Abstract
Celiac disease (CD) is an immune-mediated enteropathy that is secondary to gluten ingestion and classically associated with gastrointestinal symptoms. Diagnosis is based on serology and confirmatory duodenal biopsy, and the only treatment is lifelong avoidance of gluten. CD has been increasingly recognized to encompass a wide variety of manifestations that are relevant to women’s health, including infertility, adverse pregnancy outcomes and reduced BMD. Currently, CD is underdiagnosed, largely owing to lack of recognition of the diverse manifestations by general practitioners. Increased awareness of the clinical spectrum of this disease, as well as targeted testing in at-risk individuals (including women with unexplained infertility and previous adverse pregnancy outcomes, and in specific populations with reduced BMD) is greatly needed in order to improve rates of diagnosis.
Keywords: bone-density celiac disease, fertility, gluten, osteopenia, osteoporosis, pregnancy, screening, tissue transglutaminase
Celiac disease (CD) is an immune-mediated enteropathy triggered by ingestion of foods containing gluten, for which the only treatment is a life-long adherence to a gluten-free diet (GFD) [1]. The prevalence is thought to be 1% or higher in the general population, although fewer than 5% are diagnosed in many regions [2–4]. Classically thought to be a disease with primarily gastrointestinal manifestations and affecting children under 2 years, the epidemiology of CD has shifted such that the majority of patients are now presenting as adults with diverse symptomatology in the fourth to fifth decade of life, which accounts for the high rate of missed diagnoses [5,6]. CD is diagnosed predominantly in women. The female predominance of CD is partially due to true increased prevalence in women relative to men, but is also related to the fact that women use healthcare services more than men [7,8]. Currently, in most populations women constitute 60–70% of individuals with diagnosed CD [9,10].
The spectrum of systemic manifestations associated with CD is broad and encompasses iron deficiency anemia, hyposplenism, reduction in BMD, liver function abnormalities, neuropathy, psychological disturbances, fatigue, myalgias, arthralgias, asthma, weight loss, bloating, abdominal pain, bowel changes, alopecia, headaches, menstrual irregularities, infertility and adverse pregnancy outcomes [1,11]. Aside from the classic symptoms of abdominal pain, bloating and bowel changes, many of the nonspecific symptoms associated with CD do not routinely prompt primary care physicians to test for this disease [12]. While there are a multitude of potential complications of CD including osteoporosis, autoimmune disorders and malignancy, undiagnosed CD may be particularly devastating in women who experience unexplained infertility, recurrent abortions and perinatal complications. Excellent general reviews of CD have been published in recent years; however, little attention has been paid to the significant potential impact of CD on women’s health [1,13,14]. In this article, we review the major areas where CD and health concerns specific to women intersect.
Pathophysiology of celiac disease
The protein responsible for the immune response in CD is gluten, which is derived from wheat and similar proteins that are found in rye and barley [15]. Gliadin peptides, which are derived from gluten, contain the majority of toxic substances and are resistant to degradation by proteases, thereby allowing them to remain intact within the intestinal lumen after ingestion [16]. In individuals with CD, these peptides then enter the lamina propria, triggering chronic inflammatory changes.
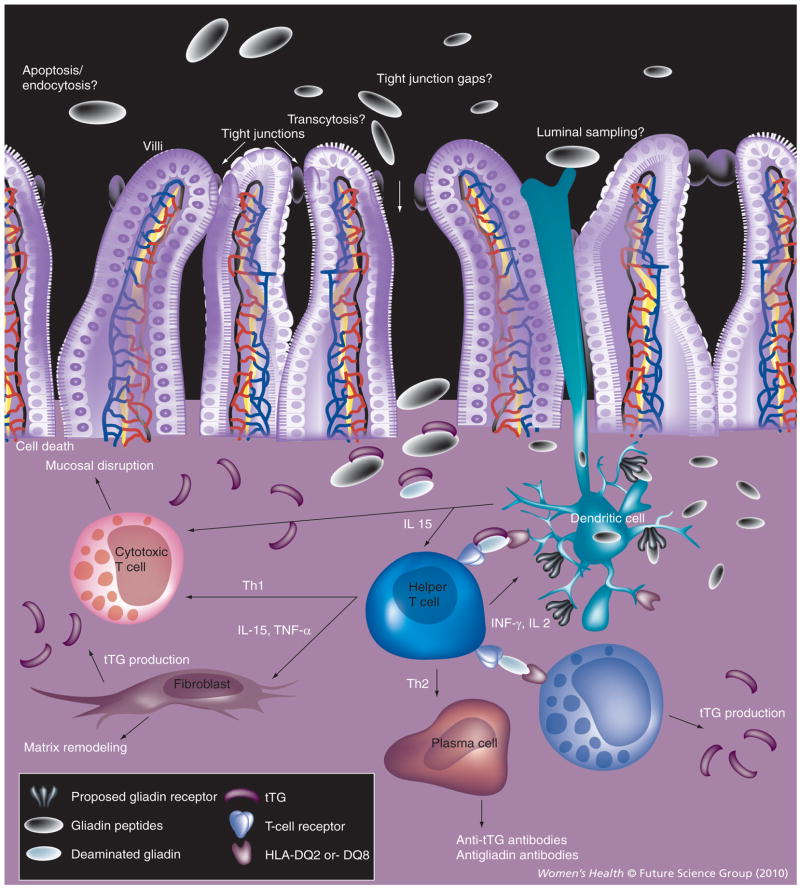
It is notable that gluten peptides in their native form are not toxic. In order for gluten peptides to cause inflammation, they must first be altered by the enzyme tissue transglutaminase (tTG), which is normally involved in tissue remodeling and protein cross-linking. tTG is normally present in nearly all organs and is increased in areas of inflammation. In the submusosa of the intestine, tTG deamidates gluten peptides, changing peptide shape and charge. These altered gluten peptides are then able to bind tightly to HLA-DQ2 and HLA-DQ8 molecules on antigen-presenting cells. This binding triggers an inflammatory reaction causing lymphocyte infiltration, villous atrophy and the production of antibodies to gliadin and tTG (Figure 1).
Figure 1. Pathogenesis of celiac disease.
Gluten peptides are deaminated by tTG and bind HLA-DQ2 and HLA-DQ8 molecules. Antigen-presenting cells activate helper T cells and trigger an inflammatory reaction causing activation of cytotoxic T cells, macrophages and plasma cells. The result is mucosal disruption, matrix remodeling, cell death and the production of antibodies to gliadin and tTG. tTG: Tissue transglutaminase.
While the exact pathogenesis of many of the complications of CD is not fully understood, data supports a direct relationship between both the nutritional deficiencies and inflammatory responses seen in CD, and observed reproductive manifestations. The nutritional deficiencies, which result from malabsorption and, secondarily, to limitations common in the GFD, can directly impact reproductive function. Specifically, zinc, selenium, iron and folate deficiencies have been implicated.
Zinc, an essential element necessary for DNA synthesis, impairs the production and secretion of follicle-stimulating hormone and luteinizing hormone, potentially leading to abnormalities in ovarian development, spontaneous abortions, eclampsia and intrauterine growth retardation [17–19]. Similarly, selenium is considered to be another crucial trace element required in adequate amounts for normal reproductive function. Selenium requirements further increase during pregnancy and lactation, and deficiencies have been associated with subfertility and spontaneous abortion in susceptible CD patients. Requirements for folate and iron also increase during pregnancy, and underlying CD may exacerbate the resulting anemia. Insufficient oxygen-carrying capacity in the blood may contribute to complications in pregnancy, and it is well known that folate deficiency is associated with neural tube defects and possible spontaneous abortion [20].
Reproductive complications also frequently occur without apparent vitamin or mineral deficiencies, making malabsorption unlikely to be the sole mechanism. Autoimmune factors have been implicated, namely the role of tTG antibodies. It has been illustrated that placental tTG is bound by maternal autoantibody, directly affecting placental function and therefore possibly impairing nutrient exchange [21,22]. Alternatively, because tTG may stabilize fragments shed from the syncytiotrophoblast through cross-linking and effective phagocytosis, interference from autoantibodies may also impair phagocytosis and lead to the release of fetoplacental antigens. This would be expected to result in altered recognition of the fetus by the maternal immune system [21]. This latter theory has yet to be validated, but is an additional possible explanation for aspects of obstetric complications in CD.
