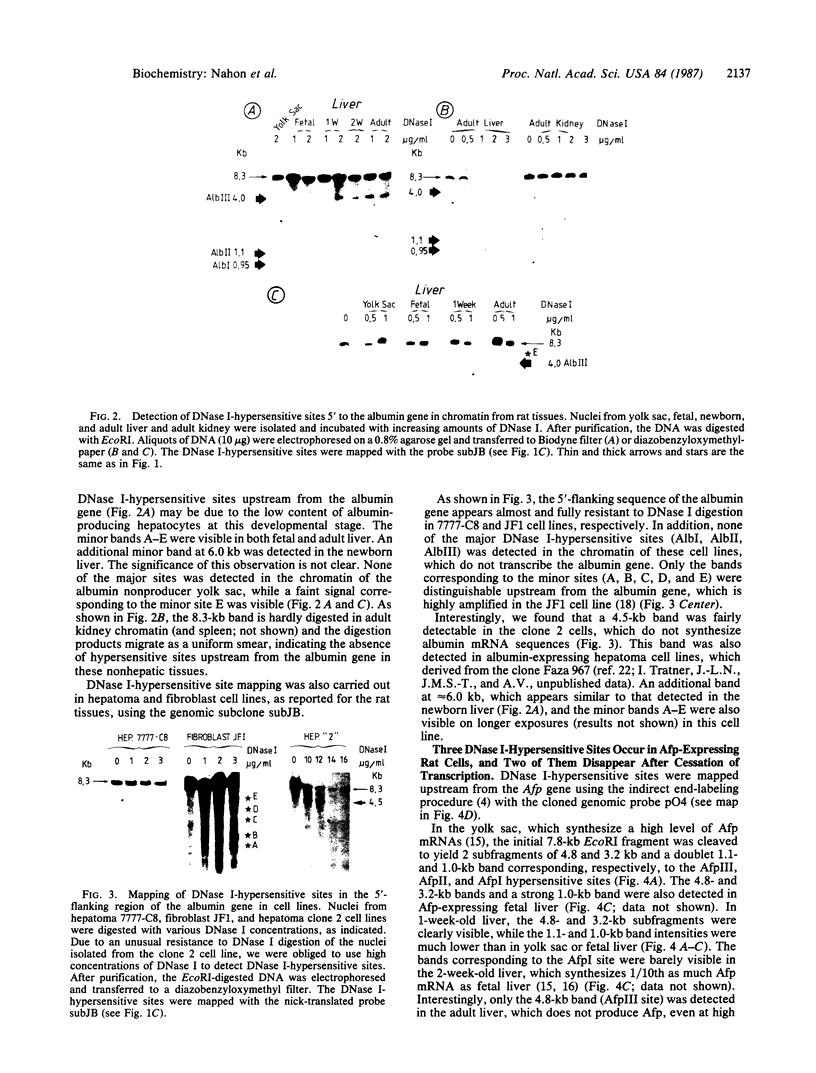
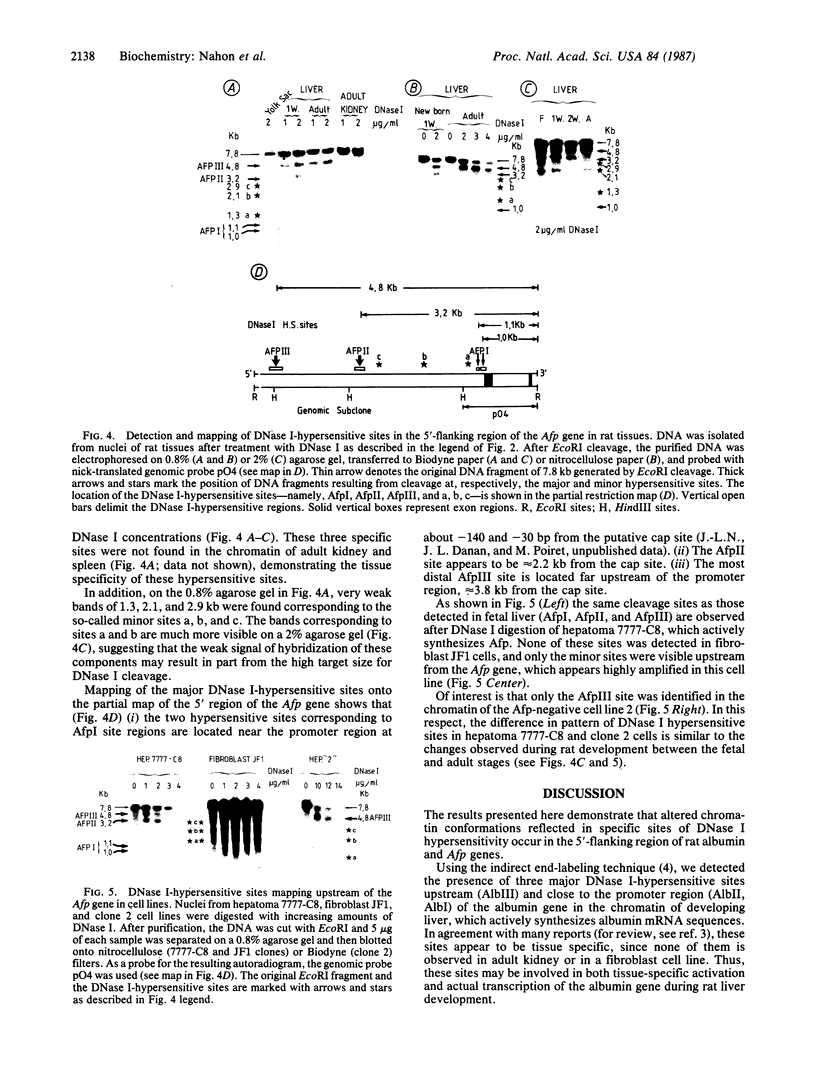
We have examined the chromatin structure of the 5'-flanking region of the albumin and alpha-fetoprotein (Afp) genes in different developing rat tissues and cloned cell lines that display various functional states of these genes. Nuclease-hypersensitive sites were probed with DNase I, using an indirect end-labeling technique. In albumin-producing rat cells two major DNase I-hypersensitive sites were found near the promoter region and one additional site was located approximately 3 kilobases (kb) upstream. Similarly, in Afp-producing rat tissues and cell lines we mapped one DNase I-hypersensitive region close to the promoter region and two cleavage sites further upstream at approximately 2.2 and approximately 3.8 kb from the cap site. The DNase I-hypersensitive sites of both genes were absent in nonhepatic rat cells and therefore appear to be tissue specific. Loss of specific sets of DNase I-hypersensitive sites accompanies the cessation of transcription for the Afp gene in adult rat liver and in a "dedifferentiated" hepatoma cell line. Likewise, specific sets of DNase I-hypersensitive sites disappear during the inactivation of the albumin gene in hepatoma cells. The distal upstream sites of the Afp and albumin genes display the same DNase I sensitivity in expressing and potentially expressible states. These findings suggest that reversible changes in short chromatin regions may be involved in the actual transcription of the albumin and Afp genes, while more permanent tissue-specific changes at other sites correlate with the capacity of these genes to be expressed during hepatic differentiation and neoplasia.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryan P. N., Olah J., Birnstiel M. L. Major changes in the 5' and 3' chromatin structure of sea urchin histone genes accompany their activation and inactivation in development. Cell. 1983 Jul;33(3):843–848. doi: 10.1016/0092-8674(83)90026-0. [DOI] [PubMed] [Google Scholar]
- Bösze Z., Venetianer A. Tumorigenicity in nude mice of dexamethasone-sensitive and -resistant, differentiated and dedifferentiated hepatoma cells. Cancer Res. 1985 May;45(5):2165–2169. [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Elgin S. C. DNAase I-hypersensitive sites of chromatin. Cell. 1981 Dec;27(3 Pt 2):413–415. doi: 10.1016/0092-8674(81)90381-0. [DOI] [PubMed] [Google Scholar]
- Fahrlander P. D., Piechaczyk M., Marcu K. B. Chromatin structure of the murine c-myc locus: implications for the regulation of normal and chromosomally translocated genes. EMBO J. 1985 Dec 1;4(12):3195–3202. doi: 10.1002/j.1460-2075.1985.tb04065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A., Nahon J. L., Gomez-Garcia M., Tratner I., Sala-Trepat J. M. Organization of the albumin and alpha-fetoprotein genes in fetal and adult rat tissues, and rat hepatomas. Differentiation. 1985;29(3):238–242. doi: 10.1111/j.1432-0436.1985.tb00322.x. [DOI] [PubMed] [Google Scholar]
