EPDCs fuse with skeletal myotubes with higher efficiency when compared to MSCs and endothelial cells. Independently from the cell origin, all nuclei recruited inside myotubes express muscle-specific genes. VCAM1 expression in nonmuscle cells is induced by soluble factors secreted by myotubes, and its function is required for fusion to occur.
Abstract
Recent studies have underscored a role for the epicardium as a source of multipotent cells. Here, we investigate the myogenic potential of adult human epicardium-derived cells (EPDCs) and analyze their ability to undergo skeletal myogenesis when cultured with differentiating primary myoblasts. Results are compared to those obtained with mesenchymal stromal cells (MSCs) and with endothelial cells, another mesodermal derivative. We demonstrate that EPDCs spontaneously fuse with pre-existing myotubes with an efficiency that is significantly higher than that of other cells. Although at a low frequency, endothelial cells may also contribute to myotube formation. In all cases analyzed, after entering the myotube, nonmuscle nuclei are reprogrammed to express muscle-specific genes. The fusion competence of nonmyogenic cells in vitro parallels their ability to reconstitute dystrophin expression in mdx mice. We additionally show that vascular cell adhesion molecule 1 (VCAM1) expression levels of nonmuscle cells are modulated by soluble factors secreted by skeletal myoblasts and that VCAM1 function is required for fusion to occur. Finally, treatment with interleukin (IL)-4 or IL-13, two cytokines released by differentiating myotubes, increases VCAM1 expression and enhances the rate of fusion of EPDCs and MSCs, but not that of endothelial cells.
INTRODUCTION
Cell therapy is among the therapeutic approaches that can be pursued to treat muscle degenerative disorders such as muscular dystrophy. Satellite cells and their myoblast progeny have the intrinsic capacity to repair multinucleated myofibers and constitute a natural reservoir of muscle precursors. Probably due to the continuous cycles of degeneration and regeneration, the proliferation potential of satellite cells from Duchenne muscular dystrophy patients is reduced. Hence, alternative cells with myogenic capacity that can be easily harvested are being searched for as candidates for cell therapy interventions.
Skeletal muscle cells inherently rely on cell fusion to generate functional tissue and may recruit non myogenic cells into cellular syncytia. Contribution by nonmuscle cells to regenerating myofibers may be determined either by autonomous transdifferentiation toward the skeletal muscle phenotype or by fusion with pre-existing skeletal myotubes. Therefore, cells able to fuse with pre-existing muscle fibers may also provide a potent means for delivering molecules to skeletal muscle tissues.
The epicardium, which comprises the outermost layer of the heart and consists of mesothelial cells, is derived from a temporary structure of precursor cells with different developmental potentials termed the proepicardium. During embryogenesis, cells from the proepicardium emigrate toward the myocardial surface to form the epicardium. A fraction of epicardial cells, referred to as epicardium-derived cells (EPDCs) then undergoes an epithelial-to-mesenchymal transition (EMT) and invades the heart, giving rise to endothelial cells, coronary smooth muscle cells, fibroblasts, as well as cells of the atrioventricular cushions. In addition, recent data show that epicardial cells also make a substantial contribution to myocytes in the ventricular septum and the atrial and ventricular walls (Cai et al., 2008; Zhou et al., 2008), although the evidence for such contribution has been recently questioned (Christoffels et al., 2009). EPDCs can be derived from adult human atrial appendages or epicardial adipose tissue (AT) biopsies and easily amplified in vitro. Cultured adult EPDCs are still able to undergo EMT and may differentiate into smooth muscle-like cells in vitro (Wada et al., 2003; van Tuyn et al., 2007) and in vivo (Limana et al., 2007, 2009; Winter et al., 2007), suggesting that these cells may retain at least part of the differentiation capacity present during embryonic development.
Previous data indicate that mesenchymal stromal cells (MSCs) derived from bone marrow (BM-MSCs) or AT (AT-MSCs) are able to generate hybrid myotubes when cocultured with differentiating myoblasts and may contribute to myofiber formation by fusing with skeletal myotubes (Schulze et al., 2005; Di Rocco et al., 2006). EPDCs in the mesenchymal status share with MSCs the ability to differentiate into smooth muscle cells and osteoblasts, when grown with specific inductive media (van Tuyn et al., 2007). Whether EPDCs can also convert to the skeletal muscle phenotype has never been investigated.
The intrinsic plasticity of EPDCs prompted us to investigate whether human EPDCs, similarly to MSCs, could be recruited from myogenic cells to form skeletal myotubes and whether they could be effectively induced to differentiate toward the myogenic lineage. Results were compared to those obtained with AT-MSCs, which were used as a positive control, and to a distinct mesoderm-derived lineage, endothelial cells. Our study, exploiting the formation of hybrid myotubes with human nonmuscle and murine myogenic cells, identifies EPDCs as a new cell type able to efficiently fuse with skeletal myotubes and provide some new hints on the process leading to the incorporation of nonmuscle cells into skeletal muscle.