Similar to fertility, the effect of CD on BMD is multifactorial. One clear factor is the vitamin D and calcium deficiencies commonly seen in CD patients owing to villous atrophy and dietary restrictions. These nutritional deficiencies may be seen with or without secondarily elevated levels of parathyroid hormone [23–26]. Also implicated in decreased BMD is a reduction in IGF, which could be due to decreased zinc absorption [27–30].
It is also likely that the chronic inflammation and proinflammatory cytokines seen in CD contribute to BMD reduction. Specifically, in the context of inflammation, cytokines are released and may affect bone remodeling through local signaling [31]. Moreno et al. demonstrated that mutations in IL-1 genes were associated with bone loss in CD [32]. Finally, a recent study suggested a link between CD and autoantibodies directed towards osteoprotegerin, which inhibits a receptor leading to osteoclast activation and bone resorption. Therefore, the inhibitory effect of osteoprotegerin is blocked and bone loss results. The authors are careful to point out that the pathogenesis of osteoporosis is complex, and the presence of these autoantibodies in CD is probably only one of many factors leading to bone loss [33].
Diagnosis & management
Celiac disease may be the cause of patients presenting at any age with nearly any complaint. For this reason, a high suspicion for CD and a low threshold for testing are necessary. Diagnosis of CD is guided by initial serological testing and confirmatory duodenal biopsy showing characteristic histological changes including crypt hyperplasia, villous atrophy and increased intraepithelial lymphocytes [34]. Indications for consideration of serological testing are listed in Box 1 [13].
Box 1. Current indications for celiac disease testing in the general population.
Chronic gastrointestinal symptoms
Past or possible diagnosis of irritable bowel syndrome
Unexplained weight loss
Iron deficiency anemia
Unexplained vitamin/mineral deficiencies
Osteoporosis in males and premenopausal females
Secondary hyperparathyroidism
Unexplained elevations in liver function tests
Certain genetic disorders (e.g., Down Syndrome, Turner’s Syndrome)
Certain autoimmune disorders (e.g., Type I diabetes mellitus and autoimmune thyroid disease) with symptoms or a change in health status
First-degree relatives with celiac disease (testing of asymptomatic adults is controversial and should be considered on a case-by-case basis)
Certain neurological disorders (e.g., unexplained peripheral neuropathy and ataxia)
Indications for celiac disease taken from [13].
Modern serologic testing for CD is accurate and cost effective. The current test of choice in most settings is IgA-tTG antibody. IgA-tTG assay is routinely used and has a 94% sensitivity and 97% specificity [35,36]. Anti-endomysial antibody (EMA) is considered to have a similarly high positive-predictive value, but given the increased expense and technical difficulty of EMA, and the fact that sensitivity and specificity are more than 95% in both assays, IgA-tTG is generally preferred [36,37]. Antigliadin antibody (AGA) testing is no longer widely used given its unacceptably low specificity (leading to a high rate of false-positive results), although a second-generation version, deamidated gliadin peptide appears to have accuracy similar to tTG and may be useful in certain situations [38,39]. It is important to recognize that most current tests are IgA based and false-negative results will occur in individuals with IgA deficiency. This is especially important since IgA deficiency is more common in CD than in the general population [40–42]. For this reason, total IgA levels are often checked along with IgA-tTG. Furthermore, all tests for CD will normalize on a GFD, so it is vital that patients are tested before starting treatment. It is currently recommended that all cases of positive celiac serology be confirmed with duodenal biopsy, and monitored for clinical and serologic improvement on a GFD.
The treatment for CD is lifelong abstinence from gluten-containing foods such as wheat, rye and barley. Adherence is often difficult, and management should include consultation with a skilled dietitian, education about the disease, identification and treatment of nutritional deficiencies, access to an advocacy group, and continuous long-term follow-up by a multidisciplinary team [43].
Case finding & screening for celiac disease
Mass screening for CD is not currently recommended since there is no clear data to suggest that patients with minor or no clinical manifestations have the same risk of malignancy and increased mortality, and the cost of diagnosis and treatment to the individual is high [44,45]. Furthermore, although there have not been studies directly measuring the burden of a GFD, it is probably substantial, and rates of noncompliance are as high as 20–50% [46,47]. Several studies have also suggested that GFD compliance is poorer in screen-detected CD versus symptom-detected CD [47–49]. There is obviously less utility in the diagnosis of CD if the GFD is unlikely to be kept. Furthermore, quality of life may be compromised when recommending such a significant lifestyle change to asymptomatic patients, especially when it is unclear if there are any considerable health benefits [50].
While it has been established that CD is vastly underdiagnosed, rather than mass screening of the general population increased awareness of the diverse clinical features of CD and targeted case finding is currently the recommended strategy [2]. We agree with this approach, since it encourages testing of individuals in certain risk groups with specific health problems who are most likely to benefit from diagnosis. This approach has been shown to be effective in increasing CD diagnosis rates in primary care settings [12].
A recent cost–effectiveness analysis suggested that when the standardized mortality ratio of untreated CD is relatively high, the cost of screening was less than US$50,000 per quality-adjusted life year (QALY), a figure that is competitive with many other accepted screening procedures [51]. Furthermore, these estimates may be conservative, since the impact of GFD on morbidity associated with malignancy, osteopenia, abortion, infertility and autoimmune diseases was not taken into account (although for some of these diseases, the beneficial effects of GFD have not yet been unequivocally established). As the standardized mortality ratio decreased, the degree of cost–effectiveness dropped dramatically, suggesting that the findings are applicable only to at-risk groups and as further support for targeted testing. Similarly, Hershcovici et al. found a gain of 0.0027 QALYs in screened versus unscreened groups, and an incremental cost–effectiveness ratio of US$48,960 per QALY in screened versus unscreened groups [52]. In addition, reduced time delay from symptom onset to diagnosis, adherence to GFD and prevalence of CD were all factors largely impacting cost–effectiveness. The authors concluded that cost–effectiveness of screening is best achieved when there is high utility of the GFD and when standard clinical diagnosis is delayed by at least 6 years. These studies demonstrate that from the standpoint of cost–effectiveness, it appears reasonable to recommend targeted screening in the setting of high likelihood of GFD adherence.
Currently, the indications for CD testing are quite broad, as mentioned earlier. There are presently no guidelines for CD testing in patients with infertility or in women with a history of adverse pregnancy outcomes, although CD prevalence has been shown to be higher in these groups than in the general population [53–55]. Owing to the higher risk of CD in these populations, and the likelihood that the GFD improves pregnancy and fertility outcomes as detailed later, we argue that given the low cost of serological screening compared with the great medical expense associated with infertility and complications of pregnancy, CD testing should be strongly considered.
Clear guidelines for CD testing in osteoporosis are also lacking, and existing data are somewhat contradictory; however, it is our opinion that celiac testing should at be considered in certain populations, as outlined later.
Fertility
Celiac disease is seldom considered in the evaluation of infertility, and the link between the two has been referred to many times in the literature as a ‘neglected clinical association’. It is estimated that approximately 7.4–14% of women are infertile in North America, with 15% of this infertility attributed to unexplained factors after hormonal and anatomical causes have been ruled out [56,57]. While women with CD may present with amenorrhea, menstrual irregularities, multiple spontaneous abortions, iron deficiency anemia or the host of other symptoms described previously, many times they are completely asymptomatic aside from infertility. Given the mean diagnosis age of 40–50 years of and the fact that diagnosis of CD is on average delayed up to 10 years, many women are only diagnosed around the time of menopause. Thus, the entire span of reproductive life may be disrupted in women with undiagnosed CD.
Is the prevalence of CD higher in women with unexplained infertility?
