The ribosome transcription activator Sfp1 is degraded by Blm10-proteasomes. Loss of BLM10 results in increased Sfp1 protein levels, increased transcription of ribosomal genes, and increased ribosome levels upon nutrient depletion. Thus Blm10-proteasome-mediated turnover of Sfp1 is a regulatory mechanism for ribosome biosynthesis repression.
Abstract
The regulation of ribosomal protein (RP) gene transcription is tightly linked to the nutrient status of the cell and is under the control of metabolic signaling pathways. In Saccharomyces cerevisiae several transcriptional activators mediate efficient RP gene transcription during logarithmic growth and dissociate from RP gene promoters upon nutrient limitation. Repression of RP gene transcription appears to be regulated predominantly by posttranslational modification and cellular localization of transcriptional activators. We report here that one of these factors, Sfp1, is degraded by the proteasome and that the proteasome activator Blm10 is required for regulated Sfp1 degradation. Loss of Blm10 results in the stabilization and increased nuclear abundance of Sfp1 during nutrient limitation, increased transcription of RP genes, increased levels of RPs, and decreased rapamycin-induced repression of RP genes. Thus we conclude that proteasomal degradation of Sfp1 is mediated by Blm10 and contributes to the repression of ribosome biogenesis under nutrient depletion.
INTRODUCTION
The proteasome is an essential protease in the cytoplasm and nuclei of eukaryotic cells. It consists of two entities: a central proteolytic core (the 20S proteasome in higher eukaryotes or the core particle [CP] in Saccharomyces cerevisiae) and a regulatory or activating complex. The CP has a barrel-shaped topology formed by four stacked rings (two inner β and two outer α rings), composed of seven subunits each. The β rings harbor three different proteolytically active subunits with different specificities: trypsin-like, chymotrypsin-like, and postacidic activity (Kisselev et al., 2006). The active sites are sequestered within the interior of the CP barrel (Groll et al., 1997). Access to the proteolytic chamber is controlled by an adjustable gate and is mediated by proteasome regulators/activators (Foerster et al., 2003). Three activator families have been described: the conserved regulatory particle (19S or PA700), the PA28 family (REG, 11S regulator) (Li and Rechsteiner, 2001), and the conserved Blm10/PA200 proteins (Ustrell et al., 2002; Schmidt et al., 2005), providing a variety of different proteasomal subspecies, which most likely target different groups of proteasomal substrates. The regulatory particle consists of 19 subunits, among them six paralogous ATPases (Finley, 2009). Opening of the CP gate by the RP is achieved by insertion of the ATPase C-termini into specific pockets at the CP surface (Smith et al., 2007; Gillette et al., 2008; Stadtmueller et al., 2009). PA28 activators are characterized by a hepta- or hexa-oligomeric ring structure of ∼200 kDa (Rechsteiner and Hill, 2005). The C-termini of the subunits of PA28 are inserted into the same α subunit pockets as the C-termini of the ATPases. However, C-terminal docking of the PA28 subunits does not trigger gate opening. Instead, internal segments known as the PA28 activation loops induce a structural rearrangement of the gate region, which allows substrate entry (Whitby et al., 2000).
Blm10 in S. cerevisiae and its human orthologue PA200 are large ∼245-kDa proteins composed of HEAT repeats, which associate with the CP/20S gate region (Ortega et al., 2005; Schmidt et al., 2005; Iwanczyk et al., 2006; Sadre-Bazzaz et al., 2010). In mammalian cells PA200 was found exclusively in a complex with the proteasome (Blickwedehl et al., 2008). We have demonstrated recently that in rapidly growing yeast cells Blm10 is part of a mature proteasome hybrid complex, where Blm10 occupies one end of the core cylinder and the regulatory particle the other end (Schmidt et al., 2005). Blm10–CP devoid of regulatory particles was not detected in unfractionated lysates, suggesting that the dominant species of Blm10-containing proteasomes is the hybrid complex. Blm10–CP complexes, purified after the dissociation of the regulatory particle from the hybrid complex, exhibit elevated CP peptidase activity (Schmidt et al., 2005; Iwanczyk et al., 2006; Li et al., 2007; Lehmann et al., 2008). Both hybrid complex formation and activation of the proteasome peptidase activity have also been described for PA200–proteasome complexes (Ustrell et al., 2002; Blickwedehl et al., 2008), suggesting that Blm10/PA200 proteins might represent a novel conserved monomeric proteasome activator family. A recent structural analysis of Blm102-CP complexes showed that similar to RP and PA28, Blm10 binding to the CP is mediated via its C-terminus. The C-terminal Blm10 residues cause structural alterations within the gate region, resulting in a partially open, disordered gate in a molecular mechanism that appears to be similar to the C-terminal docking of the proteasomal ATPases, but different from PA26 C-terminal binding to the CP, which per se does not induce gate opening (Sadre-Bazzaz et al., 2010). Furthermore, a pore within Blm10 of 13–22 Å has been detected. Whether these structural characteristics of Blm10–CP complexes are sufficient to promote protein degradation remains to be established.
The cellular functions of Blm10 are poorly understood. A proposed role for Blm10/PA200 in DNA repair (Febres et al., 2001; Ustrell et al., 2002) was not confirmed in subsequent studies in yeast and in mammalian cells (Schmidt et al., 2005; Khor et al., 2006; McCullock et al., 2006). In S. cerevisiae Blm10 binding to the proteasome occurs at a late stage during proteasome maturation (Fehlker et al., 2003; Li et al., 2007; Marques et al., 2007), which initially suggested a possible function in proteasome assembly. Loss of BLM10, however, does not result in a significant defect in proteasome assembly (Marques et al., 2007) or in gross changes in mature proteasome populations (Schmidt et al., 2005). Double mutants that combine loss of BLM10 and an assembly-defective proteasomal regulatory particle mutant show defects in 20S maturation, while either single mutant does not (Marques et al., 2007). Thus activator binding to immature CP complexes (either the regulatory particle or Blm10) appears to be an integral part of CP maturation, with Blm10 and the regulatory particle playing redundant roles during this process.
The regulation of ribosome abundance and output is crucial for the maintenance of the energy economy within a cell and thus for cell growth and size. It is estimated that ribosome biosynthesis accounts for ∼70% of total transcription and ∼25% of the total translation in rapidly growing yeast cells (Warner, 1999; Rudra et al., 2005). Thus ribosome function and biogenesis have to be tightly correlated with the cellular nutrient status. This process is mediated by the activity of the major metabolic signaling pathways, the target of rapamycin kinase (TORC1), and cyclic AMP–dependent kinase A (PKA) pathways. Inactivation of TORC1 or PKA in yeast in response to limiting nutrients results in rapid repression of ribosomal genes and inhibition of translation (Powers and Walter, 1999; Wullschleger et al., 2005). Ribosome biogenesis requires the activity of all three nuclear RNA polymerases, which mediate the expression of ribosomal proteins (RPs), rRNAs, and accessory proteins, which assist in ribosome assembly. In S. cerevisiae factors controlling ribosomal gene expression include Rrn3, regulating Pol I–driven genes (Claypool et al., 2004); Maf1, which acts upon Pol III–mediated transcription (Upadhya et al., 2002); and the Pol II–directed transcriptional regulators Rap1, Ifh1, Fhl1, Hmo1, and Sfp1 (Fingerman et al., 2003; Jorgensen et al., 2004; Marion et al., 2004; Martin et al., 2004; Schawalder et al., 2004; Wade et al., 2004; Zhao et al., 2006; Berger et al., 2007). Although TORC1/PKA signaling pathways have been implicated in the regulation of some of these factors, the precise mechanisms of gene activation and repression are not completely understood.
In this report we provide evidence for a regulatory function of proteasome-mediated turnover of Sfp1 in the repression of ribosome biogenesis upon nutrient depletion. Additionally, we demonstrate that proteasome-dependent Sfp1 turnover is mediated by the proteasome activator Blm10.
RESULTS
BLM10 deletion results in resistance to sublethal doses of cycloheximide
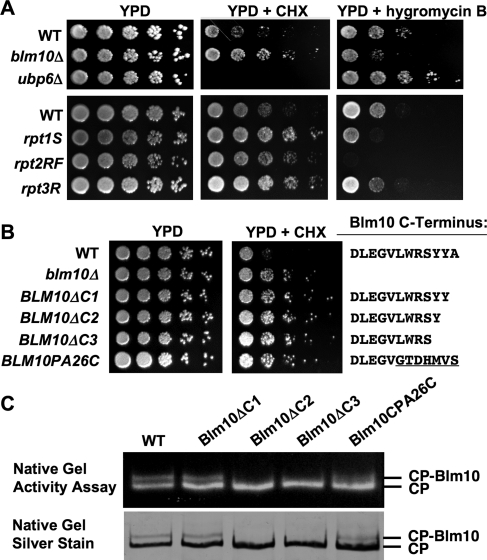
To gain insight into the cellular functions of Blm10, we performed a screen for loss-of-function phenotypes of cells deleted for BLM10. We found that blm10Δ cells exhibit resistance to sublethal doses of the translational inhibitor cycloheximide (CHX) (Figure 1A). The same phenotype has been associated with proteasome CP and regulatory particle mutants (crl mutants) defective in the turnover of ubiquitin conjugates (Gerlinger et al., 1997), derived from a screen for CHX-resistant yeast mutants (McCusker and Haber, 1988). Similar observations have been made in Arabidopsis thaliana (Kurepa et al., 2010). To corroborate that CHX resistance is a general phenotype of proteasome loss-of-function mutants, we tested the growth of the proteasomal ATPase mutants rpt1S, rpt2RF, and rpt3R (Rubin et al., 1998) under the same conditions. The respective point mutations prevent nucleotide binding to the ATPase subunits due to a point mutation within the Walker A motif, yet still allow cell growth. The ATPase mutants showed similar CHX resistance as loss of BLM10 (Figure 1A, bottom).