RESULTS
EPDCs efficiently contribute to myotube formation when cocultured with myogenic cells
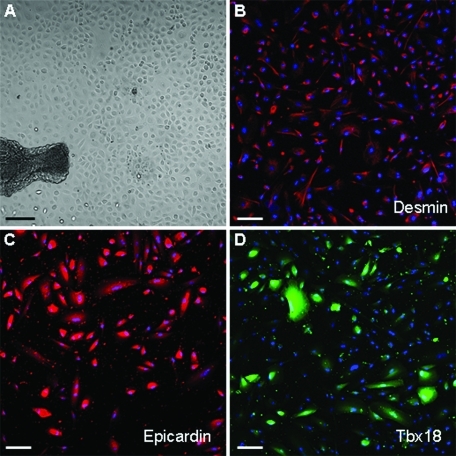
In vitro culture of epicardium dissected from human adult atrial appendages results in the outgrowth of cells with an epithelioid morphology that expresses desmin (Figure 1, A and B). After the second culturing passage, EPDCs lose the epithelial morphology and adopt a looser, mesenchymal-like appearance, suggestive of an EMT. Nonetheless, they maintain an epicardial phenotype, as indicated by the expression of the epicardial markers epicardin and Tbx18 (Figure 1, C and D).
FIGURE 1:
Characterization of cultured EPDCs. Top: EPDCs at P0, just after the migration from the explant in bright field image (A) and stained with an antibody against desmin (red, B). Bottom: EPDCs at P1, stained with antibodies against the epicardial markers Epicardin (red, C) and Tbx18 (green, D). Nuclei are stained with Hoechst (blue). Magnification: A, 10×, Bar: 100 μm; B–D, 20×, Bars: 50 μm.
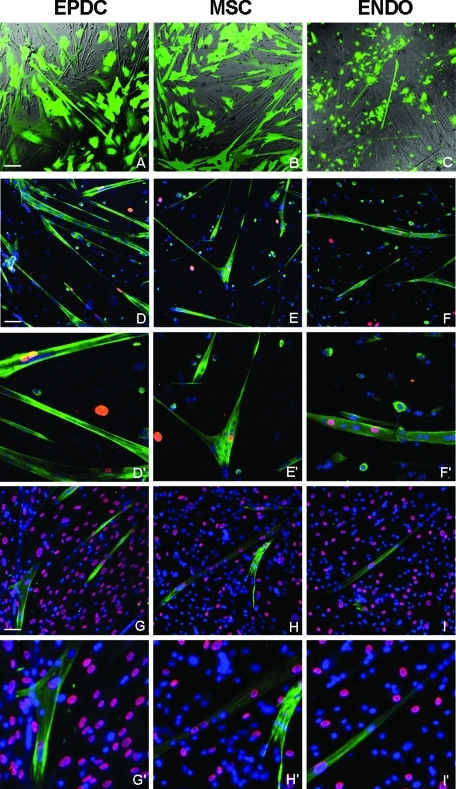
To test whether EPDCs can be directed toward a skeletal muscle phenotype, we performed experiments in which EPDCs, previously infected with a lentiviral vector harboring a green fluorescent protein (GFP) expression cassette, were cocultured with equal amounts of unlabeled differentiating mouse primary myoblasts in an 80% confluent plate. Surprisingly, after 48 h in reduced-serum differentiation medium (DM) many GFP+ contractile multinucleated cells were detected, indicating that EPDCs had been recruited to form skeletal myotubes (Figure 2A). The number of GFP+ myotubes corresponded approximately to 12% (12 ± 2%) of total myotubes, as calculated from three different experiments. When the GFP-labeled AT-MSCs were used, numerous GFP+ myotubes were also observed reaching a maximum of 5%. When GFP-labeled, endometrium-derived primary endothelial cells were used in the coculture, however, the number of GFP+ myotubes was markedly reduced compared to the other two cell types, and their number never exceeded 0.2% of total myotubes. To more precisely determine and compare the number of human nuclei incorporated into skeletal myotubes in the three cell types, coculture experiments with mouse myoblasts were performed with unlabeled human cells and by plating at lower (30%) density. Cells were then stained with an antibody against Troponin T (TnT) to identify myotubes and with an antibody specific for human laminA/C, to identify human nuclei (Figure 2, D–F). In all three cases it was possible to detect skeletal myotubes containing from 1 to 3 human nuclei together with several mouse nuclei. The fusion index, calculated as the ratio between the number of human nuclei inside myotubes and the total number of myotubes, was different, however, for the three cell types and was 11.12% ± 4.23% for EPDCs, 5.06% ± 3.22% for AT-MSCs, and 0.20% ± 0.04% for endothelial cells.