Studies show that the prevalence of CD may be as high as 4–8% in women with unexplained infertility [53,54]. A Finnish study of 98 women with unexplained infertility found that four (4.1%) had CD (p = 0.02) [53]. A similar study carried out in Sardinia found that three of 98 women with infertility had CD [54]. Of the 25 women with unexplained infertility, two were newly diagnosed with CD (prevalence: 8%; p < 0.03 compared with the general population).
Shamaly et al. conducted a study in 192 Arabic women with unexplained infertility, using EMA, total IgA and tTG antibody for screening, and diagnostic biopsy for any positive serology or for IgA deficiency. A total of five out of 192 women were diagnosed with CD, (2.6%; 95% CI: 0.85–6.0%) in contrast to one diagnosis in fertile controls (0.48%; 95% CI: 0.01–2.62%; p = 0.11) [58]. Although the increased prevalence of CD in the infertile cohort did not reach significance, the authors suggest that this may be due to small sample size.
Higher prevalence of CD in unexplained infertility was again demonstrated in an Italian study investigating the prevalence of CD in women undergoing assisted reproductive techniques (ARTs) [59]. However, as in the study by Shamaly et al., the results did not reach statistical significance. Tiboni et al. found that in 200 women undergoing ART who were screened for CD using EMA and tTG, five women (2.5%) were diagnosed with CD on biopsy compared with two (1%) in the control group (p = 0.44). Although all of these patients had alternative reasons for seeking ART, only one had successful in vitro fertilization, which ultimately resulted in miscarriage. The authors again attribute lack of statistical significance in the study to insufficient sample size.
While individual studies on the prevalence of CD in women with infertility are often under-powered, a trend toward an increased prevalence of CD is clear. Indeed, of the four studies described earlier, 17 out of 641 women with infertility were diagnosed with CD (2.4%) compared with 20 out of 2167 controls (0.9%), which is a highly significant difference (p = 0.002).
Does the GFD improve fertility rates?
One critical question for which limited data are available is whether adherence to GFD ultimately improves fertility rates. Nearly all other manifestations of CD have been shown to improve with gluten withdrawal, including BMD and risk of malignancy and mortality, so it is reasonable to expect a similar phenomenon with fertility.
A case–control study in which 68 patients were matched for age and sex showed that, prior to diagnosis and treatment, CD patients had a mean of 1.4 children and controls had a mean of 1.8 children [60]. Following diagnosis and institution of GFD, CD patients had a mean of 0.5 children (SD: 0.9) and controls had a mean of 0.7 children (SD: 1.2). The authors attribute the reduced difference in fertility following diagnosis and treatment to the effects of a GFD. However, the significantly higher rate of miscarriage in CD patients prior to diagnosis and treatment did not appear to improve with institution of a GFD. A similar study found that out of 74 patients with CD, the 20 who adhered to a GFD had reduced incidences of secondary amenorrhea, delayed menarche, early menopause and spontaneous abortion when compared with the 54 patients not adhering to a GFD [61]. The reduced period of fertility in untreated CD patients is considered to be a marker of subfertility [62]. Similarly, Rujner showed a significant decrease in the incidence of delayed menarche in young girls with CD who adhered to a GFD compared with those who did not [63].
Although the number of prospective studies directly measuring the reversibility of inferior fertility rates in CD women with a GFD is limited, the available data does seem to implicate that institution of a GFD may be beneficial in this population. Although benefit of CD diagnosis in improving fertility has yet to be conclusively shown, most experts believe that fertility improves at least modestly with treatment of CD. Larger and better controlled studies are clearly needed in this area.
Should women with unexplained infertility be screened for CD?
It is important to note that in the majority of studies, women with unexplained infertility who were found to have CD had none of the symptoms classically attributed to CD, and only half had a history of anemia [53,54,59]. For this reason, although the presence of menstrual irregularities or anemia should generally prompt CD testing, the absence of these factors in an infertile woman should not preclude consideration of CD. Given the higher prevalence of CD in women with unexplained infertility and the simplicity of testing for CD in comparison to the expensive and extensive testing typically carried in infertile couples, it is reasonable to suggest that all women with unexplained infertility be tested for CD.
Adverse outcomes in pregnancy
Aside from the effects on fertility noted earlier, CD is also associated with multiple complications in pregnancy including high miscarriage rate, intrauterine growth retardation, low birth weight and preterm birth [64–70]. Furthermore, there is reasonably good evidence to suggest that the rate of adverse outcomes is reduced with early diagnosis and treatment with GFD.
What are the pregnancy outcomes associated with CD?
Several studies support the risk of recurrent spontaneous abortion, intrauterine growth retardation and low birth weight in CD, although the risk for preterm birth is somewhat less clear. Gasbarrini et al. specifically assessed the frequency of subclinical CD in women with recurrent spontaneous abortion and intrauterine growth retardation through serological assay with EMA and tTG [64]. All control patients had negative serological testing, whereas 8% of women with recurrent spontaneous abortion and 15% of women with intrauterine growth retardation had positive celiac autoantibodies (p < 0.01). Of the nine women with positive serological testing who agreed to undergo biopsy, eight were definitively diagnosed with CD.
An Italian study found that of 845 pregnant women screened for EMA, 12 were identified as having biopsy-proven CD [65]. Seven of these pregnancies had adverse outcomes, two resulted in miscarriage and five resulted in low birth-weight babies. Of the five multiparous women with CD, four had experienced one prior miscarriage, which was significantly higher than in controls. An Italian study involving 868 women who had preterm or low birth-weight babies found that 1.60% of women with low birth-weight babies had undiagnosed CD, which was 2.25-times greater than the incidence in the control population (p < 0.04) [66]. The risk of low birth-weight babies was also found to be greater in women with undiagnosed CD (OR 6.97; 95% CI: 1.11–43.55) [66]. Notably, fewer than 20% of women with undiagnosed CD had gastrointestinal symptoms. This particular study found no difference in the rates of preterm birth among women with CD and controls.
The risk of low birth weight with CD was further supported in a large Danish population-based cohort study of all singleton live births over 25 years. Over this time period, 504,324 babies were born to 836,241 mothers, 346 of whom were born to women with diagnosed CD and 1105 of whom were born to women with undiagnosed CD [67]. It was assumed that women who were diagnosed with CD adhered to a GFD, and were therefore treated. Only women diagnosed 90 days before pregnancy were included because it was assumed that GFD treatment took this long to take effect. Women with undiagnosed CD had a higher risk of small-for-gestation-age infants (OR: 1.31; 95% CI: 1.06–1.63), very small-for-gestational-age infants (OR: 1.54; 95% CI: 1.17–2.03), and preterm birth (OR: 1.33; 95% CI: 1.02–1.72) when compared with women with a previous diagnosis of CD. In addition, infants of women with undiagnosed CD had a reduced mean birth weight of 100 g (adjusted difference: 98; 95% CI: −130 to −67).
With regard to preterm birth, smaller studies have had varied results. They have either supported this study’s finding, not supported the association between untreated CD and higher risk of preterm birth, or have not found significant risk reduction with the institution of a GFD. Overall, in regard to preterm birth, studies have had varied results and it is difficult to make any firm conclusions given the inconsistent data [66,68,69].
Does a GFD reduce the incidence of adverse outcomes in pregnancy?
As with fertility, as important as it is to recognize the association of CD with pregnancy and pregnancy outcomes, it is also critical to assess the effect of gluten withdrawal on these outcomes. Fortunately, in contrast to the issue of infertility, there is a greater pool of studies supporting a beneficial effect of a GFD in women with CD with regard to recurrent spontaneous abortion, intrauterine growth retardation and low birth weight.
For example, a Danish cohort study including 211 babies born to 127 mothers who were later diagnosed with CD showed that when compared with controls, risk of low birth weight was greater (OR: 2.6; 95% CI: 1.6–7.2) and risk of intrauterine growth was increased more than threefold (OR: 3.4; 95% CI: 1.3–5.5) [68]. Following the diagnosis of CD, there was no continued increased risk of low birth weight or intrauterine growth retardation in women with CD, a change that was attributed to treatment with the GFD in these women.