FIGURE 1:
Loss of BLM10 or disruption of its ability to bind to the proteasome results in cycloheximide (CHX) resistance. (A) Overnight cultures of WT (BY4741), blm10Δ (yMS63), and ubp6Δ (yMS222) cells (top) or WT (SUB62), rpt1S (DY106), rpt2RF (DY62), and rpt3R (DY93) (bottom) were serially diluted and spotted onto YPD in the absence or presence of 0.3 μg/ml (top) or 0.5 μg/ml (bottom) CHX or 60 μg/ml hygromycin B and incubated at 30ºC for 2 d (YPD) or 4 d (CHX, hygromycin B). (B) C-terminal Blm10 mutants exhibit a loss-of-function phenotype. Fivefold serial dilutions of overnight cultures of wild-type (WT) yeast strains, strains deleted for BLM10 (Δblm10), strains with genomically integrated C-terminal deletion mutants (BLM10ΔC1, BLM10ΔC2, and BLM10ΔC3), and a chimeric Blm10 protein where the last seven residues were exchanged against the corresponding residues of PA26 (BLM10PA26C) were spotted on YPD in the absence (left) or in the presence of 0.3 μg CHX (right). The C-terminal sequences are indicated to the right. (C) Purified WT Blm10–CP and complexes with C-terminal Blm10 mutants as indicated in (B) were purified and subjected to native gel electrophoresis, followed by an in-gel activity assay with the fluorogenic proteasome substrate Suc-LLVY-AMC (top). Subsequently the gel was stained with silver nitrate (bottom). The positions of Blm10–CP and CP are indicated.
Ubp6 is a negative regulator of proteasome function, and ubp6Δ cells are characterized by proteasome hyperactivity (Hanna et al., 2003, 2006). In contrast to proteasome hypomorphs such as the ATPase mutants, loss of UBP6 results in strong CHX sensitivity (Figure 1A, middle). Interestingly, loss of UBP6 confers resistance to a second translation inhibitor, hygromycin B (Hanna et al., 2003). The same opposing phenotype between CHX and hygromycin B is observed for loss of BLM10 or for the ATPase mutants rpt1S, rpt2SF, and to a lesser extent rpt3R (Figure 1A, right), yet sensitivity and resistance to the drugs are inverted as compared with ubp6Δ. We conclude that proteasome loss-of-function mutants (ATPase mutants) confer CHX resistance and sensitivity to hygromycin B, while proteasome hyperactivity (ubp6Δ) results in the opposite phenotypes. It appears surprising that proteasome mutants are sensitive to one class of translation inhibitors but resistant to another. However, both inhibitors are chemically and mechanistically different. Hygromycin B is an aminoglycoside, which causes miscoding (Sutcliffe, 2005) and accumulation of misfolded proteins, explaining the sensitivity of mutants with reduced proteasomal activity. CHX, on the other hand, is a glutarimide antibiotic, which prevents initiation of translation, elongation, and the removal of the nascent chain from the ribosome. It furthermore inhibits polysome breakdown and reassembly (Pestka, 1971). Thus CHX-treated cells are characterized by reduced protein synthesis. Increased proteasome activity induced by loss of UBP6 aggravates sensitivity to CHX, while proteasome loss of function alleviates the phenotype.
Because loss of BLM10 results in the same phenotype as the proteasome hypomorphic ATPase mutants (CHX resistance and sensitivity to hygromycin B), we conclude that Blm10 positively regulates proteasome activity, providing evidence for its role as a proteasome activator.
BLM10 mutations, which prevent CP binding, exhibit a loss-of-function phenotype
Recently it was demonstrated that Blm10 interaction with the CP involves C-terminal docking of Blm10 to conserved binding pockets located at the upper surface of the CP barrel (Sadre-Bazzaz et al., 2010). To investigate whether the CHX-resistant phenotype is related to the property of Blm10 to bind to the proteasome, we constructed a series of C-terminal BLM10 deletions in which either the last residue (BLM10ΔC1), the last two residues (BLM10ΔC2), or the last three residues (BLM10ΔC3) were removed. All C-terminal mutants exhibited a CHX-resistant phenotype (Figure 1B), arguing for a model in which Blm10 function is linked to its association with the proteasome. To corroborate that the mutations affect Blm10 binding to the proteasome, we purified Blm10–CP complexes and resolved them on native gels. While the deletion of the last residue of Blm10 still allowed complex formation, deletion of the last two or three residues abolished Blm10 association with the CP (Figure 1C). Interestingly, although deletion of the last residue did not prevent proteasome binding (Figure 1C), it caused a loss-of-function phenotype (Figure 1B), which could indicate that the C-terminus of Blm10 might have additional roles beyond CP binding. The same observation, intact complex formation plus a loss-of-function phenotype, is evident for a C-terminal chimera, where the last seven residues of Blm10 were exchanged against the C-terminal residues of PA26 (Figure 1, B and C).
Loss of Blm10 affects the abundance of RPs after the diauxic shift
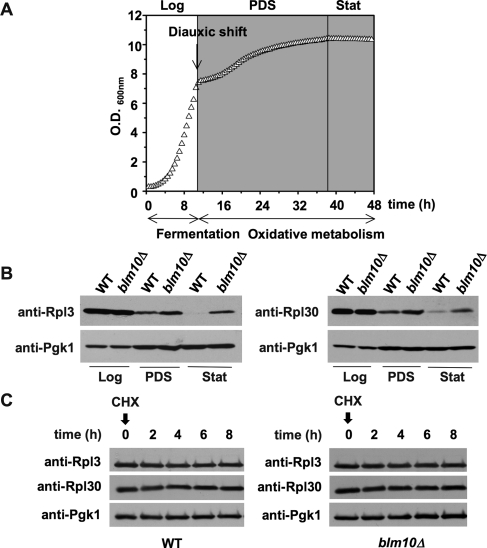
We hypothesized that the CHX-resistant phenotypes upon loss of BLM10 or inactive ATPases might be a consequence of improved ribosome function, for example, due to increased ribosome levels. The control of ribosome abundance is a highly regulated process that is sensitive to the metabolic status of the cell. S. cerevisiae growth is characterized by three defined metabolic phases, as highlighted in Figure 2A (Gasch and Werner-Washburne, 2002; Herman, 2002). Initially, yeast cells grow logarithmically and generate ATP by fermentation (logarithmic [log] phase). On nutrient depletion, ATP is generated by oxidative metabolism. This switch from fermentation to oxidative metabolism is known as the “diauxic shift” and is marked by flattening of the growth curve (Figure 2A), indicative of a reduced growth rate. After several days in the postdiauxic shift (PDS) phase, cells arrest in G0, also known as the stationary (stat) phase in yeast. Both ribosome biogenesis and abundance decrease strongly when cells pass the diauxic shift (PDS) or are treated with the Tor inhibitor rapamycin (Powers and Walter, 1999). To test for potential alterations in ribosome abundance in blm10Δ cells, we analyzed the steady-state levels of two RPs, Rpl3 (Figure 2B, top left) and Rpl30 (top right), in the different metabolic phases. As expected, RP levels are significantly reduced in PDS and stat phase (Figure 2B) in wild-type (WT) cells. A longer exposure of Figure 2B is shown in Supplemental Figure 1. Strikingly, after the diauxic shift and in stat phase, BLM10-deleted cells exhibit elevated steady-state levels of RPs (Figure 2B).
FIGURE 2:
RP abundance is increased in BLM10-deleted cells. (A) Characteristic growth curve of WT (BY4741) yeast cells in YPD obtained in a Bioscreen C MB instrument. The relevant metabolic phases of yeast growth are indicated (log, logarithmic phase; PDS, postdiauxic shift phase; stat, stationary phase). (B) RP abundance is increased in PDS and stat in BLM10-deleted cells. WT (yMS268) and blm10Δ (yMS63) cells grown in YPD were harvested in the different metabolic phases (log, PDS, and stat) and lysed. Equal amounts of protein were subjected to SDS–PAGE, followed by immunodetection with Rpl3- (left) and Rpl30-specific (right) antisera. Immunodetection of phosphoglycerate kinase 1 (Pgk1) was used as loading control (bottom). (C) RP turnover is not affected by loss of BLM10. RP levels at the time points indicated were determined after inhibition of protein synthesis by addition of 200 μg/ml CHX to log phase WT (yMS683) or blm10Δ (yMS684) cells. Cell lysates were subjected to SDS–PAGE followed by immunodetection with Rpl3- or Rpl30-specific antibodies as indicated. Pgk1 protein levels were used as loading control (bottom).
Because Blm10 activates the proteasome, a potential explanation for increased RP levels upon BLM10 deletion after the diauxic shift is a participation of Blm10-proteasomes in RP turnover. To test this hypothesis, we performed CHX chase experiments in the presence of lethal doses of the drug (Kornitzer, 2002). Incubation with high doses of CHX blocks protein synthesis, allowing for determining the rate of protein turnover. Ribosomes are very stable complexes, with an estimated half-life of several days (Warner, 1999). BLM10 deletion did not affect RP turnover (Figure 2C). Our findings are in agreement with a recent report that demonstrates that ribosome turnover is achieved via autophagy in a process involving the deubiquitinating enzyme Ubp3/Bre5 (Kraft et al., 2008).
Loss of BLM10 affects RP gene transcription
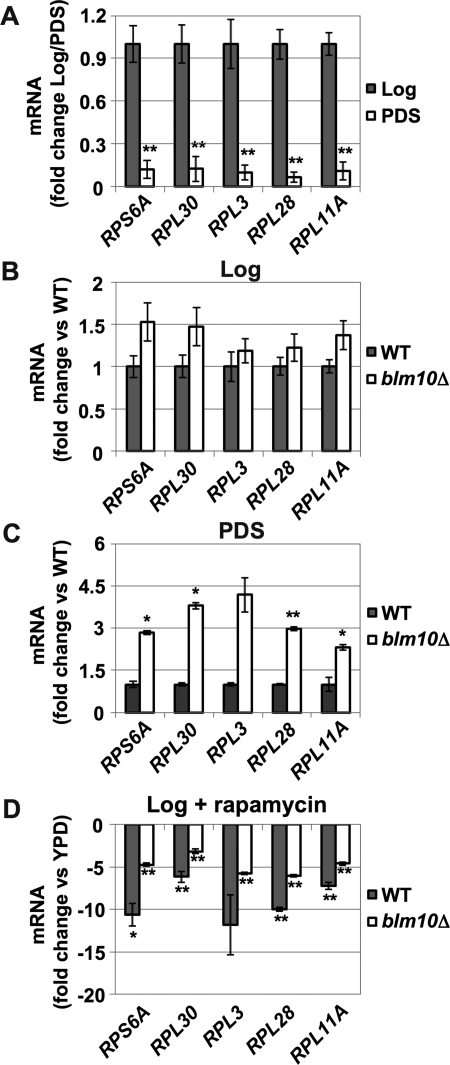
An alternative explanation for elevated RP levels in BLM10-deleted cells would be dysregulation of RP gene transcription. We therefore determined mRNA levels of five RP genes by quantitative real-time PCR (qRT-PCR) in WT and blm10Δ cells in the different growth phases. RP gene transcription is strongly repressed after the diauxic shift in WT cells (Figure 3A; Brauer et al., 2005). Loss of BLM10 did not affect RP gene transcription in log phase (Figure 3B), in agreement with unchanged RP levels observed under the same conditions (Figure 2B). After the diauxic shift, however, RP gene transcription was elevated in the absence of BLM10 (Figure 3C).
