Abstract
Inflammatory bowel disease (IBD) is a waxing and waning disease characterized by diarrhea, abdominal pain and weight loss. Recently, there has been an increased interest in the roles that sleep, circadian rhythms and melatonin could have as regulators of inflammation in the Gl tract. Advances in our understanding of the molecular machinery of the circadian clock, and the discovery of clock genes in the GI tract are opening up new avenues of research for a role of sleep in IBD. Altering circadian rhythm significantly worsens the development of colitis in animal models, and preliminary human studies have shown that patients with IBD are at increased risk for altered sleep patterns. Further research is needed to clarify the role of disturbances in IBD.
Keywords: circadian rhythms, Crohn’s disease, inflammatory bowel disease, melatonin, sleep, ulcerative colitis
Ulcerative colitis (UC) and Crohn’s disease (CD) are the two phenotypic patterns of inflammatory bowel disease (IBD) [1]. CD has a transmural pattern of inflammation that can occur anywhere in the GI tract (GIT) and is associated with the development of complications such as fistulas or strictures [2]. UC has a superficial inflammation that occurs only in the colon. This inflammation begins in the rectum and is usually limited to the left side of the colon but with time may extend over the entire colon [3]. Currently, over 2 million people in the USA are diagnosed with IBD and the last several decades have seen an increasing incidence of the disease [4]. IBD has a waxing and waning course with periods of asymptomatic remission interrupted with episodes of disease ‘flare’ where patients can present with bloody stools, diarrhea, fever and abdominal pain.
Currently the etiology of IBD is not known and thus it is not surprising that there is no cure for IBD; the primary treatment goal is to improve patients’ quality of life by treating flare ups and maintaining remission. The most effective approach to maintaining remission is to prevent episodes of flare by modifying and treating potential triggers. Although there are no undisputed triggers for flare ups, several environmental factors and social habits have been studied as triggers because they can clearly impact the disease course of IBD [5]. For example, smoking both worsens CD and decreases the likelihood of UC flare ups [6]. Another potential environmental trigger are sleep disorders that could impact disease course in IBD. If so, then diagnosis and treatment of disrupted sleep should be considered as part of a management plan for patients with IBD. In this article, we will discuss the role of circadian rhythms in sleep, the impact of circadian rhythms on the immune system of the GIT, the prevalence of sleep disruption in patients with IBD and the current hypothesis of how disrupted sleep may impact disease activity in IBD.
Overview
Recently, there has been an increased understanding in the importance of sleep disturbance in regulating many physiologic processes in the body including the GIT and new studies have shown how fragmented sleep can adversely impact normal function [7]. Several studies have shown that disruption of the normal sleep cycle is associated with a rise in obesity and the metabolic syndrome [8], and in shift workers, disrupted sleep leads to an increased risk in a number of gastrointestinal diseases such as gastroesophageal reflux disease [9], peptic ulcer disease [10] and irritable bowel syndrome [11]. In addition, altered sleep can not only negatively affect gastrointestinal function, but has the potential to modify the immune system and thus impact disease course in gastrointestinal inflammatory disorders like IBD. Inflammatory processes can in turn affect sleep pattern and thus create a vicious cycle and positive-feedback loop to maintain and perpetuate inflammation.
Inflammatory cytokines such as TNF-α and IL-1 are known to disrupt sleep [12], cause daytime sleepiness and have caused sleep alterations in a number of chronic inflammatory conditions such as systemic lupus erythematosus [13], multiple sclerosis [14], rheumatoid arthritis [15] and HIV [16]. Complaints regarding sleep and daytime fatigue occur in 50–70% of patients with rheumatoid arthritis, sleep complaints correlate with disease activity, and treating these patients with a TNF antibody helps to improve their sleep [17]. In addition, sleep restriction in animal models is associated with an increase in inflammatory cytokines such as TNF-α, IL-6 and an elevated C-reactive protein, a commonly used clinical marker of activity in IBD [18]. With prolonged sleep loss, there are elevations in monocytes and natural killer cells, which form the source for the secretion of inflammatory cytokines. Thus, disturbed sleep and chronic inflammation in IBD could form a self-perpetuating feedback loop with the chronic inflammation of IBD worsening sleep, and decreased sleep exacerbating the production of inflammatory cytokines and the inflammatory milieu. Moreover, it is well known that disrupted sleep can cause fatigue in inflammatory diseases [19] and may be one of the causes of fatigue in IBD patients. It should be noted that fatigue is one of the most common complaints in patients with IBD with a major negative impact on quality of life and this complaint cannot simply be explained by active inflammation alone [19]. Thus, consideration of sleep disruption and attempting to diagnose and treat disrupted sleep in IBD patients is a potential promising therapeutic approach that not only could improve patients’ quality of life, but may even modify disease course by preventing disease flare and prolong periods of remission.