A Swedish cohort study had comparable findings, noting that undiagnosed CD was associated with increased risk of intrauterine growth retardation (OR: 1.62; 95% CI: 1.22–2.15) and low birth weight (OR: 2.13; 95% CI: 1.66–2.75) [69]. CD diagnosed prior to pregnancy did not confer these increased risks, which was again attributed to adoption of a GFD with subsequent reduction in CD activity.
Further evidence supporting the efficacy of a GFD on pregnancy outcomes comes from a study by Ciacci et al. that compared pregnancy outcomes in 31 treated versus 94 untreated women with CD [70]. Untreated women had higher rates of miscarriage (RR: 8.90; 95% CI: 1.19–66.3) and low birth-weight babies (RR: 5.84; 90% CI: 1.07–31.9), which was unrelated to the severity of their CD. In a secondary analysis of 12 pregnant women with CD, treatment reduced the risk of miscarriage from 43.3 to 7.7% (p < 0.01; RR: 9.18; 95% CI: 1.05–79.9) and reduced the number of low birth-weight babies from 29.4% to zero (p < 0.05). Many of the women studied had no classic symptoms of CD, which has important implications for CD testing. When taking into consideration the findings of Norgard et al. and Ludvigsson et al., screening women with intra-uterine growth retardation of unknown etiology for CD may also provide better outcomes [68,69].
While the preponderance of data supports the association between untreated CD and adverse pregnancy outcomes, studies are not uniformly positive; for example, Greco et al. found a high rate of undiagnosed CD in pregnant women, but no excess risk of abortion, premature delivery, small birth weight or intrauterine growth retardation [71]. Collin et al. demonstrated a higher prevalence of CD in unexplained infertility; however, they also found that of the 150 individuals in the study who had experienced miscarriage, none of these women had CD [53]. The discordant findings reported in these studies may be due to methodological or population differences.
Should women with adverse outcomes in pregnancy be screened for CD?
Given the clear evidence for increased prevalence of recurrent spontaneous abortion, intrauterine growth retardation and low birth weight in CD, as well as the benefit of gluten withdrawal in reducing the incidence of these complications, we recommend consideration of CD testing in women who have previously experienced these adverse outcomes.
It is important to note that none of the studies discussed have examined whether adopting a GFD during pregnancy might influence pregnancy outcome. Indeed, given the proposed multifactorial etiologies of infertility and adverse pregnancy outcomes in CD, some of which may take place very early in gestation, it is likely that the benefit of CD diagnosis would only be seen when the GFD is started at least a few months prior to conception rather than during pregnancy. While it is unlikely to be cost effective to screen all otherwise asymptomatic women of childbearing age for CD, this population is an especially important one to assess for risk factors for CD. Thus, in women of childbearing age, we recommend a targeted approach, in which practitioners carefully assess for unexplained gastrointestinal symptoms, anemia, vitamin deficiencies, a personal history of related autoimmune disorders such as thyroid disease or Type I diabetes, a family history of CD or pregnancy/fertility issues including unexplained intrauterine growth retardation, low birth-weight babies, spontaneous abortion and preterm birth. Testing for CD in all healthy women of childbearing age with no established risk factors is not justified at this time. Future studies will also need to clarify the association between CD and preterm birth to determine whether previous preterm birth is a risk factor that should prompt testing for CD.
Low BMD & fractures
Osteoporosis and related skeletal fractures represent a huge burden of illness in older women. It is estimated that 15% of women will sustain a hip fracture by the age of 80 years, and in the year 2000, 9 million osteoporotic fractures were reported worldwide [72]. Although CD has been clearly shown to adversely effect BMD and most guidelines recommend BMD testing in individuals with CD, it is less certain whether testing for CD in all individuals with osteoporosis or early-onset osteopenia is efficacious [73–79]. In addition, studies have included a wide variety of demographics and site of BMD measurement has differed in these studies, and caution must be used when comparing results and making generalizations.
Is the risk of low BMD & related fractures higher in patients with CD?
Mustalahti et al. found an increased risk of low BMD in patients with CD [74]. This study measured BMD in 19 patients with screen-detected CD ranging from 23 to 69 years of age, 13 of whom were men. In addition, compared with symptomatic patients with CD, BMD was higher in the spine and the femoral neck, possibly due to longer duration of disease in asymptomatic patients. In a prospective study of 63 patients with CD (35 women and 28 men; age: 17–79 years), BMD was significantly reduced in the low forearm, the trochanter and the spine when compared with matched controls (p < 0.001) [75]. In a further study of 81 women with CD (aged: 20–70 years, 52 of whom were premenopausal and 29 of whom were postmenopausal), BMD was significantly lower in the lumbar spine and femoral neck (p < 0.001) in comparison with matched controls [76]. Femoral neck BMD was significantly lower in both premenopausal and postmenopausal women with CD when compared with controls, whereas only postmenopausal women with CD had significantly lower lumbar spine BMD.
With regard to fracture risk, a study of 83 CD patients (70% female) showed an increased risk of fracture both preceding (OR: 2; 95% CI: 1–3.9; p = 0.045) and following (OR: 2.5; 95% CI: 1.1–5.6; p = 0.026) diagnosis when compared with 166 matched controls [77]. Similarly, a much larger Swedish population-based study showed that of 13,000 patients with CD (8311 females, with a range of ages included) and 65,000 controls, the hazard ratio for hip fractures and all fractures was 2.1 (95% CI: 1.8–2.4; p < 0.001) and 1.4 (95% CI: 1.3–1.5; p < 0.001), respectively, when compared with controls [78]. Another large population-based cohort study of 4732 CD patients found that there was a significant increase in the relative risk of any fracture (30%) when compared with the general population [79]. Overall, the evidence for increased risk of osteoporosis and fracture risk in patients with CD is relatively good, as confirmed by a recent meta-analysis that evaluated 20,955 CD patients and 96,777 controls, supporting an increased risk of fracture in CD patients compared with the general population (OR: 1.43; 95% CI: 1.15–1.78) [80].
While the preponderance of data supports an association between CD and fracture, a few studies did not find this correlation. For instance, Thomason et al. found that in 244 patients with CD (171 women, with patients of heterogeneous ages) and 161 controls, the rate of one or more fractures was similar at 35 and 33%, respectively [81]. Furthermore, of the 37 patients with CD reporting fracture, there was no significant difference in rate of fracture before and after diagnosis of CD. The major shortcoming of this study was a design based on patient recall and low response rate by the general population.
In addition, while most evidence supports the finding of decreased BMD in patients with CD, several studies suggest that the incidence of CD in unselected individuals with low BMD is similar to that of the general population. A study of 96 patients (78 female; aged: 18–86 years) with idiopathic low BMD revealed seven patients with low positive EMA titers, but enteropathy consistent with CD was not seen in any of these cases [82]. A study of 135 patients with idiopathic low BMD (121 female; aged: 24–81 years) revealed 13 cases of positive EMA; however, none had biopsy results consistent with CD [83]. Similarly, a study of 127 postmenopausal women with osteoporosis showed the prevalence of CD was not significantly different when compared with 747 women recruited for a population-based study [84]. Kavuncu et al. also concluded that in 192 postmenopausal women with reduced BMD, only 0.5% had positive serology suggestive of CD, similar to the prevalence rate in the general population [85]. These studies all conclude that there is little utility in screening asymptomatic patients with low BMD for CD since the yield and cost–benefit ratio is likely to be low.
Does GFD improve BMD & reduce its complications?
For the most part, the finding of improved BMD on a GFD is supported by the literature. A study by Ciacci et al. found that in 30 women and 11 men with newly diagnosed CD, there was an increase in lumbar spine and femoral neck BMD after 1 year on a calcium-rich GFD (p < 0.001) [86]. The authors cited certain factors as significantly related to improvement in BMD with regard to treatment. Namely, gender (women had improved responses compared with men) and age (≤25 years of age at the start of treatment) were associated with greater improvements in BMD. Conversley, improvement in BMD was independent of gender and age was found in a study of 86 newly diagnosed patients with CD (22 males and 64 females), 40% of which had osteoporosis and 34% of which had osteopenia [87]. Of note, in this study there was mucosal recovery in only 57% of patients after 1 year of GFD, but there was a significant improvement in BMD in this population, including postmenopausal women.