FIGURE 3:
RP gene transcription is increased in BLM10-deleted cells after the diauxic shift. Expression of RP genes (RPS6A, RPL30, RPL3, RPL28, RPL11) was analyzed using qRT-PCR. Cycle threshold (CT) values were normalized to ACT1 expression levels. Data are reported as mean ± SEM. A single asterisk indicates a P-value < 0.05; a double asterisk indicates P < 0.01. (A) RP gene transcription is repressed in PDS. The level of RP gene expression in WT (yMS524) cells was determined in log and PDS phase in four independent experiments. PDS values are normalized to log expression levels. (B and C) Up-regulation of RP gene transcription in BLM10-deleted cells in PDS. RP gene transcription was analyzed in WT (yMS524) and blm10Δ (yMS63) in log (B) and PDS (C). RP gene expression in blm10Δ cells was normalized to WT expression in log or PDS phase. The values represent the mean of three independent experiments. (D) BLM10-deleted cells are less responsive to rapamycin-induced RP gene repression. mRNA expression of RP genes was analyzed 1 h after the addition of rapamycin (50 ng/ml) to logarithmically growing WT (yMS524) and BLM10-deleted (yMS63) cells in YPD. The values of RP gene expression levels after rapamycin treatment were normalized to the untreated control strains. The values represent the mean of three independent experiments.
RP gene transcription is rapidly down-regulated upon TORC1 inhibition by rapamycin (Powers and Walter, 1999). To investigate whether Blm10 function is required for this process, we examined rapamycin-induced RP gene repression in the presence or absence of BLM10. Rapamycin-induced RP gene down-regulation was significantly attenuated in blm10Δ cells (Figure 3D), suggesting that Blm10 is required for correct TORC1-mediated RP gene repression.
The transcription of BLM10 responds to metabolic changes
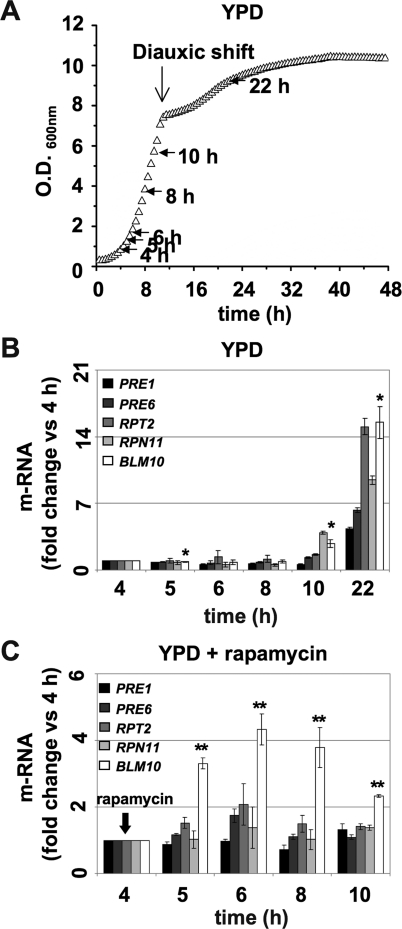
Our data suggest a regulatory function of Blm10-mediated proteasomal degradation under nutrient deprivation. We reasoned that this role might be reflected in the transcriptional profile of BLM10 expression. Proteasome protein levels increase under nutrient deprivation (Fujimuro et al., 1998). Blm10 expression has not been investigated so far. We therefore tested the expression of BLM10, regulatory particle (RPN11 and RPT2), and CP subunits (PRE1 and PRE4) via qRT-PCR during growth in complete media (Figure 4A). While BLM10 and proteasome subunit expression remained constant and at a basal level during log growth, expression of all genes tested increased after the diauxic shift (Figure 4B, 10 h and 22 h), that is, when nutrients become limiting.
FIGURE 4:
BLM10 expression is up-regulated after the diauxic shift or in the presence of rapamycin. (A) Schematic of cell sampling (marked by arrows) during growth in YPD for the qRT-PCR analysis shown in (B). (B) Expression profile of proteasome subunits and BLM10 during growth in YPD. mRNA abundance of CP subunits (PRE1 and PRE6), regulatory particle subunits (RPN11 and RPT2), and BLM10 was analyzed in WT (yMS268) via qRT-PCR at the time points indicated by arrows in (A). CT values were normalized to ACT1 expression levels. Values for each gene are presented relative to the 4-h time point. Data are reported as mean ± SEM. P-values for Blm10 expression are presented. A single asterisk indicates a P-value < 0.05; a double asterisk indicates P < 0.01. (C) Expression profile of PRE1, PRE6, RPN11, RPT2, and BLM10 in WT cells in the presence of 50 ng/ml rapamycin, which was added at the 4-h time point. Values were obtained as in (B) and at the time points indicated in (A).
Rapamycin induces an artificial starvation response even under optimal nutrient conditions. In contrast to the expression of proteasome subunits, BLM10 mRNA levels were elevated rapidly after 1 h of rapamycin addition (Figure 4C). The latter observation is corroborated by a genome-wide study that analyzed the transcriptional response to rapamycin in yeast. BLM10 was found to be among the genes most strongly induced by rapamycin (Hardwick et al., 1999). Blm10 forms a proteasome subpopulation in log phase (Schmidt et al., 2005). Because the expression of proteasomal genes is less responsive to rapamycin, the divergent regulation of proteasome subunits versus BLM10 expression might indicate a redistribution of proteasome populations during nutrient depletion.
Blm10-proteasomes participate in RP gene repression via degradation of the transcriptional activator Sfp1
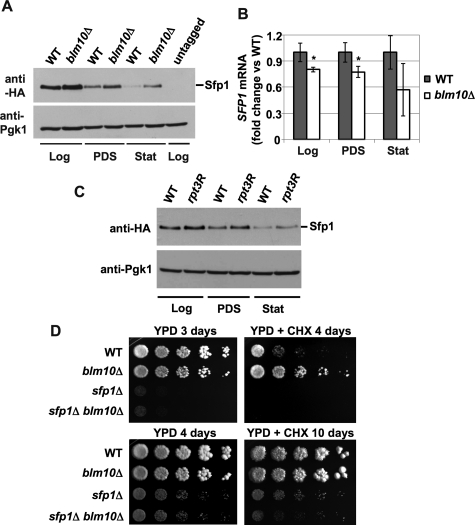
We reasoned that the elevated RP mRNA levels upon nutrient depletion in blm10Δ cells might originate from inefficient proteasome-mediated elimination of transcriptional activators during nutrient deprivation. Such a model is supported by reports that demonstrate that proteasomes participate in the regulation of transcription through the degradation of transcriptional activators (Lipford and Deshaies, 2003; Collins and Tansey, 2006). An important transcriptional activator for sustained RP gene transcription during logarithmic growth is Sfp1 (Marion et al., 2004; Lempiainen et al., 2009; Singh and Tyers, 2009). We tested the steady-state levels of Sfp1 in the different metabolic phases in WT cells. A strong reduction of Sfp1 protein levels was apparent in WT cells after the diauxic shift and in stat phase (Figure 5A), indicating that Sfp1 function might be regulated at the protein level. In blm10Δ cells increased protein levels of Sfp1 were found in all metabolic phases analyzed as compared with WT (log, PDS, and stat) (Figure 5A), suggesting a function for Blm10 in proteasome-mediated turnover of Sfp1. To rule out that BLM10 deletion results in elevated SFP1 transcription, which could explain an increase in Sfp1 abundance, we tested SFP1 mRNA levels and found them largely unaffected or even reduced by BLM10 deletion (Figure 5B). Thus the elevated Sfp1 protein levels observed upon BLM10 deletion are likely caused by a defect in protein turnover. To investigate whether Sfp1 stabilization is a general consequence of proteasome dysfunction, we tested the steady-state level of Sfp1 in the proteasomal ATPase mutant (rpt3R; Rubin et al., 1998). As with BLM10 deletion, loss of the ATPase activity of Rpt3 results in elevated Sfp1 levels (Figure 5C).
FIGURE 5:
Deletion of BLM10 results in elevated Sfp1 levels. (A) Sfp1 steady-state levels are increased in BLM10-deleted cells. SFP1-HA3 (yMS908) and SFP1-HA3 blm10Δ (yMS909) strains were grown to the different metabolic phases in YPD and lysed. Equal protein amounts were separated by SDS–PAGE and subjected to immunodetection with an anti-HA antibody to detect SFP1-HA3 (top). Pgk1 protein levels were used as a loading control (bottom). (B) SFP1 transcription is not significantly altered upon loss of BLM10. WT (yMS524) and BLM10-deleted cells (yMS63) were grown to the different metabolic phases, and SFP1 mRNA levels were determined via qRT-PCR. CT values were normalized to ACT1 expression levels. SFP1 mRNA levels in blm10Δ were normalized to the WT levels. Data are reported as mean ± SEM. A single asterisk indicates a P-value < 0.05; a double asterisk indicates P < 0.01. (C) Sfp1 protein levels are increased in rpt3R mutants. WT (yMS1092) and rpt3R (yMS1093) were grown to the different metabolic phases and analyzed as in Figure 2B. (D) Epistatic genetic interaction between BLM10 and SFP1. Log phase WT (yMS268), blm10Δ (yMS131), sfp1Δ (yMS1011), and sfp1Δ blm10Δ (yMS1012) strains were serially diluted and spotted onto YPD media in the absence (left) or presence of 0.2 μg/ml CHX (right).
The CHX-resistant phenotype of blm10Δ cells is lost upon SFP1 deletion
SFP1-deleted cells exhibit a strong growth defect in the presence of low doses of CHX (Fingerman et al., 2003), while BLM10-deleted cells exhibit CHX resistance (Figures 1A and 5D). To investigate an epistatic relationship between the two genes, we constructed double mutants and tested them for growth on low doses CHX. The growth advantage of blm10Δ in the presence of low doses of CHX is lost in the absence of SFP1 (Figure 5D). Considering the results shown above, we propose that the CHX-resistant phenotype of BLM10-deleted cells originates from Sfp1 stabilization.