Sleep, circadian organization & the GI tract
Sleep disorders are common. NIH studies have estimated that 50–70 million Americans currently suffer from sleep disorders, and intermittent sleep disorders can adversely affect people’s health [20]. Circadian rhythm sleep disorders (CRSDs) are sleep disturbances that lead to excessive sleepiness, insomnia or impairment of normal function like work and social activities. CRSDs result from a misalignment between the timing of the internal endogenous circadian clock and desired sleep times in the social world. Numerous different types of CRSDs exist such as advanced sleep phase disorder, delayed sleep phase disorder, free running sleep disorder or irregular sleep–wake disorder. Jet lag and shift work are also considered CRSDs that often lead to sleep impairment. The specifics of each of these sleep disorders have been reviewed in detail elsewhere [21]. Recently, we have advanced our understanding of the molecular machinery of circadian rhythms, and with these new tools we are increasing our understanding of the biologic importance of circadian rhythms in health and disease.
The rhythm of the sleep–wake cycle in humans is tightly controlled by the circadian system, which fluctuates with the light–dark cycle. The circadian system controls the temporal organization of many aspects of human physiology including gastrointestinal motility, digestive enzyme secretion and metabolism. The core molecular machinery of the circadian clock is within many tissues and organs of the body including the heart, kidneys, liver and gut. On a molecular level, the mechanism of the circadian clock that generates this fluctuation is a transcriptional–translational feedback loop consisting of interlocking transcription factors CLOCK and BMAL1 that bind to an E-box protein and form a heterodimer. This heterodimer activates transcription of genes PERIOD (Per1, Per2 and Per3) and CRYTOCHROME (Cry1 and Cry2). The resultant transcribed PER and CRY proteins bind to each other and this complex translocates into the nucleus to inhibit CLOCK/BMAL1 activity, which in turn results in the repression of the Per and Cry genes [22]. Overall, circadian genes are responsible for modifying the daily expression of around 10% of genes in the body. Thus, the impact in changes in circadian function may be dramatic. Changes in circadian rhythms, either directly, by alteration of the normal molecular function or indirectly, through changes in the sleep patterns, may affect normal gastrointestinal physiology and mucosal immune balance.
The central circadian clock is located in the hypothalamic suprachiasmatic nuclei (SCN). The SCN is the master clock that coordinates the expression and timing of multiple peripheral circadian molecular rhythms, including the circadian clock genes in the GIT. The central clock alternates on roughly a 24 h (circadian) cycle entrained by light–dark signals. The SCN also sends nerve impulses to the pineal gland and regulates production of neurohormones like melatonin, whose production is commonly used as a marker of the central circadian clock. The recent finding of the circadian clock machinery in the GIT [23] has opened new avenues of research for GIT diseases. The peripheral clock of the GIT is entrained mainly by local stimuli such as food intake, which has been found to be a potent synchronizer or ‘zeitgeber’ for the peripheral clock system [24].
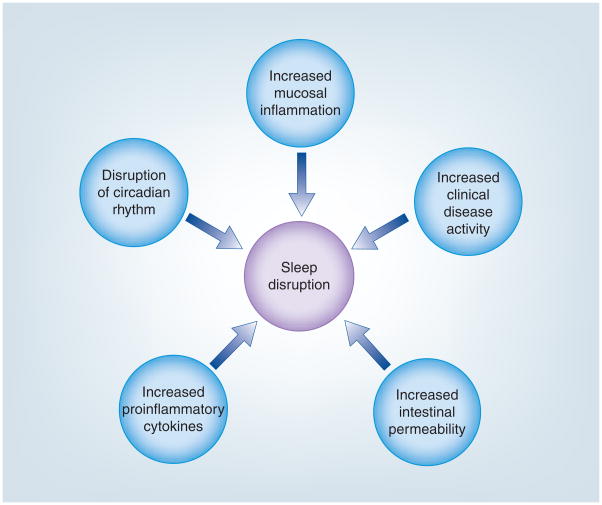
The impact of circadian variation on bodily function is evidenced in clinically relevant ways on a daily basis. The daily rhythmic variation in serum cortisol levels is one example well known to clinicians, but the circadian impact on the GIT is also of importance. For example, the presence of nocturnal diarrhea is a frequently asked question in a gastroenterologist’s office, often to help distinguish an inflammatory from functional cause for the diarrhea. This is due in part because gastrointestinal motility, and therefore bowel movements, are suppressed during slow wave sleep and then colonic motility is increased when we wake [25]. This daily variation in motility is just one example of the importance circadian rhythms play in maintaining gastrointestinal health. Another reason to support a potentially critical role of circadian and sleep patterns in diseases like IBD is the effect of circadian rhythms on immune function [26]. The immune system of the gut is also under circadian regulation and inflammatory mediators such as TNF-α have been found to suppress clock gene expression [27]. In addition, our recent finding that circadian clock genes can impact intestinal epithelial cell permeability provides additional evidence for the importance of circadian machinery as a potential trigger for IBD flare ups [Swanson GR, Unpublished Data]. Gut leakiness is considered an important mechanism for initiating the inflammatory cascades seen in patients with IBD during a disease flare, and could be a factor to disrupt sleep (Figure 1). The identification of important markers in this complex network of central and peripheral circadian rhythms has opened up new areas of study in gastrointestinal diseases such as IBD. Below, we summarize findings from our laboratory and from the literature to demonstrate a potential link between circadian machinery, melatonin and sleep disturbances in IBD disease course.