FIGURE 2:
Human EPDCs, MSCs, and endothelial cells are incorporated into mouse skeletal myotubes and are reprogrammed to express human skeletal muscle specific genes. Adult EPDCs were cocultured with differentiating mouse primary myoblasts. Equal numbers of MSCs and endothelial cells were used for comparison. (A–C) Combined bright-field and green fluorescent protein (GFP) fluorescent (green) images of living cells. Human cells were labeled with a lentiviral vector expressing GFP. Several GFP-labeled multinucleated myotubes, indicating that human cells are participating in myotube formation, are visible with all three cell types. The number of green myotubes is much lower in the case of endothelial cells compared to EPDCs and MSCs. (D–F) Immunofluorescence of fixed cells labeled with antibodies that recognize both mouse and human Troponin T (hTNT, green) but only human LaminA/C (red). Several hybrid myotubes containing both human (red) and mouse (Hoechst blue) nuclei are visible. G–I: Fixed cells were stained with antibodies specific for hTnT (green) and human LaminA/C (red). Hoechst (blue) was used to visualize all nuclei. Independently from the cell origin, human nuclei inside hybrid myotubes are phenotypically reprogrammed to express skeletal muscle specific proteins. ENDO: endometrium-derived endothelial cells. Magnification: 20×, Bars: 50 μm. D′–F′, G′– I′: 2× enlarged boxes of D–F and G–I, respectively.
This result suggests that different cell types, although all of mesodermal derivation, display a different propensity to the recruitment by skeletal myotubes. Because myotubes with only human nuclei could not be detected, it could be assumed that the recruitment of EPDCs, as well as of AT-MSCs and endothelial cells, into skeletal myotubes depends on fusion with mouse myotubes and not on cell-autonomous myogenic differentiation. To confirm this point, coculture experiments were performed in Transwell dishes (Corning, Corning, NY), in which myogenic and nonmyogenic cells were separated by a 0.4-μm filter, which does not allow cell mixing. In these conditions, no skeletal muscle differentiation markers were detectable in the tested human cells, indicating that the direct contact with mouse myoblasts is required to induce the expression of myogenic markers (unpublished data).
To test whether the fusion efficiency varies with age or, in the case of MSCs, with the source tissue, we repeated the coculture experiments with cells derived from patients of different ages and with stromal cells extracted from the cardiac biopsy instead of from subcutaneous abdominal fat depots. In some cases we directly compared MSCs and EPDCs extracted from the same biopsy. We observed that, whereas age may actually have an adverse effect on the number of cells that overgrow from the biopsy and on their life span, it does not affect their ability to fuse with myotubes. Also, the fusion efficiency does not vary significantly if MSCs are extracted from subcutaneous AT or derived from cardiac biopsies. In all cases it never reached the level obtained with EPDCs. Finally, we tested different preparation of endometrium-derived endothelial cells as well as commercially available human umbilical venous cord endothelial cells (HUVECs). In all cases the percentage of fused cells was approximately 0.2–0.3%. From our results we can therefore conclude that the high fusion efficiency is an intrinsic property of epicardial cells and that different cell types, although sharing a common mesodermal origin, may have different fusion propensity.
Human nuclei inside myotubes are reprogrammed to express human skeletal muscle–specific genes downstream of MyoD
To test whether human nuclei recruited from mouse myotubes were reprogrammed to express skeletal muscle–specific proteins, coculture plates were immunostained with an antibody against human TnT (hTnT) that recognizes the human but not the mouse protein. Results show that hTnT is synthesized by all myotubes containing human nuclei, independently from their cell origin. As it can be observed in Figure 2, G–I, hTnT staining is always localized in the proximity of human nuclei (identified by the reaction with the anti–human lamin A/C antibody) and fades around mouse nuclei. As expected, no staining was detected when EPDCs, endothelial cells, and AT-MSCs were cultured alone or in plates containing only mouse myotubes. These results show that, independently of cell origin, after entering the myotube nuclei are reprogrammed to express human muscle-specific genes.