Proteasome-mediated Sfp1 degradation requires Blm10
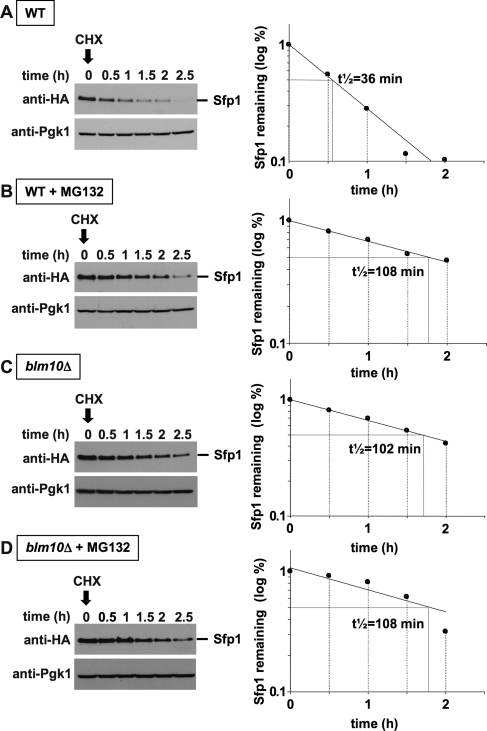
To corroborate that Sfp1 turnover is mediated by proteasomes and is dependent on Blm10, we performed CHX chase experiments (Kornitzer, 2002) in rapidly growing cells. The experimental approach involves lethal doses of CHX, which blocks translation and cell cycle and results in a growth arrest (McCusker and Haber, 1988; Supplemental Figure 2). In the absence of new synthesis, the half-life of a protein can be determined. We found that in WT cells Sfp1 has a short half-life of ∼35 min, indicating continuous turnover of Sfp1 (Figure 6A). In the presence of MG132, a proteasome inhibitor, Sfp1 half-life increased to ∼108 min (Figure 6B). Thus Sfp1 degradation is mediated by the proteasome. A similar increase in Sfp1 half-life was detected in blm10Δ cells, demonstrating that Sfp1 degradation is executed most likely by Blm10-proteasomes (Figure 6C). Because the addition of MG132 did not increase Sfp1 half-life further in BLM10-deleted cells (Figure 6D), our data argue for a model in which Sfp1 degradation might be specifically mediated by Blm10-proteasomes, but not by other proteasome complexes. Although MG132 is readily taken up by yeast cells, it is also rapidly exported from the cell by specific transporters. To investigate the effect of MG132, a PDR5 deletion strain, a gene that codes for an export pump had to be used (Fleming et al., 2002). To ascertain that the lethal CHX dose used for the chase experiment indeed inhibits cell growth in a pdr5Δ strain background, we tested the strains used in Figure 6, A–D, for sensitivity to CHX. The pdr5Δ strains are exquisitely sensitive to CHX, independent of the presence or absence of BLM10 (Supplemental Figure 3).
FIGURE 6:
Proteasomal degradation of Sfp1 requires the activator Blm10. Logarithmically growing SFP1-HA pdr5Δ (yMS957) or SFP1-HA blm10Δ pdr5Δ (yMS958) cells were grown in the absence (A and C) or presence of the proteasome inhibitor MG132 (B and D) for 3 h at 30°C. Subsequently, translation was blocked with 200 μg/ml CHX, and aliquots were harvested and processed at the time points indicated. Equal amounts of protein were subjected to SDS–PAGE, followed by immunodetection with an HA-specific antibody to detect Sfp1 protein levels (left). Pgk1 immunodetection was used as a loading control (bottom). A densitometric analysis of the Sfp1 protein levels is depicted on the right. Sfp1 half-life (t½) was calculated from an exponential decay curve with SigmaPlot 11.0.
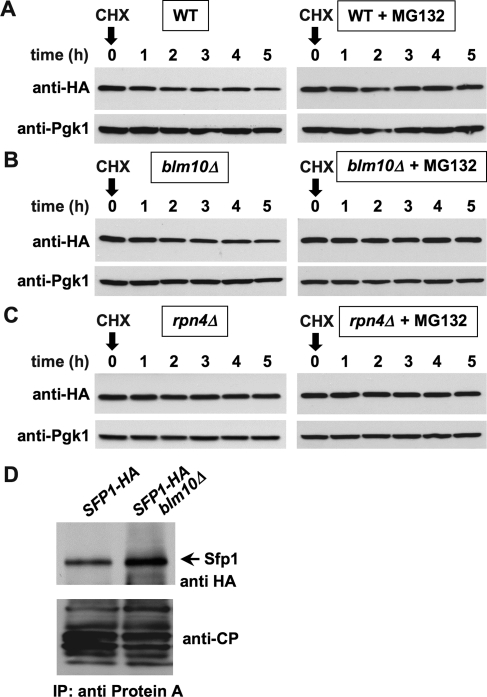
Blm10 has been implicated in proteasome assembly (Fehlker et al., 2003; Li et al., 2007; Marques et al., 2007). In consequence, impaired Sfp1 turnover could potentially be explained by a general impairment of proteasome structural integrity upon loss of BLM10. To compare the proteolytic capacity of proteasomes in WT and blm10Δ cells, we tested the turnover of a general proteasome substrate, Ubc6 (Walter et al., 2001; Ravid et al., 2006). Loss of BLM10 did not influence the turnover of Ubc6 (Figure 7, A and B), whereas deletion of the proteasome-related transcription factor Rpn4, which results in reduced proteasome abundance (Xie and Varshavsky, 2001; Schmidt et al., 2005), effectively inhibited Ubc4 turnover. Thus, in the absence of Blm10, proteasomes are fully functional, arguing against impaired proteasome assembly in blm10Δ cells. The result additionally corroborates a model in which Blm10-proteasomes might target a subgroup of proteasome substrates (Schmidt et al., 2005). To further investigate the interaction between Sfp1 and Blm10-proteasomes, we mapped their physical interaction by CP immunoprecipitation (Figure 7D, right) in the absence or presence of BLM10. We found that Sfp1 copurifies with the proteasome in both WT and blm10Δ cells, indicating that Sfp1 interaction with the proteasome is not mediated by direct interaction of Sfp1 with Blm10. However, in blm10Δ cells the Sfp1 protein level, which copurified with the CP, was elevated, demonstrating that Blm10 positively affects the regulated turnover of Sfp1 at the proteasome.
FIGURE 7:
Loss of BLM10 does not lead to a general impairment of proteasome function (A and B). Turnover of the proteasome substrate Ubc6 was determined in WT (UBC6-HA3 pdr5Δ [yMS792]) or in blm10Δ (UBC6-HA3 blm10Δ pdr5Δ [yMS1089]). (C) Ubc6 is stabilized in rpn4Δ cells. Turnover of the proteasome substrate Ubc6 in rpn4Δ (UBC6-HA3 rpn4Δ pdr5Δ [yMS1364]) strain. Pgk1 immunodetection was used as a loading control (bottom). (D) Sfp1 interacts with Blm10-proteasomes. For CP pull-down cells from yMS1189 and from yMS1190 containing protein A–tagged Pre1 and carrying SFP1–HA3 in the presence (yMS1189) or in the absence (yMS1190) of BLM10 were harvested in logarithmic (log) phase, lysed, and subjected to immune precipitation. The samples were separated by SDS–PAGE and probed with anti-HA (top) or anti-CP (bottom) antibodies.
Loss of BLM10 leads to increased ribosome function
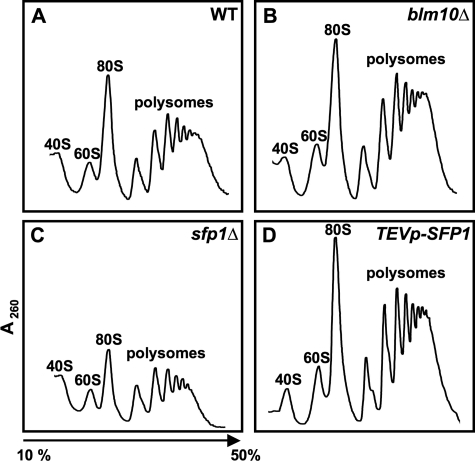
It is well established that changes in Sfp1 levels affect ribosome abundance. The sfp1Δ cells show reduced levels of 80S ribosomes and higher-order polyribosomes compared with WT cells (Fingerman et al., 2003), while elevated Sfp1 level results in increased transcription of RP genes (Jorgensen et al., 2004). To test whether the elevated RP levels we observed upon Sfp1 stabilization in the absence of BLM10 lead to an increase in functional ribosomes, we compared the sucrose gradient profiles of ribosomal subunits and polyribosomes in WT, blm10Δ, sfp1Δ, and SFP1– overexpressing cells. Loss of SFP1 results in a reduced pool of 80S ribosomes and higher-order polyribosomes (Figure 8C), as previously described (Fingerman et al., 2003). Cells overexpressing SFP1, on the other hand, exhibit increased 80S ribosomes and polyribosomes. Consistent with the data reported above, BLM10 deletion results in a profile similar to SFP1 overexpression (Figure 8B).
FIGURE 8:
Polyribosome profiles of cells with varying Sfp1 levels. Polyribosomes profiles were recorded for WT (A), blm10D (B), sfp1D (C), and SFP1–overexpressing cells (TEVpSFP1) (D).