Mustalahti et al. showed that following 1 year on a GFD, there was an increase in BMD, with eight out of 19 and ten out of 19 patients having significant improvements in BMD in the spine and femoral neck, respectively [74]. Valdimarsson et al. found similar results, showing that after 1 year on a GFD there were significant improvements in BMD in all sites examined (i.e., the low forearm, trochanter and spine) [75].
Meyer et al. showed that of 105 women and 23 men with CD, osteoporosis was present in the lumbar spine of 34% of patients and osteopenia was present in 38% [88]. Osteoporosis and osteopenia were present in 27 and 44% at the femoral neck, respectively. This study also found no initial difference in BMD in patients on a GFD versus no treatment, but there was an increase of 7.5% at the femoral neck after 16 ± 2 months on a GFD (p < 0.02).
In addition to improvement in BMD, fracture risk also appears to be reduced with the institution of a GFD. A study of 165 patients (143 women; aged: 16–74 years) with CD found that 25% had at least one fracture in the peripheral skeleton compared with 8% of matched controls (95% CI: 1.8–7.2; p < 0.0001) [89]. A total of 80% of these fractures occurred in patients prior to diagnosis of CD or in patients not adherent to a GFD. In CD patients adherent to a GFD, the rate of fractures was reduced to 7%, which is similar to that of the general population.
Is there any advantage in screening women with osteoporosis for CD?
Screening for CD is variably supported in patients with osteopenia and osteoporosis. Nuti et al. showed that in 255 postmenopausal women with idiopathic osteoporosis, 2.4% had biopsy-proven CD [90]. Lindh et al. screened 92 patients with osteoporosis and found a prevalence rate of 3.3% [91]. These rates are higher than the 1% prevalence estimated in the general population. A more recent study supported this finding in a largely postmenopausal female population where prevalence of CD was 3.4% in osteoporotics and 0.2% in nonosteoporotics. The majority of osteoporotic patients diagnosed with CD also had symptoms of weight loss, gastrointestinal disturbance or anemia. Similar to the findings of the studies mentioned earlier, BMD improved within 1 year of starting a GFD in this cohort.
As suggested by Sanders et al., a case-finding approach may be the most practical approach in terms of optimizing cost–effectiveness and screening of CD in patients with low BMD [92]. In a prospective study, 978 participants selected (936 female) under the age of 70 years were evaluated in order to reduce the likelihood of age-related osteoporosis negatively skewing the prevalence of CD. Patients undergoing dual energy x-ray absorptiometry scan as referred from primary or secondary care had various serological assays drawn, including AGA and EMA. The prevalence of histologically proven CD was 1.2%. The prevalence of CD in those with a normal dual energy x-ray absorptiometry scan was 0.7%. In those patients with reduced BMD, prevalence of CD was 1.4% (OR: 2.3; 95% CI: 0.5–10.4; p = 0.347). Importantly, all patients with undiagnosed CD had anemia and/or gastrointestinal symptoms. At this time, data suggests that screening of all patients with osteoporosis is likely to have a low yield in revealing patients with CD. Rather, we suggest that individuals who are found to have low BMD should be assessed for a history of signs or symptoms typical of CD and tested if these are positive. This strategy is also supported by the findings of Stenson et al., where almost all osteoporotic patients diagnosed with CD had anemia, weight loss or gastrointestinal symptoms [93].
Reduced BMD in postmenopausal, or peri-menopausal women specifically, is also much less likely to be due to a secondary cause such as CD, as opposed to an age-related effect. Therefore, while it is probably not practical to screen every asymptomatic osteoporotic postmenopausal woman for CD, it may be effective to consider CD testing in otherwise asymptomatic premenopausal women with low BMD. In summary, although the literature is not decisive in the matter of screening for CD in osteoporosis, the best strategy according to our current knowledge is targeted screening in premenopausal women with low BMD (the current recommendation), as well as in postmenopausal women with low BMD and symptoms suggestive of CD.
Conclusion
The epidemiology of CD has shifted from a solely gastrointestinal disease to include a wide range of nonclassical symptoms, some of which are specifically relevant to women’s health such as infertility, adverse pregnancy outcomes and osteoporosis. Given the high rates of missed diagnoses, it is critical for practitioners to recognize at-risk groups and to appropriately test these patients in the hopes of instituting treatment and preventing significant complications. Obviously, a major limitation of the argument for testing these additional populations is the lack of data regarding cost–effectiveness of this strategy. This factor aside, based on current literature, we recommend that all women with unexplained infertility and history of unexplained intrauterine growth retardation, low birth-weight babies and spontaneous abortion be tested for CD. Furthermore, all pre-menopausal women with low BMD should be screened for CD consistent with current recommendations [73]. Postmenopausal women with reduced BMD and symptoms suggestive of CD should also be tested. Practitioners in general should maintain a low threshold to test for CD, given the myriad of presentations and the low overall rate of diagnosis. See Table 1 for a list of conditions that should elicit consideration for CD testing.
Table 1.
Proposed indications for celiac disease testing in women.
| Population | Indications for celiac disease testing† |
|---|---|
| Women of childbearing age | Unexplained gastrointestinal symptoms or weight loss |
| Anemia | |
| Vitamin deficiencies | |
| Family history of celiac disease | |
| Previous spontaneous abortion | |
| History of unexplained intrauterine growth retardation | |
| Previous low birth-weight babies | |
| History of preterm birth | |
| Women with infertility | Unexplained gastrointestinal symptoms or weight loss |
| Anemia | |
| Vitamin deficiencies | |
| Autoimmune disorders | |
| Family history of celiac disease | |
| No alternative hormonal or anatomical cause for infertility | |
| Women with low BMD | All premenopausal women |
| Significant vitamin D deficiency | |
| Poor response to standard treatment | |
| Family history of celiac disease | |
| Unusually severe presentation of low BMD | |
| Postmenopausal women with anemia, weight loss or gastrointestinal symptoms | |
The standard initial test for celiac disease in most populations is IgA-tissue transglutaminase.
Future perspective
As CD awareness increases in the primary care setting, the currently neglected clinical associations of CD will become more prominent in the general medical world. This will probably prompt better attention to targeted testing, which is greatly needed given the low rate of diagnosis.
To further assess the utility of standard CD testing in the populations, we propose that specific areas of future research are required. First and foremost, studies addressing cost–analyses in each of these populations must be performed, since cost–effectiveness is a key component in recommending any screening program. In addition, large, well-designed studies assessing potential improvement in fertility with a GFD are lacking. Furthermore, while literature in general suggests that treatment of CD improves adverse fetal outcomes, longer term studies supporting this conclusion are needed. It would be useful to see that CD patients are able to complete a normal reproductive life on a GFD, rather than assessing only the first year postdiagnosis, as is commonly reported.
In addition, no clear association has been made between CD and preterm birth consistently, which is an area that deserves further attention with larger population studies. It is unlikely to be effective to screen all women prenatally for CD at this time, since it is unclear if institution of a GFD during pregnancy would be sufficient to influence outcomes. As more studies are performed to assess the impact of a GFD in pregnancy, the utility of instituting a GFD perinatally will need to be explored before suggesting that screening become part of routine antenatal or prenatal testing. Future studies might also address the utility of screening nulliparous women with risk factors such as anemia, autoimmune disease and family history in order to prevent adverse pregnancy outcomes.
Finally, data support an association between CD osteoporosis, osteopenia and risk of fractures. However, it is unclear under what circumstances individuals with low BMD or a history of fractures should be tested for CD. Well-designed prospective studies evaluating the cost–effectiveness of CD testing in this population will be needed before recommendations can be made for general clinical practice.