Figure 1.
Factors causing sleep disruption in inflammatory bowel disease.
IBD & melatonin
One over-the-counter sleep aid that has been of particular interest in IBD is melatonin. Melatonin is important in the regulation of circadian rhythms and has multiple important hormonal functions. Melatonin was first discovered in 1958 by Lerner and colleagues [28], and is secreted at night time from the pineal gland. It is a derivate of serotonin and inhibits the increase in gastrointestinal motility and smooth muscle cell contraction caused by serotonin [29]. Melatonin has been found in multiple areas of the body including the entire GIT. While night-time melatonin is derived mainly from the pineal gland, there is melatonin in the GIT at levels of 400 times higher than the pineal gland [30]. Melatonin is a powerful antioxidant and free radical scavenger [31] and has also been found to decrease levels of TNF-α [32], two pathways know to be important in IBD.
Melatonin is produced by the pineal gland each night, normally rising in the few hours before the onset of nocturnal sleep. As daytime melatonin levels are low, the rapid increase towards elevated nocturnal levels has shown to be a reliable circadian phase marker. Estimates of the phase of the central circadian clock are usually made by measuring at least the first 6 h of the approximately 12 h melatonin secretion in the plasma or saliva. Importantly, endogenous melatonin levels are suppressed by light and so the measurement of the onset of melatonin secretion must occur in dim light (‘dim light melatonin onset’ [DLMO]). Measuring the DLMO can help to determine if patients have a normal circadian alignment or if their central circadian rhythm and desired sleep cycle are misaligned [33], as can measuring other factors such as core body temperature. In addition, melatonin itself has several physiologic effects such as lowering core body temperature by vasodilation [34], its ability to modify signaling pathways or tissue injury as an inhibitor of NF-κB [35], and as an antioxidant [36]. Notably, synthetic exogenous melatonin is available over the counter in the USA and when taken can mimic the effects of endogenous melatonin. When timed appropriately it can also shift the timing of the central clock [37]. Thus, melatonin has been used as a potential treatment in patients with CRSD, depression and other medical problems such as colitis.
Because of these mechanisms of action, the therapeutic effect of melatonin was examined in the mice model of colitis induced by dextran sodium sulfate (DSS). Melatonin was found to be able to prevent DSS-induced colitis and to prevent its formation when given as a pretreatment [38]. These findings have been duplicated multiple times in several different mice and rat models looking at different mechanisms. Specifically, melatonin was able to reverse both the increase in intestinal permeability and influx of bacterial endotoxins, and decrease myeloperoxidase (MPO) and TNF-α activity, which leads to ulcerations [39,40]. The ability of melatonin to modulate the inflammatory cascade, scavenge reactive oxygen radicals and its ability to prevent and treat animal models of colitis have provided a compelling rationale to study its effectiveness in human IBD. Indeed, melatonin is an attractive therapeutic intervention in human patients with IBD not only for its mechanistic properties, but also because melatonin is readily available, inexpensive and has a low side-effect profile.