Sfp1 accumulates in the nucleus in BLM10-deleted cells
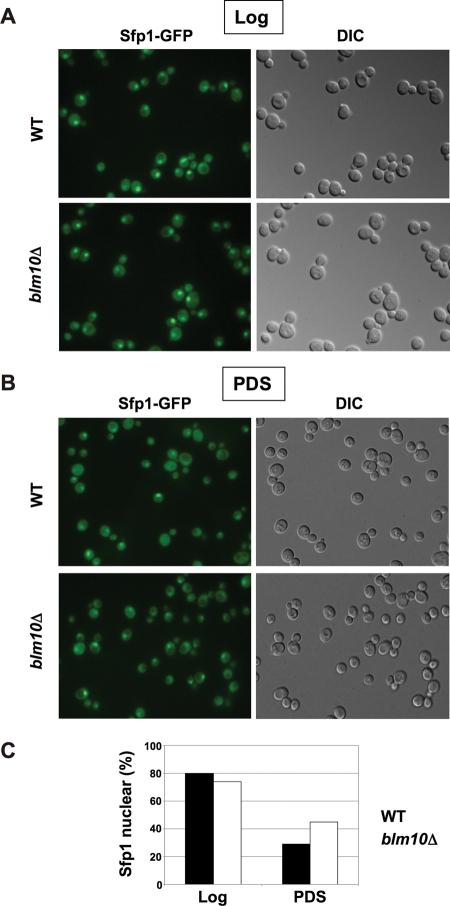
Sfp1 function appears to be controlled by differential localization. While the protein is localized predominantly to the nucleus if nutrients are abundant, it is found evenly distributed in the cell after the diauxic shift or upon rapamycin treatment (Jorgensen et al., 2004; Marion et al., 2004). A current model for the repression of RP gene transcription under nutrient-limiting conditions involves the dissociation of Sfp1 from RP gene promoters, followed by nuclear export of the protein. We demonstrate here that Sfp1 is degraded after the diauxic shift, and if degradation is abrogated due to loss of Blm10 or upon proteasome inhibition, the protein is stabilized and the repression of RP gene transcription is attenuated. The higher levels of RP mRNA in blm10Δ cells suggest that a significant fraction of Sfp1 must remain functional and bound to RP gene promoters even under repressive conditions. To test Sfp1 localization in BLM10-deleted cells, we tagged the C-terminus of Sfp1 with green fluorescent protein (GFP) and performed live-cell fluorescence microscopy. As reported previously in WT cells (Marion et al., 2004; Lempiainen et al., 2009; Singh and Tyers, 2009), the predominant nuclear localization of Sfp1 during log growth (Figure 9A, top) is lost in PDS (Figure 9B, top). In blm10Δ log phase cells, Sfp1 is also detected predominantly in the nucleus (Figure 9A, bottom). In PDS, however, loss of BLM10 results in a high fraction of Sfp1 retained in the nucleus (Figure 9B, bottom, and 9C). The higher Sfp1 levels in blm10Δ cells after the diauxic shift are also reflected in increased intensity of Sfp1-GFP fluorescence if the cells are imaged with identical exposure times (Supplemental Figure 4).
FIGURE 9:
Impaired Sfp1 localization in BLM10-deleted cells after the diauxic shift. (A) SFP1-GFP (yMS928) and SFP1-GFP blm10Δ (yMS929) cells were grown in synthetic complete media. Sfp1 localization was visualized in log phase via live-cell fluorescence. Differential interference contrast (DIC) images are shown on the right. (B) Sfp1 localization in PDS phase cells was analyzed in SFP1-GFP (yMS928) and SFP1-GFP blm10Δ (yMS929) as in (A). (C) Quantification of cells with nuclear Sfp1 localization in log and PDS in WT (yMS928) and blm10Δ (yMS929) from 10 independent fluorescent micrographs with ∼500 cells each, using ImageJ software 1.42q for visualization.
DISCUSSION
Nutrient depletion in S. cerevisiae results in a rapid cellular response characterized by a metabolic switch from fermentation to oxidative metabolism (Herman, 2002). The major targets of this metabolic adaptation are the mitochondria and the ribosomes. While mitochondrial activity is induced after the diauxic shift to improve the efficiency of ATP generation, ribosome biogenesis and function are down-regulated due to the high energy investment in ribosome synthesis and translation (Warner, 1999). Ribosome synthesis requires the expression of three tightly regulated gene clusters: the rRNA genes, the 137 RP genes, and the >200 ribosome biogenesis (Ribi) genes (Jorgensen et al., 2004; Wade et al., 2004). After the diauxic shift, the transcription of these genes is rapidly repressed (Brauer et al., 2005).
In S. cerevisiae the machinery that activates ribosomal gene transcription involves several transcription factors and activators. The repression of ribosomal gene transcription is less well understood. Recent findings indicate that differential localization of Sfp1 mediates the repression of RP genes (Lempiainen et al., 2009; Singh and Tyers, 2009). Sfp1 is a split-zinc finger transcription factor, which might represent the functional analog of c-Myc in yeast (Jorgensen et al., 2004). It was identified as a factor that strongly influences cell size and growth. The underlying reason for the small-cell-size phenotype of sfp1Δ cells is improper regulation of RP and Ribi gene expression (Marion et al., 2004). Under favorable nutrient conditions, Sfp1 is localized to the nucleus and binds to RP gene promoters (Marion et al., 2004; Lempiainen et al., 2009; Singh and Tyers, 2009). Nutrient depletion results in its relocalization to the cytoplasm. Mutations in putative Sfp1 TORC1 phosphorylation sites partially abrogate its nuclear localization (Lempiainen et al., 2009), and nuclear accumulation of Sfp1 is prevented in the presence of rapamycin (Marion et al., 2004; Singh and Tyers, 2009). Thus TORC1-dependent phosphorylation is thought to mediate localization and promoter binding of Sfp1. As loss of SFP1 results in the mislocalization of two other critical factors for RP gene transcription, Fhl1 and Ifh1 (Jorgensen et al., 2004), Sfp1 function and localization appear to play a central role in the regulation of RP gene transcription.
Here we report that in rapidly growing cells Sfp1 has a short half-life of ∼38 min. Sfp1 turnover is impeded by proteasome inhibition or upon loss of the proteasome activator Blm10. Thus Sfp1 levels are regulated by proteasomal degradation in a process that is mediated by Blm10. Loss of BLM10 results in increased RP gene transcription, increased RP levels, increased abundance of active ribosomes, and resistance to low doses of CHX. Furthermore, blm10Δ cells exhibit increased nuclear abundance of Sfp1 during nutrient depletion, suggesting that nuclear export of Sfp1 per se is not sufficient to terminate its function. In summary, our results argue for a functional participation of proteasomes in the repression of ribosome biogenesis during nutrient depletion through degradation of Sfp1. Interestingly, proteasome recruitment to RP genes has been demonstrated recently (Auld et al., 2006). It is therefore tempting to speculate that Sfp1 turnover might occur at the actively transcribed gene, potentially to remove transcriptional complexes after transcription initiation or during the termination of transcription.
The proteasome has different functions in eukaryotic cells: 1) clearance of abnormal proteins to avoid proteotoxicity, 2) a metabolic function by providing building blocks for protein synthesis under starvation conditions, and 3) a regulatory signaling function exerted by the temporally controlled elimination of specific proteins. Ribosomes and proteasomes represent two macromolecular complexes with opposite functions to maintain proteostasis. Thus it could be expected that the first two functions largely determine the relationship between both complexes. Indeed, the proteasome degrades defective ribosomal products (DRiPs) and thus prevents the accumulation of damaged proteins produced by the ribosome (Schubert et al., 2000). Proteasome levels are also up-regulated under starvation conditions to supply amino acids for protein synthesis (Lecker et al., 2006). However, there is increasing evidence that proteasomes also exhibit regulatory functions during ribosome biogenesis. In genome-wide analyses in S. cerevisiae, proteasome subunits were detected at promoters of highly transcribed genes in general, and of RP genes in particular (Auld et al., 2006). Heterozygous proteasomal gene deletions result in a large cell phenotype indicative of increased ribosome function (Jorgensen et al., 2004), which in the light of the present study is most likely caused by impaired Sfp1 degradation. Furthermore, proteasome inhibition in mammalian cells revealed dysregulated ribosome maturation such as the accumulation of 90S preribosomes and altered nucleolar morphology (Stavreva et al., 2006). A significant portion of RPs is degraded by the proteasome before ribosome formation in mammalian cells (Lam et al., 2007). Proteasome-mediated degradation of Sfp1, as reported in this study, adds an additional layer to the complex interaction between the proteasome and ribosome biogenesis and function by providing a regulatory mechanism for the repression of RP gene transcription under nutrient limitation.
The precise impact of Blm10 on proteasome function is unknown. Previous reports suggest that Blm10 might play a role in proteasome assembly (Fehlker et al., 2003) and thus might represent a proteasome chaperone required for correct proteasome biogenesis. Several lines of evidence, however, point to a function beyond maturation. 1) blm10Δ cells do not exhibit assembly defects (Marques et al., 2007). 2) In contrast to the nine identified proteasome assembly chaperones, which either dissociate or are degraded after correct structure formation (Bedford et al., 2010), Blm10 copurifies with mature proteasomes and forms a stable proteasome subpopulation (Schmidt et al., 2005; Lehmann et al., 2008). 3) Different from proteasome chaperones, Blm10 binds to the CP gate region, a critical regulatory entry point for proteasome substrates (Schmidt et al., 2005; Iwanczyk et al., 2006) 4) On Blm10 binding, the CP gate region undergoes structural changes resulting in partial gate opening, as evident from the Blm102–CP crystal structure (Sadre-Bazzaz et al., 2010). 5) Blm10 activates the proteasomal peptidase activity (Schmidt et al., 2005; Iwanczyk et al., 2006; Lehmann et al., 2008; Sadre-Bazzaz et al., 2010). These observations are in line with typical characteristics of proteasome activators, such as the RP or the PA28 activator family.
The data reported here provide additional support for a potential proteasome activator function of Blm10 and suggest that Blm10 promotes the degradation of specific proteasome substrates. Loss of BLM10 results in the stabilization of Sfp1. Furthermore, treatment of blm10Δ cells with proteasome inhibitors does not result in additional stabilization of Sfp1, arguing for a Blm10-proteasome–specific mechanism. In contrast, turnover of the proteasome substrate Ubc6 is unaffected by loss of BLM10, while Ubc6 is stabilized in rpn4Δ cells, which are characterized by low proteasome levels. These findings argue for fully functional proteasomes in the absence of Blm10 and against a major role of Blm10 in proteasome assembly.
If Blm10 functions as an activator for proteasomal protein degradation, then the interaction between Blm10 and the CP should involve opening of the CP gate. Indeed, an open or partially open gate has been observed in Blm102–CP cryo-electron microscopy and crystal structures (Iwanczyk et al., 2006; Sadre-Bazzaz et al., 2010). Previous studies suggest two distinct gate-opening mechanisms. C-terminal docking of PA26 is not sufficient for gate opening but requires a second internal loop structure. The C-termini of the RP ATPases, on the other hand, directly structurally alter the gate region to promote gate opening (Rabl et al., 2008). The cocrystal structure of Blm102–CP suggests that Blm10 C-terminal docking has similar consequences at the molecular level for gating as binding of the proteasomal ATPases (Sadre-Bazzaz et al., 2010). Thus alterations within the Blm10 C-terminus might result in disrupted binding or gating, and C-terminal mutants might exhibit a loss-of-function phenotype. We addressed this hypothesis with a phenotypic characterization of Blm10 C-terminal mutants. They fall in two classes. C-terminal mutants, which abrogate CP binding (BLM10ΔC2 and BLM10ΔC3), exhibit a loss-of-function phenotype (CHX resistance), indicating that proteasomal degradation of Sfp1 requires physical interaction of Blm10 with the CP. The second class of C-terminal mutants (BLM10ΔC1 and BLM10PA26C) is proteasome-binding competent yet displays CHX resistance. Although we cannot exclude alternative scenarios, we speculate that the loss-of-function phenotype of both mutants, especially the BLM10PA26C chimera, where the last seven residues have been exchanged against the gating-inactive C-terminal residues of PA26, might reflect impaired gate opening of these Blm10 mutants and thus impaired Sfp1 turnover. In summary, our data provide the first evidence indicating that the physical interaction of Blm10 with the proteasomes might promote the degradation of specific proteasome substrates.