In conclusion, CD is a rapidly evolving field. With improved diagnostic tests and a better understanding of the true spectrum of disease, both awareness of CD and the diagnosed population are quickly growing. In the near future, aspects of CD will become relevant for nearly all clinicians, and novel therapeutics may offer benefits beyond the GFD alone [94]. Given the female predominance of CD and the multiple potential impacts on gender-specific issues, this is especially true for practitioners in the field of women’s health.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
Daniel Leffler is a consultant to Alba Therapeutics, Alvine Pharmaceuticals, Shire Pharmaceuticals and Prometheus Laboratories. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–188. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 2.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multi-center study. Arch Intern Med. 2003;163(3):286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ, Valdovinos M, Petterson TM, et al. Epidemiology of celiac sprue: a community-based study. Am J Gastroenterol. 1994;89(6):843–846. [PubMed] [Google Scholar]
- 4.Collin P, Reunala T, Rasmussen M, et al. High incidence and prevalence of adult celiac disease Augmented diagnostic approach. Scand J Gastroenterol. 1997;32(11):1129–1133. doi: 10.3109/00365529709002992. [DOI] [PubMed] [Google Scholar]
- 5.Catassi C, Fabriani E. The spectrum of celiac disease in children. Baillieres Clin Gastroenterol. 1997;11(3):485–507. doi: 10.1016/s0950-3528(97)90028-2. [DOI] [PubMed] [Google Scholar]
- 6.Rampertab SD, Pooran N, Brar P, et al. Trends in the presentation of celiac disease. Am J Med. 2006;119(4):355, e9–e14. doi: 10.1016/j.amjmed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 7.Pinkhasov RM, Wong J, Kashanian J, et al. Are men shortchanged on health? Perspective on health care utilization and health risk behavior in men and women in the United States. Int J Clin Pract. 2010;64(4):475–487. doi: 10.1111/j.1742-1241.2009.02290.x. [DOI] [PubMed] [Google Scholar]
- 8.Schappert SM, Burt CW. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001–2002. Vital Health Stat. 2006;13:159, 1–66. [PubMed] [Google Scholar]
- 9.Megiorni F, Mora B, Bonamico M, et al. HLA-DQ and susceptibility to celiac disease: evidence for gender differences and parent-of-origin effects. Am J Gastroenterol. 2008;103:997–1003. doi: 10.1111/j.1572-0241.2007.01716.x. [DOI] [PubMed] [Google Scholar]
- 10.Green PHR, Stavropoulos SN, Panagi SG, et al. Characteristics of adult celiac disese in the USA: results of a national survey. Am J Gastroenterol. 2001;96(1):126–131. doi: 10.1111/j.1572-0241.2001.03462.x. [DOI] [PubMed] [Google Scholar]
- 11.Leffler D, Kelly CP. In: Celiac Disease: What The Last Few Years Have Taught us. Advances in Digestive Disease. Howden CW, editor. AGA Institute Press; 2007. [Google Scholar]
- 12••.Catassi C, Kryszak D, Louis-Jacques O, et al. Detection of celiac disease in primary care: a multicenter case-finding study in North America. Am J Gastroenterol. 2007;102(7):1454–1460. doi: 10.1111/j.1572-0241.2007.01173.x. Multicenter, prospective study demonstrating the utility of an active case-finding strategy in the primary care setting. [DOI] [PubMed] [Google Scholar]
- 13•.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357(17):1731–1743. doi: 10.1056/NEJMra071600. Excellent general review of celiac disease (CD) [DOI] [PubMed] [Google Scholar]
- 14.Green PH, Jabri B. Coeliac disease. Lancet. 2003;362(9381):383–391. doi: 10.1016/S0140-6736(03)14027-5. [DOI] [PubMed] [Google Scholar]
- 15.Schuppan D, Dennis MD, Kelly CP. Celiac disease: epidemiology, pathogenesis, diagnosis, and nutritional management. Nutr Clin Care. 2005;8(2):54–69. [PubMed] [Google Scholar]
- 16.Shan L, Molberg Ø, Parrot I, et al. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–2279. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- 17.Bedwal RSBA. Zinc, copper and selenium in reproduction. Experientia. 1994;50:626–640. doi: 10.1007/BF01952862. [DOI] [PubMed] [Google Scholar]
- 18.Bougle D, Proust A. Iron and zinc supplementation during pregnancy: interactions and requirements. Contracept Fertil Steril. 1999;27:537–543. [PubMed] [Google Scholar]
- 19.Singhal N, Alam S, Sherwani R, et al. Serum zinc levels in celiac disease. Indian Pediatr. 2008;45:319–321. [PubMed] [Google Scholar]
- 20.Hirson C. Coeliac infertility- folic acid therapy. Lancet. 1970;1(7643):412. doi: 10.1016/s0140-6736(70)91537-0. [DOI] [PubMed] [Google Scholar]
- 21.Anjum N, Baker PN, Robinson NJ, et al. Maternal celiac disease autoantibodies bind directly to syncytiotrophoblast and inhibit placental tissue transglutaminase activity. Reprod Biol Endocrinol. 2009;7:16. doi: 10.1186/1477-7827-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Simone N, Silano M, Castellani R, et al. Anti-tissue transglutaminase antibodies from celiac patients are responsible for trophoblast damage via apoptosisin vitro. Am J Gastroenterol. 2010 doi: 10.1038/ajg.2010.233. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 23.Molteni N, Bardella MT, Vezzoli G, et al. Intestinal calcium absorption as shown by stable strontium test in celiac disease before and after gluten-free diet. Am J Gastroenterol. 1995;90:2025–2028. [PubMed] [Google Scholar]
- 24.Ciacci C, Cirillo M, Mellone M, et al. Hypocalciuria in overt and subclinical celiac disease. Am J Gastroenterol. 1995;90:1408–1484. [PubMed] [Google Scholar]
- 25.Keaveny AP, Freaney R, McKenna MJ, et al. Bone remodeling indices and secondary hyperparathyroidism in celiac disease. Am J Gastroenterol. 1996;91:1226–1231. [PubMed] [Google Scholar]
- 26.Selby PL, Davies M, Adams JE, et al. Bone loss in celiac disease is related to secondary hyperparathyroidism. J Bone Miner Res. 1999;14:652–657. doi: 10.1359/jbmr.1999.14.4.652. [DOI] [PubMed] [Google Scholar]
- 27.Moreno ML, Crusius JBA, Chernavsky A, et al. The IL-1 gene family and bone involvement in celiac disease. Immunogenetics. 2005;57:618–620. doi: 10.1007/s00251-005-0033-x. [DOI] [PubMed] [Google Scholar]
- 28.Jameson S. Coeliac disease, insulin-like growth factor, bone mineral density, and zinc. Scand J Gastroenterol. 2000;35:894–896. [PubMed] [Google Scholar]
- 29.Valdimarsson T, Arnqvist HJ, Toss G, et al. Low circulating insulin-like growth factor I in coeliac disease and its relation to bone mineral density. Scand J Gastroenterol. 1999;34:904–908. doi: 10.1080/003655299750025381. [DOI] [PubMed] [Google Scholar]
- 30.Devine A, Rosen C, Mohan S, et al. Effects of zinc and other nutritional factors on insulin-like growth factor I and insulin-like growth factor-binding proteins in post-menopausal women. Am J Clin Nutr. 1998;68:200–206. doi: 10.1093/ajcn/68.1.200. [DOI] [PubMed] [Google Scholar]
- 31.Taranta A, Fortunati D, Longo M, et al. Imbalance of osteoclastogenesis-regulating factors in patients with celiac disease. J Bone Miner Res. 2004;19:1112–1121. doi: 10.1359/JBMR.040319. [DOI] [PubMed] [Google Scholar]
- 32.Moreno ML, Crusius JBA, Cherñavsky A, et al. The IL-1 gene family and bone involvement in celiac disease. Immunogenetics. 2005;57:618–620. doi: 10.1007/s00251-005-0033-x. [DOI] [PubMed] [Google Scholar]