The first case report of melatonin supplementation for treatment of IBD was in a patient with UC who used melatonin supplementation for jet lag and incidentally noted an improvement in his UC symptoms [41]. This case raised a question concerning whether melatonin is only effective if melatonin homeostasis is disrupted. If so, then melatonin should only be used in those IBD patients exhibiting an abnormal melatonin profile. However, there is no study that has comprehensively evaluated melatonin profiles in IBD patients. Accordingly, we examined endogenous melatonin profiles in a small sample of asymptomatic patients with IBD [42]. Melatonin profiles are currently the preferred method to measure the timing of the central circadian clock [43], and our study found that one in four patients had an abnormal melatonin secretion profile with no clear circadian rhythm and thus no clear DLMO. Alterations in melatonin did not correlate with any characteristics of the subjects’ IBD. Our pilot study suggests that abnormal melatonin profile occurs in a subgroup of patients with IBD. We believe that in vitro and in vivo animal studies and a few human case reports now provide a strong rationale for conducting future randomized clinical trials to determine whether melatonin supplementation is effective in treating IBD flare ups and/or maintaining remission. However, these future studies should consider the endogenous melatonin profile, stratify patients based on whether they have an abnormal melatonin profile and include patients with both active and inactive IBD. This is essential to avoid a potential negative finding and type II error.
Sleep, circadian rhythms & inflammatory bowel disease
Recent studies examining the quality of sleep in patients with IBD have shown that their sleep is altered more than healthy subjects. In one study of 80 patients with inactive IBD (47 with UC and 33 with CD) using a validated questionnaire to study sleep quality – the Pittsburgh Sleep Quality Index– we found that IBD subjects subjectively reported worse overall sleep quality than healthy controls [44]. IBD subjects reported significantly prolonged sleep latency, frequent sleep fragmentation, higher rate of using sleeping pills, decreased daytime energy and increased tiredness. The degree of sleep disturbance did not correlate with disease activity, and the magnitude and frequency of disrupted sleep in patients with inactive IBD was similar to patients with irritable bowel syndrome. As this study only included subjects with inactive IBD, this finding is not surprising, as patients with active IBD, possibly with more disturbed sleep, were not included for comparison. This study was a ‘proof of concept’ study and further studies of sleep disturbance in IBD patients with active and inactive disease are needed. Patients with inactive IBD also had objective evidence of disrupted sleep [45]. In another study of 16 patients with inactive IBD with a single overnight polysomnography we found significant disruption of sleep patterns in IBD patients similar to those patients with diarrhea secondary to irritable bowel syndrome. Patients with inactive IBD had decreased total sleep time, and an increase in stage 1 sleep and overall arousal index, reinforcing that patients with IBD have an altered sleep pattern. Further studies are needed first to determine the mechanism of disrupted sleep in IBD patients and second to determine whether treatment of disrupted sleep can positively impact disease course in IBD patients. Studies are also needed to determine the role of circadian clock genes on disrupted sleep in IBD and whether those patients with disrupted sleep and circadian rhythms have more severe disease activity, course and complications.
It is reasonable to hypothesize that disruption of sleep and altered circadian rhythms can be associated with a more severe disease course because of their known deleterious effects on health. The largest and best studies to determine the effects of disrupted circadian rhythms are those conducted on shift workers who often have chronic circadian misalignment. Previous studies in shift workers have shown a possible association with shift work and the risk of poor health and inflammatory disorders [46]. More specifically, one study reported an increase risk of IBD with occupations associated with disruption of circadian rhythms. The study found that electricians and food and beverage industry workers had an odds ratio of 1.6–1.7 for the development of UC and CD [47]. The authors suggested that increased exposure to artificial light and irregular work hours may be important environmental factors in the development of the disease. However, another study with a similar design did not find an association between IBD risk and these occupations [48]. This is interesting as clinically we have noted a strong association between worsening of disease course in two patients (one policeman, one female nurse) with UC and one patient with CD (firewomen) who started shift work and two patients with CD and multiple periods of jet lag and an improvement when they return to a normal schedule.
This uncontrolled, anecdotal observation of course requires further investigation using a prospective nested study design to determine if shift work and disrupted circadian rhythms worsen IBD disease course. Recently, two animal studies have highlighted the impact of circadian disruption and shift work on the GIT. In the same DSS mice model of colitis in which melatonin supplementation was effective in preventing and treating colitis, we examined the effect that chronic disruption of circadian rhythms by a continuous 12-h phase shift in the light–dark cycle every 5 days for 3 months (modeling shift work) would have on the severity of colitis. The other group of DSS mice stayed on a constant 12:12-h light–dark cycle as a control. We found that the phase-shifted animals developed a much more severe form of colitis that was associated with more significant weight loss and an overall higher mortality compared with those mice with normal circadian phase [49]. The severity of colitis was confirmed by tissue histopathologic scoring and was also associated with an increase in neutrophil activation as evidenced by elevation in MPO activity. In a related study, we then repeated a similar experiment examining the effect of sleep deprivation on colitis in DSS-treated mice. In this study the mice were placed in a rotating mechanical wheel to keep them awake either acutely for one 24-h period at day 0, or chronically for 6 h every other day [50]. The results showed a worsening of DSS colitis in mice with either acute or chronic sleep deprivation, but a much more clinically significant worsening of the DSS colitis in the chronically sleep deprived animals as manifested again by greater weight loss and MPO activity in the colon. Limitations of the previous studies include the use of the DSS model of colitis which may be a poor model for Crohn’s disease, but does mimic the disruption of the intestinal barrier central to disease flare in IBD.