MATERIALS AND METHODS
Strains, media, growth conditions, and chemicals
All strains and plasmids used in this work are listed in Table 1. They were obtained using standard genetic techniques. Unless otherwise noted, strains are isogenic to BY4741 or BY4742 (Brachmann et al., 1998) and are S288C derived. DY93 and DY106 and their parental strain SUB62 were kind gifts from Daniel Finley (Rubin et al., 1998). Complete gene deletion, promoter exchange, or tag integration were constructed at the genomic locus by homologous recombination using standard techniques (Longtine et al., 1998; Goldstein and McCusker, 1999). Primer sequences are available upon request. Unless otherwise noted, strains were grown at 30°C in yeast peptone dextrose (YPD) and were harvested at OD660 nm 1 for log phase cells, at OD660 nm ∼12 for PDS phase cells, and after 5 d for stat phase cells unless otherwise noted. CHX was purchased from Sigma (St. Louis, MO), hygromycin B from Invitrogen (Carlsbad, CA), rapamycin from A. G. Scientific (San Diego, CA), MG132 from Calbiochem (Darmstadt, Germany), and N-succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (Suc-LLVY-AMC) from Bachem (Torrance, CA).
Table 1:
Strains used in this study
| Strain | Genotype | Source |
| SUB62 | Matalys2–801 leu2–3, 2–112 ura3–52 his3-Δ200 trp1-1(am) | Finley et al., 1994 |
| BY4741 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Brachmann et al., 1998 |
| BY4742 | Matα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | Brachmann et al., 1998 |
| DY106 | Matalys2–801 leu2–3, 2–112ura3–52his3-Δ200 trpl-1(am)rpt1K256S | Rubin et al., 1998 |
| DY62 | Matalys2–801 leu2–3, 2–112 ura3–52 his3-Δ200 trpl-1(am)rpt2K229RS241F | Rubin et al., 1998 |
| DY93 | Matalys2–801 leu2–3, 2–112 ura3–52 his3-Δ200 trpl-1(am) rpt3K219R | Rubin et al., 1998 |
| yMS31 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 PRE1TEVPROA::HIS3 | Schmidt et al., 2005 |
| yMS63 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 blm10Δ::natMX | Schmidt et al., 2005 |
| yMS94 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 PRE1TEVPROA::HIS3 blm10Δ::natMX | Schmidt et al., 2005 |
| yMS131 | Matα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 blm10Δ::natMX | This study |
| yMS222 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ubp6Δ::KanMX | This study |
| yMS268 | Matα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | This study |
| yMS524 | Matahis3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | This study |
| yMS565 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 BLM10ΔC1::KanMX | This study |
| yMS566 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 BLM10ΔC2::KanMX | This study |
| yMS567 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 BLM10ΔC3::KanMX | This study |
| yMS568 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 PRE1TEVPROA::HIS3BLM10ΔC1::KanMX | This study |
| yMS569 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 PRE1TEVPROA::HIS3BLM10ΔC2::KanMX | This study |
| yMS570 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 PRE1TEVPROA::HIS3BLM10ΔC3::KanMX | This study |
| yMS573 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 PRE1TEVPROA::HIS3BLM10PA26C::KanMX | This study |
| yMS598 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 BLM10PA26C::KanMX | This study |
| yMS792 | Matahis3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 UBC6-HA3::KanMX pdr5Δ::HphMX | This study |
| yMS908 | Matahis3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 SFP1-HA3::KanMX | This study |
| yMS909 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 SFP1-HA3::KanMXblm10Δ::natMX | This study |
| yMS928 | Matα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 SFP1-GFP::KanMX | This study |
| yMS929 | Matα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 SFP1-GFP::KanMXblm10Δ::natMX | This study |
| yMS957 | Matahis3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 SFP1-HA3::KanMXpdr5Δ::HphMX | This study |
| yMS958 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 SFP1-HA3::KanMXblm10Δ::natMXpdr5Δ::HphMX | This study |
| yMS1011 | Matα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 sfp1Δ::KanMX | This study |
| yMS1012 | Matα his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 sfp1Δ::KanMXblm10Δ::natMX | This study |
| yMS1013 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 sfp1Δ::KanMX | This study |
| yMS1089 | Matahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 UBC6-HA3::KanMXpdr5Δ::HphMXblm10Δ::natMX | This study |
| yMS1090 | Matahis3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 kanMX::TEVpSFP1 | This study |
| yMS1092 | Matalys2–801 leu2–3, 2–112 ura3–52 his3-Δ200 trpl-1(am)SFP1-HA3::KanMX | This study |
| yMS1093 | Matalys2–801 leu2–3, 2–112 ura3–52 his3-Δ200 trp1-1 (am) rpt3K219RSFP1-HA3::KanMX | This study |
| yMS1189 | Matα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 SFP1-HA3::KanMXPRE1TEVPROA::HIS3 | This study |
| yMS1190 | Matα his3Δ1 leu2Δ0 ura3Δ0 SFP1-HA3::KanMXPRE1TEVPROA::HIS3blm10Δ::natMX | This study |
| yMS1364 | Matahis3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 UBC6-HA3::KanMXpdr5Δ::HphMX rpn4Δ::natMX | This study |
Blm10-proteasome purification and in situ assay to determine CP peptidase activity
For the purification of WT and mutant Blm10–CP complexes, cells from yMS31, yMS568, yMS569, yMS570, and yMS573 containing protein A–tagged Pre1 were collected and lysed using French press cell disruption. Purification was performed as described previously (Schmidt et al., 2005). Briefly, the cleared lysate was batch incubated with IgG Affinity gel (MP Biomedicals, Solon, OH), and the beads were collected and washed. Proteasome complexes were eluted with tobacco etch virus (TEV) protease (Invitrogen, Carlsbad, CA). Subsequently, the purified complexes were first resolved on 3.5% acrylamide native gels (Schmidt et al., 2005), followed by an in-gel activity assay with the fluorogenic proteasome substrate Suc-LLVY-AMC as described previously and silver staining of the gel (Schmidt et al., 2005).
Phenotypic analysis of gene deletion
Strains were grown overnight in YPD and diluted in 96-well plates to a density of 6 × 106 cells per well followed by fivefold serial dilutions. They were spotted onto YPD plates in the absence or presence of CHX or hygromycin B. The concentration is indicated in the respective figure legend.
Growth curves
Cultures were inoculated overnight in YPD. In the morning the culture was diluted to an OD660 nm = 0.1. Subsequently, four aliquots of 150 μl of the culture were added to an HC2 plate. Growth was recorded in a Bioscreen C MB machine (Growth Curves USA, Piscataway, NJ) for 48 h at 30ºC under continuous shaking and with absorbance readings at 600 nm every 30 min.
Immunoprecipitation
For the proteasome CP pull-down cells (strains yMS1189, yMS1190, and untagged control BY4742) were harvested in log phase, resuspended in lysis buffer (50 mM Tris, pH 8, 50 mM NaCl, 5 mM MgCl2, 1 mM EDTA) supplemented with protease inhibitor cocktail (complete, Roche, Indianapolis, IN), 10 mg/ml pepstatin A and 1 mg/ml antipain, and drop-frozen in liquid nitrogen. Frozen yeast cells were lysed in an MM301 grinding mill (Retsch, Haan, Germany) following the manufacturer’s protocol. Cell extracts were cleared at 11,000 rpm for 30 min at 4°C and filtered through cheesecloth (VWR International, San Diego, CA). The supernatants were mixed with rabbit immunoglobulin G resin (MP Biomedicals, Solon, OH) and incubated for 2 h at 4°C. The resin was collected at 500 rpm for 2 min at 4°C and washed five times with cold lysis buffer, resuspended in 2× Laemmli sample buffer, and boiled for 5 min, and the supernatants were collected by centrifugation (500 rpm, 3 min). The proteins were separated by SDS–PAGE, followed by immunodetection for Sfp1-HA and for CP.
Gel electrophoresis and immunoblotting
Cells from WT and mutant strains were harvested in the respective growth phases and stored at −80°C. Cells were disrupted by alkaline lysis as described previously (Kushnirov, 2000). Protein concentration was determined using a Bradford protein assay (Biorad, Hercules, CA). Equal protein amounts were subjected to SDS–PAGE and immunodetection. Antibodies used were anti-CP (BIOMOL, Plymouth Meeting, PA), anti-Rpl3, and anti-Rpl30, kindly provided by Jonathan Warner, and anti-HA 12C5 (Roche, Indianapolis, IN). Anti–phosphoglycerate kinase 1 (Pgk1) (Invitrogen, Carlsbad, CA) was used as a loading control. Signals were detected via enhanced chemiluminescence using a kit (Pierce, Rockford, IL).