- 33.Riches PL, McRorie E, Fraser WD, et al. Osteoporosis associated with neutralizing autoantibodies against osteoprotegerin. N Engl J Med. 2009;361(15):1459–1465. doi: 10.1056/NEJMoa0810925. [DOI] [PubMed] [Google Scholar]
- 34.Marsh MN. Gluten, major histocompatibility complex, and the small intestine A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 35.Zintzaras E, Germenis AE. Performance of antibodies against tissue transglutaminase for the diagnosis of celiac disease: meta-analysis. Clin Vaccine Immunol. 2006;13(2):187–192. doi: 10.1128/CVI.13.2.187-192.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leffler DA, Schuppan D. Update on serologic testing in celiac disease. Am J Gastroenterol. 2010 doi: 10.1038/ajg.2010.276. In press. [DOI] [PubMed] [Google Scholar]
- 37.Rostom A, Dubé C, Cranney A, et al. The diagnostic accuracy of serologic tests for celiac disease: a systematic review. Gastroenterology. 2005;128:S38–S46. doi: 10.1053/j.gastro.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 38.Sugai E, Vazquez H, Nachman F, et al. Accuracy of testing for antibodies to synthetic gliadin-related peptides in celiac disease. Clin Gastroenterol Hepatol. 2006;4(9):1112–1117. doi: 10.1016/j.cgh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Prince HE. Evaluation of the INOVA diagnostics enzyme-linked immunosorbent assay kits for measuring serum immunoglobulin G(IgG) and IgA to deamidated gliadin peptides. Clin Vaccine Immunol. 2006;13(1):150–151. doi: 10.1128/CVI.13.1.150-151.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cataldo F, Marino V, Bottaro G, et al. Celiac disease and selective immunoglobulin A deficiency. J Pediatr. 1997;131:306–308. doi: 10.1016/s0022-3476(97)70172-0. [DOI] [PubMed] [Google Scholar]
- 41.Heneghan MA, Stevens FM, Cryan EM, et al. Celiac sprue and immunodeficiency states: a 25-year review. J Clin Gastroenterol. 1997;25:421–425. doi: 10.1097/00004836-199709000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Cataldo F, Marino V, Ventura A, et al. Italian society of paediatric gastroenterology and hepatology(SIGEP) and ‘club del tenue’ working groups on coeliac disease Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Gut. 1998;42:362–365. doi: 10.1136/gut.42.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Institutes of Health Consensus Development Conference statement on celiac disease, June 28–30, 2004. Gastroenterology. 2005;128(Suppl 1):S1–S9. doi: 10.1053/j.gastro.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Cranney A, Rostom A, Sy R, et al. Consequences of testing for celiac disease. Gastroenterology. 2005;128(4 Suppl 1):S109–S120. doi: 10.1053/j.gastro.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 45.Corrao G, Corazza GF, Bagnardi V, et al. Mortality in patients with CD and their relatives: a cohort study. Lancet. 2001;358(9279):356–361. doi: 10.1016/s0140-6736(01)05554-4. [DOI] [PubMed] [Google Scholar]
- 46.Hogberg L, Grodzinsky E, Stenhammar L. Better dietary compliance in patients with celiac disease diagnosed in early childhood. Scand J Gastroenterol. 2003;38:751–754. doi: 10.1080/00365520310003318. [DOI] [PubMed] [Google Scholar]
- 47.Ciacci C, Cirillo M, Cavallaro R, et al. Long-term follow-up of celiac adults on gluten-free diet: prevalence and correlates of intestinal damage. Digestion. 2002;66:178–185. doi: 10.1159/000066757. [DOI] [PubMed] [Google Scholar]
- 48.Fabriani E, Catassi C, Villari A, et al. Dietary compliance in screening-detected celiac disease adolescents. Acta Paediatr Suppl. 1996;412:65–67. doi: 10.1111/j.1651-2227.1996.tb14256.x. [DOI] [PubMed] [Google Scholar]
- 49.Fabriani E, Taccari LM, Ratsch IM, et al. Compliance with GFD in adolescents with screening-detected celiac disease: a 5-year follow up study. J Pediatr. 2000;136(6):841–843. [PubMed] [Google Scholar]
- 50.Whitaker JKH, West J, Holmes GKT, Logan RFA. Patient perceptions of the burden of coeliac disease and its treatment in the UK. Aliment Pharmacol Ther. 2009;29:1131–1136. doi: 10.1111/j.1365-2036.2009.03983.x. [DOI] [PubMed] [Google Scholar]
- 51•.Shamir R, Hernell O, Leshno M. Cost–effectiveness analysis of screening for celiac disease in the adult population. Med Decis Making. 2006;26:282–293. doi: 10.1177/0272989X06289012. Addresses the cost–effectiveness of mass screening in CD. [DOI] [PubMed] [Google Scholar]
- 52.Hershcovici T, Leshno M, Goldin E, et al. Cost-effectiveness of mass screening for celiac disease is determined by time-delay to diagnosis and quality of life on a gluten free diet. Aliment Pharmacol Ther. 2010;1(8):901–910. doi: 10.1111/j.1365-2036.2010.04242.x. [DOI] [PubMed] [Google Scholar]
- 53.Collin P, Vilska S, Heinonen PK, et al. Infertility and celiac disease. Gut. 1996;39:382–384. doi: 10.1136/gut.39.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meloni GF, Dessole S, Vargiu N, et al. The prevalence of celiac disease in infertility. Hum Reprod. 1999;14(11):2759–2761. doi: 10.1093/humrep/14.11.2759. [DOI] [PubMed] [Google Scholar]
- 55.McCarthy FP, Khashan AS, Quigley E, et al. Undiagnosed maternal celiac disease in pregnancy and an increased risk of fetal growth restriction. J Clin Gastroenterol. 2009;43:792–793. doi: 10.1097/MCG.0b013e3181a51a1b. [DOI] [PubMed] [Google Scholar]
- 56.Stephen EH, Chandra A. Declining estimates of infertility in the United States: 1982–2002. Fertil Steril. 2006;86(3):516–523. doi: 10.1016/j.fertnstert.2006.02.129. [DOI] [PubMed] [Google Scholar]
- 57.Mosher WD, Pratt WF. Fecundity and infertility in the United States 1965–1988. National Center for Health Statistics; Hyattsville, MD, USA: 1990. [Google Scholar]
- 58.Shamaly H, Mahameed A, Sharony A, et al. Infertility and celiac disease: do we need more than one serological marker? Acta Obstet Gynecol Scand. 2004;83:1184–1188. doi: 10.1111/j.0001-6349.2004.00592.x. [DOI] [PubMed] [Google Scholar]
- 59.Tiboni GM, Grazia de Vita M, Faricelli R, et al. Serological testing for celiac disease in women undergoing assisted reproduction techniques. Hum Reprod. 2006;21(2):376–379. doi: 10.1093/humrep/dei314. [DOI] [PubMed] [Google Scholar]
- 60.Sher KS, Mayberry JF. Female fertility, obstetric and gynecological history in celiac disease: a case control study. Acta Paediatr Suppl. 1996;412:76–77. doi: 10.1111/j.1651-2227.1996.tb14258.x. [DOI] [PubMed] [Google Scholar]
- 61.Ferguson R, Holmes GK, Cooke WT. Coeliac disease: fertility and pregnancy. Scand J Gastroenterol. 1982;17(1):65–68. doi: 10.3109/00365528209181045. [DOI] [PubMed] [Google Scholar]
- 62.Bona G, Marinello D, Oderda G. Mechanisms of abnormal puberty in coeliac disease. Horm Res. 2002;57(2):63–65. doi: 10.1159/000058103. [DOI] [PubMed] [Google Scholar]
- 63.Rujner J. Age at menarche in girls with celiac disease. Ginekol Pol. 1999;70(5):359–362. [PubMed] [Google Scholar]
- 64.Gasbarrini A, Torre ES, Trivellini C, et al. Recurrent spontaneous abortion and intrauterine fetal growth retardation as symptoms of celiac disease. Lancet. 2000;356(9227):399–400. doi: 10.1016/S0140-6736(00)02535-6. [DOI] [PubMed] [Google Scholar]