We hypothesize from these two studies that key immuno-inflammatory genes, such as NF-κB, and cytokines may be under circadian regulation and be altered by changes in sleep patterns. When an animal is in a state of chronic phase shifting and loses the ability to entrain to its environment, dysregulation of the inflammatory cascade leads to exacerbation of mucosal immune inflammation in animal models of colitis. Another possibility is disrupted circadian-induced loss of normal intestinal barrier function because disruption of intestinal barrier integrity is the primary mechanism of DSS model of colitis. It is plausible that genetic factors can dictate the susceptibility to the effects of changes in circadian homeostasis. For example, extensive polymorphisms in the circadian clock genes in humans including PER genes has been recently reported. Interestingly, polymorphisms in the PER 2 gene have been shown to be involved in increased susceptibility to alcohol-induced changes in circadian rhythms in rodents [51]. This observation raises the possibility that such polymorphisms could underlie the differential response to changes in circadian homeostasis in pathological states such as IBD. This is one hypothesis from the current available data, and further studies are needed to answer these questions (Table 1). It must be stated that many fundamental questions regarding the link between circadian rhythms, mucosal immune regulation diseases and gut leakiness in IBD need to be clarified. These preliminary studies give a rationale to proceed with future clinical trials examining the effect of sleep and circadian disruption on disease course in IBD and to see if disruption of the sleep/circadian homeostasis triggers IBD flare. We believe these in vitro and in vivo animal studies have identified potential new therapeutic targets for the treatment of patients with IBD, and future human randomized clinical trials are now needed to substantiate this promise.
Table 1.
Studies that either directly or indirectly support a role for circadian disruption in inflammatory bowel disease.
| Study (year) | Subjects | Intervention | Sleep measure | Circadian modification | Clinical outcome | Main finding | Histologic measure | Ref. |
|---|---|---|---|---|---|---|---|---|
| Selected pharmacologic interventional studies (melatonin) | ||||||||
| Li et al. (2005) | TNBS-treated rats | Melatonin | Improved colitis | Mediated through NF-κB | [39] | |||
| Mei et al. (2002) | TNBS-treated rats | Melatonin | Improved colitis | Reduced oxidative injury | [40] | |||
| Observation studies in sleep and IBD | ||||||||
| Keefer et al. (2006) | Inactive IBD, IBS, healthy controls | Polysomnography PSQI | Decreased total sleep time and sleep efficiency | Sleep impacted IBD-Q | [44] | |||
| Ranjbaran et al. (2007) | Inactive IBD, IBS, healthy controls | PSQI | Poor overall sleep quality | Sleep impacted IBD-Q | [45] | |||
| Burgess et al. (2010) | Inactive IBD | Wrist actigraphy DLMO | Decreased sleep efficiency | Some abnormal melatonin secretion | [42] | |||
| Biological physiological studies | ||||||||
| Preuss et al. (2008) | DSS-treated mice | Phase shift | Worsened colitis increased mortality | Increased MPO | [49] | |||
| Tang et al. (2009) | DSS-treated mice | Acute and chronic sleep deprivation | Worsened colitis increased mortality | Increased MPO | [50] | |||
DLMO: Dim light melatonin onset; DSS: Dextran sodium sulfate; IBD: Inflammatory bowel disease; IBD-Q: Inflammatory bowel disease questionnaire; IBS: Irritable bowel syndrome; MPO: Myeloperoxidase; PSQI: Pittsburgh Sleep Quality Index; TNBS: Trinitrobenzene sulfonic acid.
Expert commentary
Although we spend roughly a third of our lives sleeping, and many aspects of our lives are regulated by daily circadian rhythmicity, the importance of the sleep and circadian rhythms in clinical diseases such as IBD are only beginning to be understood. Sleep deprivation and shift work or circadian misalignment are also becoming more common in today’s modern society; total sleep time has steadily decreased over the last 25 years in the modern work environment and increases in frequent long flight and mobility have challenged our circadian homeostasis. Recent advances in the molecular machinery that regulates circadian rhythms in both the central and peripheral systems of the GIT have opened up new avenues for the exploration of interaction between immune activation, sleep and daily circadian rhythms. Disruption of the circadian system can cause immune activation and the release of inflammatory cytokines such as TNF-α and has led to an increased interest in the role sleep disruption could play in chronic inflammatory diseases such as IBD.