Protein degradation assay (CHX chase)
Protein turnover was determined using a CHX chase assay (Ravid et al., 2006). To analyze proteasome-dependent degradation, log phase cultures were supplemented with 40 μM for Sfp1 and 75 μM for Ubc6 of MG132 or vehicle (dimethyl sulfoxide [DMSO]) for 3 h or 30 min, respectively, at 30ºC before the addition of 0.2 mg/ml CHX. Aliquots were harvested at the times indicated and either immediately frozen at −20ºC (Figure 6) or lysed directly (Figure 7). After alkaline lysis (Kushnirov, 2000), equal protein amounts were subjected to SDS–PAGE and immunodetection using an anti-HA antibody to detect Sfp1 or Ubc6 levels. The immunoblots were scanned and band intensity was quantified using ImageJ 1.42q.
qRT-PCR
Total RNA was isolated after an enzymatic digest of the outer cell wall with zymolase (Seikagaku, Tokyo, Japan) using the RNeasy kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Subsequently, 1 µg RNA was treated with DNAse (Invitrogen), followed by reverse transcription using the High Capacity cDNA kit (Applied Biosystems, Carslbad, CA). TaqMan primers and probes were designed using the software Primer3Plus (Untergasser et al., 2007). The sequences are available upon request. In an Applied Biosystems 7900HT instrument, 5 ng cDNA was subjected to qRT-PCR. The reactions were performed in 40 cycles of a two-step PCR (95°C for 15 s and 60°C for 1 min) after an initial activation with 50°C for 2 min and 95°C for 10 min. Negative controls were run simultaneously for each reaction. Data were analyzed using ABI Prism 7900 SDS 2.1v (Applied Biosystems) software. To compare the relative mRNA expression between the individual genes and the endogenous reference gene ACT1, the comparative threshold cycle (CT) method was used. The amount of target, relative to the reference gene as described in the individual figure legends, is given by 2−ΔΔCT. All reactions were performed in quadruplicates. Error bars indicate the mean ± SEM of at least three independent experiments. Statistical significance of the obtained data was determined with the independent-samples t-test analysis using SPSS version 16 software (SPSS, Chicago, IL). P-values < 0.05 were considered statistically significant.
Polyribosome profile analysis
For polyribosome preparation, 50 ml cultures of BY4741, yMS63, yMS1013, and yMS1090 were grown to log phase (OD660 0.8–1.0), and CHX was added to a final concentration of 100 μg/ml. The cells were chilled immediately on ice. After centrifugation at 5000 rpm for 5 min at 4°C, the cell pellets were washed once with 10 ml ice-cold LHB buffer (0.1 M NaCl, 0.03 M MgCl2, 0.01 M Tris, pH 7.4, 100 μg/ml CHX, 200 μg/ml heparin) and resuspended in 0.5 ml cold LHB buffer. A 700-μl volume of glass beads was added, and the cells were vortexed 1 × 1 min in a BeadBeater. The lysates were spun down briefly to reduce foam and diluted with LHB to a final volume of 1.5 ml. After centrifugation in a microcentrifuge for 10 min at maximum speed and 4°C, A260 was measured, and 10 OD260 units were loaded onto a sucrose gradient (11 ml 10–50% sucrose in 0.05 M Tris-Ac, pH 7.0, 0.05 M NH4Cl, 0.012 M MgCl2). The gradients were centrifuged in a Beckman SW41 rotor at 40,000 rpm for 2.5 h, and A260 of gradient fractions was read using an ISCO UA-5 absorbance detector.
Microscopy
yMS928 and yMS929 carrying C-terminally GFP-tagged Sfp1 were grown overnight in synthetic dextrose at 30ºC to PDS. Cells were either rediluted in the morning to an OD660 nm 0.1 and grown for additional 3 h to obtain log phase cultures or were harvested immediately. Live-cell fluorescence of the strains was monitored using a fluorescence microscope (Olympus BX61) at the Albert Einstein Imaging Facility with a 60× NA 1.4 objective (PlanApo). Fluorescence or differential interference contrast (DIC) images were captured with a cooled CCD camera (Sensicam QE, Cooke, Romulus, MI) using IPlab 4.0 software. Images were processed using ImageJ software 1.42q.
Supplementary Material
Acknowledgments
We thank Jonathan Warner for providing us with antibodies and access to the Bioscreen C MB instrument, Kerri Macintosh for support with the polyribosome profiles, Daniel Finley for yeast mutant strains, and Philip Rommel for technical support. This work was supported by grants from the National Institute of Health GM-084228 to MS and a fellowship from the Spanish Ministerio de Ciencia e Innovación to ADL.
Abbreviations used:
- CHX
cycloheximide
- CP
core particle
- CT
cycle threshold
- DIC
differential interference contrast
- DMSO
dimethyl sulfoxide
- GFP
green fluorescent protein
- HA
hemagglutinin
- logarithmic
log
- PDS
postdiauxic shift
- Pgk1
phosphoglycerate kinase 1
- PKA
cyclic AMP–dependent kinase A
- qRT-PCR
quantitative real-time PCR
- RP
ribosomal protein
- stat
stationary
- Suc-LLVY-AMC
N-succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin
- TEV
tobacco etch virus
- TOR
target of rapamycin
- WT
wild type
- YPD
yeast peptone dextrose
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-04-0352) on January 5, 2011.
REFERENCES
- Auld KL, Brown CR, Casolari JM, Komili S, Silver PA. Genomic association of the proteasome demonstrates overlapping gene regulatory activity with transcription factor substrates. Mol Cell. 2006;21:861–871. doi: 10.1016/j.molcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Bedford L, Paine S, Sheppard PW, Mayer RJ, Roelofs J. Assembly, structure, and function of the 26S proteasome. Trends Cell Biol. 2010;20:391–401. doi: 10.1016/j.tcb.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AB, Decourty L, Badis G, Nehrbass U, Jacquier A, Gadal O. Hmo1 is required for TOR-dependent regulation of ribosomal protein gene transcription. Mol Cell Biol. 2007;27:8015–8026. doi: 10.1128/MCB.01102-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blickwedehl J, Agarwal M, Seong C, Pandita RK, Melendy T, Sung P, Pandita TK, Bangia N. Role for proteasome activator PA200 and postglutamyl proteasome activity in genomic stability. Proc Natl Acad Sci USA. 2008;105:16165–16170.. doi: 10.1073/pnas.0803145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD. Designer deletion strains derived from S. cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 1998;14:115–132. doi: 10.1002/(SICI)1097-0061(19980130)14:2<115::AID-YEA204>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Brauer MJ, Saldanha AJ, Dolinski K, Botstein D. Homeostatic adjustment and metabolic remodeling in glucose-limited yeast cultures. Mol Biol Cell. 2005;16:2503–2517. doi: 10.1091/mbc.E04-11-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool JA, French SL, Johzuka K, Eliason K, Vu L, Dodd JA, Beyer AL, Nomura M. Tor pathway regulates Rrn3p-dependent recruitment of yeast RNA polymerase I to the promoter but does not participate in alteration of the number of active genes. Mol Biol Cell. 2004;15:946–956. doi: 10.1091/mbc.E03-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GA, Tansey WP. The proteasome: a utility tool for transcription? Curr Opin Genet Dev. 2006;16:197–202. doi: 10.1016/j.gde.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Febres DE, Pramanik A, Caton M, Doherty K, McKoy J, Garcia E, Alejo W, Moore CW. The novel BLM3 gene encodes a protein that protects against lethal effects of oxidative damage. Cell Mol Biol (Noisy-le-grand) 2001;47:1149–1162. [PubMed] [Google Scholar]
- Fehlker M, Wendler P, Lehmann A, Enenkel C. Blm3 is part of nascent proteasomes and is involved in a late stage of nuclear proteasome assembly. EMBO Rep. 2003;4:959–963. doi: 10.1038/sj.embor.embor938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingerman I, Nagaraj V, Norris D, Vershon AK. Sfp1 plays a key role in yeast ribosome biogenesis. Eukaryot Cell. 2003;2:1061–1068. doi: 10.1128/EC.2.5.1061-1068.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D, Sadis S, Monia BP, Boucher P, Ecker DJ, Crooke ST, Chau V. Inhibition of proteolysis and cell cycle progression in a multiubiquitination-deficient yeast mutant. Mol Cell Biol. 1994;14:5501–5509. doi: 10.1128/mcb.14.8.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JA, Lightcap ES, Sadis S, Thoroddsen V, Bulawa CE, Blackman RK. Complementary whole-genome technologies reveal the cellular response to proteasome inhibition by PS-341. Proc Natl Acad Sci USA. 2002;99:1461–1466. doi: 10.1073/pnas.032516399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster A, Whitby FG, Hill CP. The pore of activated 20S proteasomes has an ordered 7-fold symmetric conformation. EMBO J. 2003;22:4356–4364. doi: 10.1093/emboj/cdg436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimuro M, Takada H, Saeki Y, Toh-e A, Tanaka K, Yokosawa H. Growth-dependent change of the 26S proteasome in budding yeast. Biochem Biophys Res Commun. 1998;251:818–823. doi: 10.1006/bbrc.1998.9560. [DOI] [PubMed] [Google Scholar]
- Gasch AP, Werner-Washburne M. The genomics of yeast responses to environmental stress and starvation. Funct Integr Genomics. 2002;2:181–192. doi: 10.1007/s10142-002-0058-2. [DOI] [PubMed] [Google Scholar]
- Gerlinger UM, Guckel R, Hoffmann M, Wolf DH, Hilt W. Yeast cycloheximide-resistant crl mutants are proteasome mutants defective in protein degradation. Mol Biol Cell. 1997;8:2487–2499. doi: 10.1091/mbc.8.12.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette TG, Kumar B, Thompson D, Slaughter CA, DeMartino GN. Differential roles of the COOH termini of AAA subunits of PA700 (19 S regulator) in asymmetric assembly and activation of the 26 S proteasome. J Biol Chem. 2008;283:31813–31822. doi: 10.1074/jbc.M805935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AL, McCusker JH. Three new dominant drug resistance cassettes for gene disruption in S. cerevisiae. Yeast. 1999;15:1541–1553. doi: 10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik HD, Huber R. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature. 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- Hanna J, Leggett DS, Finley D. Ubiquitin depletion as a key mediator of toxicity by translational inhibitors. Mol Cell Biol. 2003;23:9251–9261. doi: 10.1128/MCB.23.24.9251-9261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Hathaway NA, Tone Y, Crosas B, Elsasser S, Kirkpatrick DS, Leggett DS, Gygi SP, King RW, Finley D. Deubiquitinating enzyme Ubp6 functions noncatalytically to delay proteasomal degradation. Cell. 2006;127:99–111. doi: 10.1016/j.cell.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Hardwick JS, Kuruvilla FG, Tong JK, Shamji AF, Schreiber SL. Rapamycin-modulated transcription defines the subset of nutrient-sensitive signaling pathways directly controlled by the Tor proteins. Proc Natl Acad Sci USA. 1999;96:14866–14870. doi: 10.1073/pnas.96.26.14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman PK. Stationary phase in yeast. Curr Opin Microbiol. 2002;5:602–607. doi: 10.1016/s1369-5274(02)00377-6. [DOI] [PubMed] [Google Scholar]