- Godbout R., Ingram R., Tilghman S. M. Multiple regulatory elements in the intergenic region between the alpha-fetoprotein and albumin genes. Mol Cell Biol. 1986 Feb;6(2):477–487. doi: 10.1128/mcb.6.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Propagation of globin DNAase I-hypersensitive sites in absence of factors required for induction: a possible mechanism for determination. Cell. 1982 Aug;30(1):131–139. doi: 10.1016/0092-8674(82)90019-8. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. The calcium dependent endonuclease activity of isolated nuclear preparations. Relationships between its occurrence and the occurrence of other classes of enzymes found in nuclear preparations. Biochem Biophys Res Commun. 1973 May 15;52(2):475–481. doi: 10.1016/0006-291x(73)90736-5. [DOI] [PubMed] [Google Scholar]
- Krumlauf R., Hammer R. E., Tilghman S. M., Brinster R. L. Developmental regulation of alpha-fetoprotein genes in transgenic mice. Mol Cell Biol. 1985 Jul;5(7):1639–1648. doi: 10.1128/mcb.5.7.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnath L., Locker J. DNaseI sensitivity of the rat albumin and alpha-fetoprotein genes. Nucleic Acids Res. 1985 Jan 11;13(1):115–129. doi: 10.1093/nar/13.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucotte G., Gal A., Nahon J. L., Sala-Trepat J. M. Eco RI restriction-site polymorphism of the albumin gene in different inbred strains of rat. Biochem Genet. 1982 Dec;20(11-12):1105–1115. doi: 10.1007/BF00498935. [DOI] [PubMed] [Google Scholar]
- Mathis D., Oudet P., Chambon P. Structure of transcribing chromatin. Prog Nucleic Acid Res Mol Biol. 1980;24:1–55. doi: 10.1016/s0079-6603(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Muglia L., Rothman-Denes L. B. Cell type-specific negative regulatory element in the control region of the rat alpha-fetoprotein gene. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7653–7657. doi: 10.1073/pnas.83.20.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahon J. L., Gal A., Erdos T., Sala-Trepat J. M. Differential DNase I sensitivity of the albumin and alpha-fetoprotein genes in chromatin from rat tissues and cell lines. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5031–5035. doi: 10.1073/pnas.81.16.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahon J. L., Gal A., Frain M., Sell S., Sala-Trepat J. M. No evidence for post-transcriptional control of albumin and alpha-fetoprotein gene expression in developing rat liver neoplasia. Nucleic Acids Res. 1982 Mar 25;10(6):1895–1911. doi: 10.1093/nar/10.