Inflammatory bowel disease is a chronic inflammatory condition characterized by frequent flare ups associated with diarrhea, weight loss and bleeding. As there is currently no cure, maintaining remission in IBD patients is crucial to reduce the risk of complications. Recent studies have shown that disordered sleep is more common in IBD, and treatment of sleep disruption with a melatonin supplement to improve sleep has been documented to improve animal models of colitis. Two recent studies examining the role of circadian desynchronization and sleep deprivation in an animal model of colitis have shown that disordered sleep can have a significant impact, increaseing the activity of the mucosal immune system and activating the inflammation system. Human clinical trials in patients with IBD are needed to determine if management of sleep disorders and restoration of normal circadian rythmicity could diminish the frequency and severity of disease flare ups and thus improve patients’ quality of life and decrease the risk for disease-related complications such as hospitalizations and surgery.
Five-year view
To date, there have only been initial investigations into the role of circadian rhythms in gastrointestinal diseases. The function of the circadian clock in controlling local physiologic function and the immune system in the GIT is only beginning to be understood. IBD is one gastrointestinal disease in which sleep may have an important impact on disease course and severity. As we increase our understanding of how the circadian clock regulates inflammation in the GIT and new targets for treatment, future medical therapy may focus on modifying the circadian clock (chronotherapeutics) to treat IBD and minimize complications related to disease flare.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
The authors are supported by NIH grant RO-1 AA020216-01. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Sandborn WJ. Current directions in IBD therapy: what goals are feasible with biological modifiers? Gastroenterology. 2008;135(5):1442–1447. doi: 10.1053/j.gastro.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 2.Cummings JR, Keshav S, Travis SP. Medical management of Crohn’s disease. Br Med J. 2008;336 (10):1062–1066. doi: 10.1136/bmj.39547.603218.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci. 1993;38(6):1137–1146. doi: 10.1007/BF01295733. [DOI] [PubMed] [Google Scholar]
- 4.Loftus EV, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR. Crohn’s disease in Olmsted County, Minnesota, 1940–1993: incidence, prevalence, and survival. Gastroenterology. 1998;114(6):1161–1168. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- 5.Sandler RS, Eisen CM. Epidemiology of inflammatory bowel disease. In: Kirsner JB, editor. Inflammatory Bowel Disease. 4. Saunder; PA, USA: 2000. pp. 89–112. [Google Scholar]
- 6.Birrenbach T, Böcker U. Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflamm Bowel Dis. 2004;10(6):848–859. doi: 10.1097/00054725-200411000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Hoogerwerf WA. Role of biological rhythms in gastrointestinal health and disease. Rev Endocr Metab Disord. 2009;10(4):293–300. doi: 10.1007/s11154-009-9119-3. [DOI] [PubMed] [Google Scholar]
- 8••.Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308(5724):1043–1045. doi: 10.1126/science.1108750. Landmark article to first demonstrate that the circadian clock plays a crucial role in energy metabolism and gastrointestinal hormones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demeter P, Visy KV, Gyulai N, et al. Severity of gastroesophageal reflux disease influences daytime somnolence: a clinical study of 134 patients underwent upper panendoscopy. World J Gastroenterol. 2004;10(12):1798–1801. doi: 10.3748/wjg.v10.i12.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segawa K, Nakazawa S, Tsukamoto Y, et al. Peptic ulcer is prevalent among shift workers. Dig Dis Sci. 1987;32(5):449–453. doi: 10.1007/BF01296025. [DOI] [PubMed] [Google Scholar]
- 11••.Nojkov B, Rubenstein J, Chey WD, Hoogerwerf W. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am J Gastroenterol. 2010;105(4):842–847. doi: 10.1038/ajg.2010.48. Demonstrated how a circadian sleep disorder can increase the prevalence of gastrointestinal diseases like irritable bowel syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoham S, Davenne D, Cady AB, Dinarello CA, Krueger JM. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am J Physiol. 1987;253(1):R142–R149. doi: 10.1152/ajpregu.1987.253.1.R142. [DOI] [PubMed] [Google Scholar]