- Iwanczyk J, Sadre-Bazzaz K, Ferrell K, Kondrashkina E, Formosa T, Hill CP, Ortega J. Structure of the Blm10–20 S proteasome complex by cryo-electron microscopy. Insights into the mechanism of activation of mature yeast proteasomes. J Mol Biol. 2006;363:648–659. doi: 10.1016/j.jmb.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen P, Rupes I, Sharom JR, Schneper L, Broach JR, Tyers M. A dynamic transcriptional network communicates growth potential to ribosome synthesis and critical cell size. Genes Dev. 2004;18:2491–2505. doi: 10.1101/gad.1228804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khor B, et al. Proteasome activator PA200 is required for normal spermatogenesis. Mol Cell Biol. 2006;26:2999–3007. doi: 10.1128/MCB.26.8.2999-3007.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisselev AF, Callard A, Goldberg AL. Importance of the different proteolytic sites of the proteasome and the efficacy of inhibitors varies with the protein substrate. J Biol Chem. 2006;281:8582–8590. doi: 10.1074/jbc.M509043200. [DOI] [PubMed] [Google Scholar]
- Kornitzer D. Monitoring protein degradation. Methods Enzymol. 2002;351:639–647. doi: 10.1016/s0076-6879(02)51874-7. [DOI] [PubMed] [Google Scholar]
- Kraft C, Deplazes A, Sohrmann M, Peter M. Mature ribosomes are selectively degraded upon starvation by an autophagy pathway requiring the Ubp3p/Bre5p ubiquitin protease. Nat Cell Biol. 2008;10:602–610. doi: 10.1038/ncb1723. [DOI] [PubMed] [Google Scholar]
- Kurepa J, Karangwa C, Duke LS, Smalle JA. Arabidopsis sensitivity to protein synthesis inhibitors depends on 26S proteasome activity. Plant Cell Rep. 2010;29:249–259. doi: 10.1007/s00299-010-0818-8. [DOI] [PubMed] [Google Scholar]
- Kushnirov VV. Rapid and reliable protein extraction from yeast. Yeast. 2000;16:857–860. doi: 10.1002/1097-0061(20000630)16:9<857::AID-YEA561>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lam YW, Lamond AI, Mann M, Andersen JS. Analysis of nucleolar protein dynamics reveals the nuclear degradation of ribosomal proteins. Curr Biol. 2007;17:749–760. doi: 10.1016/j.cub.2007.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- Lehmann A, Jechow K, Enenkel C. Blm10 binds to preactivated proteasome core particles with open gate conformation. EMBO Rep. 2008;9:1237–1243. doi: 10.1038/embor.2008.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempiainen H, Uotila A, Urban J, Dohnal I, Ammerer G, Loewith R, Shore D. Sfp1 interaction with TORC1 and Mrs6 reveals feedback regulation on TOR signaling. Mol Cell. 2009;33:704–716. doi: 10.1016/j.molcel.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Li J, Rechsteiner M. Molecular dissection of the 11S REG (PA28) proteasome activators. Biochimie. 2001;83:373–383. doi: 10.1016/s0300-9084(01)01236-6. [DOI] [PubMed] [Google Scholar]
- Li X, Kusmierczyk AR, Wong P, Emili A, Hochstrasser M. β-Subunit appendages promote 20S proteasome assembly by overcoming an Ump1-dependent checkpoint. EMBO J. 2007;26:2339–2349. doi: 10.1038/sj.emboj.7601681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipford JR, Deshaies RJ. Diverse roles for ubiquitin-dependent proteolysis in transcriptional activation. Nat Cell Biol. 2003;5:845–850. doi: 10.1038/ncb1003-845. [DOI] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in S. cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Marion RM, Regev A, Segal E, Barash Y, Koller D, Friedman N, O’Shea EK. Sfp1 is a stress- and nutrient-sensitive regulator of ribosomal protein gene expression. Proc Natl Acad Sci USA. 2004;101:14315–14322. doi: 10.1073/pnas.0405353101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AJ, Glanemann C, Ramos PC, Dohmen RJ. The C-terminal extension of the β7 subunit and activator complexes stabilize nascent 20 S proteasomes and promote their maturation. J Biol Chem. 2007;282:34869–34876. doi: 10.1074/jbc.M705836200. [DOI] [PubMed] [Google Scholar]
- Martin DE, Soulard A, Hall MN. TOR regulates ribosomal protein gene expression via PKA and the Forkhead transcription factor FHL1. Cell. 2004;119:969–979. doi: 10.1016/j.cell.2004.11.047. [DOI] [PubMed] [Google Scholar]
- McCullock S, Kinard T, McCullough L, Formosa T. blm3-1 is an allele of UBP3, a ubiquitin protease that appears to act during transcription of damaged DNA. J Mol Biol. 2006;363:660–672. doi: 10.1016/j.jmb.2006.08.073. [DOI] [PubMed] [Google Scholar]
- McCusker JH, Haber JE. Cycloheximide-resistant temperature-sensitive lethal mutations of S. cerevisiae. Genetics. 1988;119:303–315. doi: 10.1093/genetics/119.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega J, Heymann JB, Kajava AV, Ustrell V, Rechsteiner M, Steven AC. The axial channel of the 20S proteasome opens upon binding of the PA200 activator. J Mol Biol. 2005;346:1221–1227. doi: 10.1016/j.jmb.2004.12.049. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Powers T, Walter P. Regulation of ribosome biogenesis by the rapamycin-sensitive TOR-signaling pathway in S. cerevisiae. Mol Biol Cell. 1999;10:987–1000. doi: 10.1091/mbc.10.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabl J, Smith DM, Yu Y, Chang SC, Goldberg AL, Cheng Y. Mechanism of gate opening in the 20S proteasome by the proteasomal ATPases. Mol Cell. 2008;30:360–368. doi: 10.1016/j.molcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravid T, Kreft SG, Hochstrasser M. Membrane and soluble substrates of the Doa10 ubiquitin ligase are degraded by distinct pathways. EMBO J. 2006;25:533–543. doi: 10.1038/sj.emboj.7600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechsteiner M, Hill CP. Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends Cell Biol. 2005;15:27–33. doi: 10.1016/j.tcb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Rubin DM, Glickman MH, Larsen CN, Dhruvakumar S, Finley D. Active site mutants in the six regulatory particle ATPases reveal multiple roles for ATP in the proteasome. EMBO J. 1998;17:4909–4919. doi: 10.1093/emboj/17.17.4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra D, Zhao Y, Warner JR. Central role of Ifh1p-Fhl1p interaction in the synthesis of yeast ribosomal proteins. EMBO J. 2005;24:533–542. doi: 10.1038/sj.emboj.7600553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadre-Bazzaz K, Whitby FG, Robinson H, Formosa T, Hill CP. Structure of a Blm10 complex reveals common mechanisms for proteasome binding and gate opening. Mol Cell. 2010;37:728–735. doi: 10.1016/j.molcel.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schawalder SB, Kabani M, Howald I, Choudhury U, Werner M, Shore D. Growth-regulated recruitment of the essential yeast ribosomal protein gene activator Ifh1. Nature. 2004;432:1058–1061. doi: 10.1038/nature03200. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Haas W, Crosas B, Santamaria PG, Gygi SP, Walz T, Finley D. The HEAT repeat protein Blm10 regulates the yeast proteasome by capping the core particle. Nat Struct Mol Biol. 2005;12:294–303. doi: 10.1038/nsmb914. [DOI] [PubMed] [Google Scholar]
- Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- Singh J, Tyers M. A Rab escort protein integrates the secretion system with TOR signaling and ribosome biogenesis. Genes Dev. 2009;23:1944–1958. doi: 10.1101/gad.1804409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Chang SC, Park S, Finley D, Cheng Y, Goldberg AL. Docking of the proteasomal ATPases’ carboxyl termini in the 20S proteasome’s α ring opens the gate for substrate entry. Mol Cell. 2007;27:731–744. doi: 10.1016/j.molcel.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtmueller BM, Ferrell K, Whitby FG, Heroux A, Robinson H, Myszka DG, Hill CP. Structural models for interactions between the 20S proteasome and its PAN/19S activators. J Biol Chem. 2009;285:13–17. doi: 10.1074/jbc.C109.070425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavreva DA, et al. Potential roles for ubiquitin and the proteasome during ribosome biogenesis. Mol Cell Biol. 2006;26:5131–5145. doi: 10.1128/MCB.02227-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JA. Improving on nature: antibiotics that target the ribosome. Curr Opin Microbiol. 2005;8:534–542. doi: 10.1016/j.mib.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007;35:W71–74. doi: 10.1093/nar/gkm306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhya R, Lee J, Willis IM. Maf1 is an essential mediator of diverse signals that repress RNA polymerase III transcription. Mol Cell. 2002;10:1489–1494. doi: 10.1016/s1097-2765(02)00787-6. [DOI] [PubMed] [Google Scholar]
- Ustrell V, Hoffman L, Pratt G, Rechsteiner M. PA200, a nuclear proteasome activator involved in DNA repair. EMBO J. 2002;21:3516–3525. doi: 10.1093/emboj/cdf333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade JT, Hall DB, Struhl K. The transcription factor Ifh1 is a key regulator of yeast ribosomal protein genes. Nature. 2004;432:1054–1058. doi: 10.1038/nature03175. [DOI] [PubMed] [Google Scholar]
- Walter J, Urban J, Volkwein C, Sommer T. Sec61p-independent degradation of the tail-anchored ER membrane protein Ubc6p. EMBO J. 2001;20:3124–3131. doi: 10.1093/emboj/20.12.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JR. The economics of ribosome biosynthesis in yeast. Trends Biochem Sci. 1999;24:437–440. doi: 10.1016/s0968-0004(99)01460-7. [DOI] [PubMed] [Google Scholar]
- Whitby FG, Masters EI, Kramer L, Knowlton JR, Yao Y, Wang CC, Hill CP. Structural basis for the activation of 20S proteasomes by 11S regulators. Nature. 2000;408:115–120. doi: 10.1038/35040607. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Oppliger W, Hall MN. Molecular organization of target of rapamycin complex 2. J Biol Chem. 2005;280:30697–30704. doi: 10.1074/jbc.M505553200. [DOI] [PubMed] [Google Scholar]
- Xie Y, Varshavsky A. RPN4 is a ligand, substrate, and transcriptional regulator of the 26S proteasome: a negative feedback circuit. Proc Natl Acad Sci USA. 2001;98:3056–3061. doi: 10.1073/pnas.071022298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, McIntosh KB, Rudra D, Schawalder S, Shore D, Warner JR. Fine-structure analysis of ribosomal protein gene transcription. Mol Cell Biol. 2006;26:4853–4862. doi: 10.1128/MCB.02367-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.