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M. O., Sperling L., Herbomel P., Yaniv M., Weiss M. C. Tissue-specific expression is conferred by a sequence from the 5' end of the rat albumin gene. EMBO J. 1984 Nov;3(11):2505–2510. doi: 10.1002/j.1460-2075.1984.tb02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R. Transcriptionally active chromatin. Biochim Biophys Acta. 1984 Sep 10;782(4):343–393. doi: 10.1016/0167-4781(84)90044-7. [DOI] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Dever J., Sargent T. D., Thomas K., Sell S., Bonner J. Changes in expression of albumin and alpha-fetoprotein genes during rat liver development and neoplasia. Biochemistry. 1979 May 29;18(11):2167–2178. doi: 10.1021/bi00578a006. [DOI] [PubMed] [Google Scholar]
- Sargent T. D., Jagodzinski L. L., Yang M., Bonner J. Fine structure and evolution of the rat serum albumin gene. Mol Cell Biol. 1981 Oct;1(10):871–883. doi: 10.1128/mcb.1.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent T. D., Wu J. R., Sala-Trepat J. M., Wallace R. B., Reyes A. A., Bonner J. The rat serum albumin gene: analysis of cloned sequences. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3256–3260. doi: 10.1073/pnas.76.7.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubach W., Groudine M. Alteration of c-myc chromatin structure by avian leukosis virus integration. Nature. 1984 Feb 23;307(5953):702–708. doi: 10.1038/307702a0. [DOI] [PubMed] [Google Scholar]
- Scott R. W., Vogt T. F., Croke M. E., Tilghman S. M. Tissue-specific activation of a cloned alpha-fetoprotein gene during differentiation of a transfected embryonal carcinoma cell line. Nature. 1984 Aug 16;310(5978):562–567. doi: 10.1038/310562a0. [DOI] [PubMed] [Google Scholar]
- Sell S., Sala-Trepat J. M., Sargent T. D., Thomas K., Nahon J. L., Goodman T. A., Bonner J. Molecular mechanisms of control of albumin and alphafetoprotein production: a system to study the early effects of chemical hepatocarcinogens. Cell Biol Int Rep. 1980 Mar;4(3):235–254. doi: 10.1016/0309-1651(80)90056-9. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Belayew A. Transcriptional control of the murine albumin/alpha-fetoprotein locus during development. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5254–5257. doi: 10.1073/pnas.79.17.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venetianer A., Poliard A., Poiret M., Erdös T., Hermesz E., Sala-Trepat J. M. Activation of alpha-fetoprotein synthesis in rat hepatoma cells with reduced sensitivity to dexamethasone. Differentiation. 1986;32(2):148–156. doi: 10.1111/j.1432-0436.1986.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Larsen A., Groudine M. Alpha-Globin-gene switching during the development of chicken embryos: expression and chromosome structure. Cell. 1981 May;24(2):333–344. doi: 10.1016/0092-8674(81)90323-8. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]