- 13.Chandrasekhara PKS, Jayachandran NV, Rajasekhar L, Thomas J, Narsimulu G. The prevalence and associations of sleep disturbances in patients with systemic lupus erythematosus. Mod Rheumatol. 2009;19(4):407–415. doi: 10.1007/s10165-009-0185-x. [DOI] [PubMed] [Google Scholar]
- 14.Brass SD, Duquette P, Proulx-Therrien J, Auerbach S. Sleep disorders in patients with multiple sclerosis. Sleep Med Rev. 2010;14(2):121–129. doi: 10.1016/j.smrv.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Abad VC, Sarinas PSA, Guilleminault C. Sleep and rheumatologic disorders. Sleep Med Rev. 2008;12(3):211–228. doi: 10.1016/j.smrv.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Salahuddin N, Barroso J, Leserman J, Harmon J, Pence B. Daytime sleepiness, nighttime sleep quality, stressful life events, and HIV-related fatigue. J Assoc Nurses AIDS Care. 2009;20(1):6–13. doi: 10.1016/j.jana.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells G, Li T, Maxwell L, Maclean R, Tugwell P. Responsiveness of patient reported outcomes including fatigue, sleep quality, activity limitation, and quality of life following treatment with abatacept for rheumatoid arthritis. Ann Rheum Dis. 2008;67(2):260–265. doi: 10.1136/ard.2007.069690. [DOI] [PubMed] [Google Scholar]
- 18.Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- 19.Marcus SB, Strople JA, Neighbors K, et al. Fatigue and health-related quality of life in pediatric inflammatory bowel disease. Clin Gastroenterol Hepatol. 2009;7(5):554–561. doi: 10.1016/j.cgh.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Chilcott LA, Shapiro CM. The socioeconomic impact of insomnia. Pharmacoeconomics. 1996;10(Suppl 1):1–14. doi: 10.2165/00019053-199600101-00003. [DOI] [PubMed] [Google Scholar]
- 21.Lu BS, Zee PC. Circadian rhythm sleep disorders. Chest. 2006;130(6):1915–1923. doi: 10.1378/chest.130.6.1915. [DOI] [PubMed] [Google Scholar]
- 22.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134(5):728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Sládek M, Rybová M, Jindráková Z, et al. Insight into the circadian clock within rat colonic epithelial cells. Gastroenterology. 2007;133(4):1240–1249. doi: 10.1053/j.gastro.2007.05.053. One of the first two studies demonstrating the presence of the circadian clock genes in the gastrointestinal tract. [DOI] [PubMed] [Google Scholar]
- 24••.Hoogerwerf W, Hellmich H, Cornélissen G, et al. Clock gene expression in the murine gastrointestinal tract: endogenous rhythmicity and effects of a feeding regimen. Gastroenterology. 2007;133(4):1250–1260. doi: 10.1053/j.gastro.2007.07.009. One of the first two studies demonstrating the presence of the circadian clock genes in the gastrointestinal tract. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa Y, Cook IJ, Panagopoulos V, McEvoy RD, Sharp DJ, Simula M. Relationship between sleep patterns and human colonic motor patterns. Gastroenterology. 1994;107(5):1372–1381. doi: 10.1016/0016-5085(94)90539-8. [DOI] [PubMed] [Google Scholar]
- 26.Burgess HJ, Revell VL, Molina TA, Eastman CI. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocrinol Metab. 2010;95(7):3325–3331. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann NY Acad Sci. 2010;1193(1):48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- 28.Lerner AB, Case JD, Lee TH, Mori W. Isolation of melatonin, the pineal factor that lightens melanocytes. J Am Chem Soc. 1958;80:2587. [Google Scholar]
- 29.Bubenik GA. Thirty four years since the discovery of gastrointestinal melatonin. J Physiol Pharmacol. 2008;59(Suppl 2):33–51. [PubMed] [Google Scholar]
- 30.Thor PJ, Krolczyk G, Gil K, Zurowski D, Nowak L. Melatonin and serotonin effects on gastrointestinal motility. J Physiol Pharmacol. 2007;58(Suppl 6):97–103. [PubMed] [Google Scholar]
- 31.Aydogan S, Yerer MB, Goktas A. Melatonin and nitric oxide. J Endocrinol Invest. 2006;29(3):281–287. doi: 10.1007/BF03345555. [DOI] [PubMed] [Google Scholar]