- 65.Martinelli P, Troncone R, Paparo F, et al. Coeliac disease and unfavourable outcome of pregnancy. Gut. 2000;46:332–335. doi: 10.1136/gut.46.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salvatore S, Finazzi S, Radaelli G, et al. Prevalence of undiagnosed celiac disease in the parents of preterm and/or small for gestational age infants. Am J Gastroenterol. 2007;102:168–173. doi: 10.1111/j.1572-0241.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 67••.Khashan AS, Henriksen TB, Mortensen PB, et al. The impact of maternal celiac disease on birthweight and preterm birth: a Danish population-based cohort study. Hum Reprod. 2010;25(2):528–534. doi: 10.1093/humrep/dep409. Large population-based cohort study, that demonstrates the association between CD and reduced birthweight. [DOI] [PubMed] [Google Scholar]
- 68.Norgard B, Fonager K, Sorensen HT, et al. Birth outcomes of women with celiac disease: a nationwide historical cohort study. Am J Gastroenterol. 1999;94(9):2435–2440. doi: 10.1111/j.1572-0241.1999.01370.x. [DOI] [PubMed] [Google Scholar]
- 69•.Ludvigsson JF, Montgomery SM, Ekbom A. Celiac disease and risk of adverse fetal outcome: a population-based cohort study. Gastroenterology. 2005;129:454–463. doi: 10.1016/j.gastro.2005.05.065. Population-based cohort study demonstrating the association between CD and intrauterine growth retardation and reduced birthweight. [DOI] [PubMed] [Google Scholar]
- 70.Ciacci C, Cirillo M, Auriemma G, et al. Celiac disease and pregnancy outcome. Am J Gastroenterol. 1996;91(4):718–722. [PubMed] [Google Scholar]
- 71.Greco L, Veneziano A, Di Donato L, et al. Undiagnosed celiac disease does not appear to be associated with unfavorable outcome of pregnancy. Gut. 2004;53(1):149–151. doi: 10.1136/gut.53.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 73.Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology. 2003;124(3):795–841. doi: 10.1053/gast.2003.50106. [DOI] [PubMed] [Google Scholar]
- 74.Mustalahti K, Collin P, Sievanen H. Osteopenia in patients with clinically silent celiac disease warrants screening. Lancet. 1999;354(9180):744–745. doi: 10.1016/S0140-6736(99)01990-X. [DOI] [PubMed] [Google Scholar]
- 75.Valdimarsson T, Lofman O, Toss G, et al. Reversal of osteopenia with diet in adult celiac disease. Gut. 1996;38(3):322–327. doi: 10.1136/gut.38.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pistorius LR, Sweidan WH, Purdie DW, et al. Celiac Disease and bone mineral density in adult female patients. Gut. 1995;37(5):639–642. doi: 10.1136/gut.37.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jafri MR, Nordstrom CW, Murray JA, et al. Long-term fracture risk in patients with celiac disease: a population-based study in Olmsted County, Minnesota. Dig Dis Sci. 2008;53(4):964–971. doi: 10.1007/s10620-007-9976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ludvigsson JF, Michaelsson K, Ekbom A, et al. Celiac disease and the risk of fractures- a general population-based cohort study. Aliment Pharmacol Ther. 2007;25(3):273–285. doi: 10.1111/j.1365-2036.2006.03203.x. [DOI] [PubMed] [Google Scholar]
- 79.West J, Logan RF, Card TR, et al. Fracture risk in people with celiac disease: a population-based cohort study. Gastroenterology. 2003;125(2):429–436. doi: 10.1016/s0016-5085(03)00891-6. [DOI] [PubMed] [Google Scholar]
- 80••.Olmos M, Antelo M, Vazquez H, et al. Systematic review and meta-analysis of observational studies on the prevalence of fractures in celiac disease. Dig Liver Dis. 2008;40(1):46–53. doi: 10.1016/j.dld.2007.09.006. Meta-analysis demonstrating the association between CD and fracture. [DOI] [PubMed] [Google Scholar]
- 81.Thomason K, West J, Logan RF, et al. Fracture experience of patients with celiac disease: a population based study. Gut. 2003;52(4):518–522. doi: 10.1136/gut.52.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mather KJ, Meddings JB, Beck PL, et al. Prevalence of IgA-antiendomysial antibody in asymptomatic low bone mineral density. Am J Gastroenterol. 2001;96(1):120–125. doi: 10.1111/j.1572-0241.2001.03461.x. [DOI] [PubMed] [Google Scholar]
- 83.Karakan T, Ozyemisci-Taskiran O, Gunendi Z, et al. Prevalence of IgA-antiendomysial antibody in a patient cohort with idiopathic low bone mineral density. World J Gastroenterol. 2007;13(21):2978–2982. doi: 10.3748/wjg.v13.i21.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzalez D, Sugai E, Gomez JC, et al. Is it necessary to screen for celiac disease in postmenopausal osteoporotic women? Calcif Tissue Int. 2002;71(2):141–144. doi: 10.1007/s00223-001-1027-9. [DOI] [PubMed] [Google Scholar]
- 85.Kavuncu V, Dundar U, Ciftci IH, et al. Is there any requirement for celiac disease screening routinely in postmenopausal women with osteoporosis? Rheumatol Int. 2009;29(7):841–845. doi: 10.1007/s00296-008-0797-z. [DOI] [PubMed] [Google Scholar]
- 86.Ciacci C, Maurelli L, Klain M, et al. Effects of dietary treatment on bone mineral density in adults with celiac disease: factors predicting response. Am J Gastroenterol. 1997;92(6):992–996. [PubMed] [Google Scholar]
- 87.Sategna-Guidetti C, Grosso SB, Gross S, et al. The effects of 1-year gluten withdrawal on bone mass, bone metabolism and nutritional status in newly-diagnosed adult celiac disease patients. Aliment Pharmacol Ther. 2000;14(1):35–43. doi: 10.1046/j.1365-2036.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- 88.Meyer D, Stravropolous S, Diamond B, et al. Osteoporosis in a North American population with celiac disease. Am J Gastroenterol. 2001;96(1):112–119. doi: 10.1111/j.1572-0241.2001.03507.x. [DOI] [PubMed] [Google Scholar]
- 89.Vasquez H, Mazure R, Gonzalez D, et al. Risk of fractures in celiac disease patients: a cross-sectional, case–control study. Am J Gastroenterol. 2000;95(1):183–189. doi: 10.1111/j.1572-0241.2000.01682.x. [DOI] [PubMed] [Google Scholar]
- 90.Nuti R, Martini G, Valenti R, et al. Prevalence of undiagnosed celiac syndrome in osteoporotic women. J Intern Med. 2001;250(4):361–366. doi: 10.1046/j.1365-2796.2001.00895.x. [DOI] [PubMed] [Google Scholar]
- 91.Lindh E, Ljunghall S, Larsson K, et al. Screening for antibodies against gliadin in patients with osteoporosis. J Intern Med. 1992;231(4):403–406. doi: 10.1111/j.1365-2796.1992.tb00951.x. [DOI] [PubMed] [Google Scholar]
- 92••.Sanders DS, Patel D, Khan FB, et al. Case-finding for adult celiac disease in patients with reduced bone mineral density. Dig Dis Sci. 2005;50(3):587–592. doi: 10.1007/s10620-005-2479-y. Supports the strategy of targeted testing for CD in patients with reduced BMD. [DOI] [PubMed] [Google Scholar]
- 93•.Stenson WF, Newberry R, Lorenz R, et al. Increased prevalence of celiac disease and need for routine screening among patients with osteoporosis. Arch Intern Med. 2005;165(4):393–399. doi: 10.1001/archinte.165.4.393. Study demonstrating a higher prevalence of CD in a largely postmenopausal osteoporotic population, most of whom had other symptoms of CD, with improvement of BMD with gluten withdrawal. [DOI] [PubMed] [Google Scholar]
- 94.Schuppan D, Junker Y, Barisani D. Celiac disease, from pathogenesis to novel therapies. Gastroenterology. 2009;137(6):1912–1933. doi: 10.1053/j.gastro.2009.09.008. [DOI] [PubMed] [Google Scholar]