- 32.Di Stefano A, Paulesu L. Inhibitory effect of melatonin on production of IFN-γ or TNF-α in peripheral blood mononuclear cells of some blood donors. J Pineal Res. 1994;17(4):164–169. doi: 10.1111/j.1600-079x.1994.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 33.Van Someren EJW, Nagtegaal E. Improving melatonin circadian phase estimates. Sleep Med. 2007;8(6):590–601. doi: 10.1016/j.sleep.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Aoki K, Zhao K, Yamazaki F, et al. Exogenous melatonin administration modifies cutaneous vasoconstrictor response to whole body skin cooling in humans. J Pineal Res. 2008;44(2):141–148. doi: 10.1111/j.1600-079X.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- 35.Markus RP, Ferreira ZS, Fernandes PA, Cecon E. The immune-pineal axis: a shuttle between endocrine and paracrine melatonin sources. Neuroimmunomodulation. 2007;14(3–4):126–133. doi: 10.1159/000110635. [DOI] [PubMed] [Google Scholar]
- 36.Nishida S. Metabolic effects of melatonin on oxidative stress and diabetes mellitus. Endocrine. 2005;27(2):131–136. doi: 10.1385/endo:27:2:131. [DOI] [PubMed] [Google Scholar]
- 37.Huether G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia. 1993;49(8):665–670. doi: 10.1007/BF01923948. [DOI] [PubMed] [Google Scholar]
- 38.Terry PD, Villinger F, Bubenik GA, Sitaraman SV. Melatonin and ulcerative colitis: evidence, biological mechanisms, and future research. Inflamm Bowel Dis. 2009;15(1):134–140. doi: 10.1002/ibd.20527. [DOI] [PubMed] [Google Scholar]
- 39•.Li JH, Yu JP, Yu HG, et al. Melatonin reduces inflammatory injury through inhibiting NF-κB activation in rats with colitis. Mediators Inflamm. 2005;4:185–193. doi: 10.1155/MI.2005.185. Showed how melatonin through NF-κB can reduce inflammation in an animal model of colitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mei Q, Yu JP, Xu JM, Wei W, Xiang L, Yue L. Melatonin reduces colon immunological injury in rats by regulating activity of macrophages. Acta Pharmacol Sin. 2002;23(10):882–886. [PubMed] [Google Scholar]
- 41.Jan J, Freeman R. Re: Mann–melatonin for ulcerative colitis? Am J Gastroenterol. 2003;98(6):1446–1446. doi: 10.1111/j.1572-0241.2003.07508.x. [DOI] [PubMed] [Google Scholar]
- 42.Burgess HJ, Swanson GR, Keshavarzian A. Endogenous melatonin profiles in asymptomatic inflammatory bowel disease. Scand J Gastroenterol. 2010;45(6):759–761. doi: 10.3109/00365521003749818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Someren EJ, Nagtegaal E. Improving melatonin circadian phase estimates. Sleep Med. 2007;8(6):590–601. doi: 10.1016/j.sleep.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 44•.Keefer L, Stepanski E, Ranjbaran Z, Benson LM, Keshavarzian A. An initial report of sleep disturbance in inactive inflammatory bowel disease. J Clin Sleep Med. 2006;2(4):409–416. First study quantifying sleep disturbances in patients with inflammatory bowel disease. [PubMed] [Google Scholar]
- 45.Ranjbaran Z, Keefer L, Farhadi A, Stepanski E, Sedghi S, Keshavarzian A. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol. 2007;22(11):1748–1753. doi: 10.1111/j.1440-1746.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- 46.Sookoian S, Gemma C, Gianotti TF, et al. Effects of rotating shift work on biomarkers of metabolic syndrome and inflammation. J Intern Med. 2007;261(3):285–292. doi: 10.1111/j.1365-2796.2007.01766.x. [DOI] [PubMed] [Google Scholar]
- 47.Sonnenberg A. Occupational distribution of inflammatory bowel disease among German employees. Gut. 1990;31(9):1037–1040. doi: 10.1136/gut.31.9.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boznańska P, Wichan P, Stepień A, et al. 24-hour urinary 6-hydroxymelatonin sulfate excretion in patients with ulcerative colitis. Pol Merkur Lekarski. 2007;22(131):369–372. [PubMed] [Google Scholar]
- 49••.Preuss F, Tang Y, Laposky AD, Arble D, Keshavarzian A, Turek F. Adverse effects of chronic circadian desynchronization in animals in a ‘challenging’ environment. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R2034–R2040. doi: 10.1152/ajpregu.00118.2008. One of two studies demonstrating for the first time that circadian disruption can worsen mucosal colitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Tang Y, Preuss F, Turek FW, Jakate S, Keshavarzian A. Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep Med. 2009;10(6):597–603. doi: 10.1016/j.sleep.2008.12.009. One of two studies demonstrating for the first time that circadian disruption can worsen mucosal colitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perreau-Lenz S, Zghoul T, DE Fonseca FR, Spanagel R, Bilbao A. Circadian regulation of central ethanol sensitivity by the mPer2 gene. Addict Biol. 2009;14:253–259. doi: 10.1111/j.1369-1600.2009.00165.x. [DOI] [PubMed] [Google Scholar]