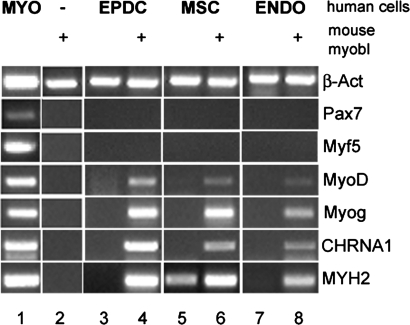
To determine the extent of human nuclear reprogramming by mouse myotubes, coculture experiments with human nonmuscle cells and mouse primary myoblasts were analyzed by reverse transcription–polymerase chain reaction (RT-PCR) with human specific primers for several muscle markers (Figure 3). Results were similar with all three cell types tested. Among the analyzed markers, the expression of the muscle-specific transcription factor MyoD and myogenin, as well as of late differentiation markers, such as the cholinergic nicotinic receptor CHRNA1 and the skeletal muscle myosin heavy chain MYH2, was detected in the presence but not in the absence of mouse myoblasts. A low level of MYH2, but not of other myogenic markers, could be consistently detected for AT-MSCs also in the absence of mouse skeletal muscle cells (Figure 3, lane 5). No human transcripts were detected, however, for early myogenic markers, such as Pax7 and Myf5, suggesting that reprogramming is limited to myogenic markers downstream from the master transcription factor MyoD.
FIGURE 3:
RT-PCR analysis for skeletal muscle markers of human cells cocultured with mouse myoblasts. Human cells were cultured for 48 h in DM in the absence or presence (+) of differentiating mouse primary myoblasts. mRNA expression was evaluated by RT-PCR analysis. With the exception of β-actin (β-act) in lane 2, primers were selected so to be specific for human transcripts only. MYO: human skeletal muscle positive control; ENDO: endometrium-derived endothelial cells; Myog: Myogenin; CHRNA1: cholinergic nicotinic receptor A1; MYH2: adult skeletal myosin heavy chain.
Transplantation of EPDCs and MSCs but not endothelial cells rescues dystrophin expression in mdx mice
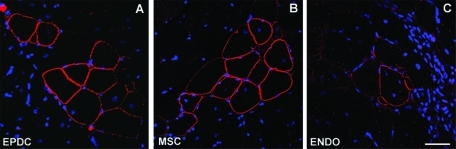
To further assess the myogenic differentiation potential of EPDCs, we wanted to investigate whether EPDCs can be recruited to form skeletal myofibers in vivo. EPDCs cultured for not more than two passages were injected into the tibialis anterior (TA) muscle of pharmacologically immunosuppressed mdx mice. As in the previous experiment, AT-MSCs and endothelial cells at P2 were used for comparison. Ten days after injection, less than 0.1% dystrophin-positive fibers, corresponding to spontaneously revertant fibers, were observed in sections from age-matched phosphate-buffered saline (PBS)-injected mice. Conversely, dystrophin was detected in up to 10% (10.3 ± 2.3% in three different experiments) of the myofibers analyzed, corresponding to 1% of the entire area, on sections from EPDC-transplanted muscles, as revealed by immunofluorescence with an antibody against the C-terminal portion of mouse dystrophin (Figure 4). Dystrophin-positive fibers were organized in clusters, suggesting clonal proliferation of donor cells. Analogous results, although with a reduced number of dystrophin-positive fibers (5.7 ± 1.5%, approximately 0.5% of the entire area), were obtained with AT-MSCs. Conversely, with endothelial cells the number of dystrophin-positive fibers was never higher than that of spontaneous revertants, corresponding to 0.1 ± 0.05% fibers (<0.01% of the area) in the 2-mo-old mdx mice used for our experiments.
FIGURE 4:
Transplantation of EPDCs and MSCs but not endothelial cells rescues dystrophin expression in mdx mice. TA transverse sections from transplanted mdx mice. Staining for dystrophin (red) was performed 7 d after injection. Nuclei are visualized by Hoechst (blue). Several clusters of dystrophin-positive fibers can be detected in mice that received injections with EPDCs and MSCs, whereas the number of dystrophin-positive fibers detected with endothelial cells (ENDO) does not goes beyond the number of revertant fibers present in uninjected controls. Magnification: 40×, Bar: 25 μm.
These results show that administration of EPDCs effectively induces the formation of dystrophin-expressing muscle fibers in mdx mice with a twofold efficiency compared to that of MSCs.
VCAM1 expression levels in human cells correlate with their fusion propensity and are modulated by soluble factors secreted by differentiating myoblasts
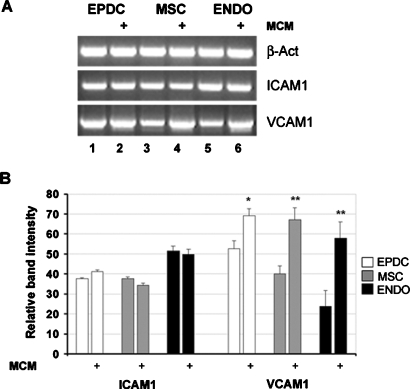
To understand the basis for the differential fusion ability of distinct cell types, we searched for fusion-related molecules that are not specific to the skeletal muscle phenotype and that could be differentially expressed in the three cell types. Several adhesion molecules are known to be involved in cell fusion processes. Among those, the α4 integrin receptor vascular cell adhesion molecule 1 (VCAM1) has been reported to have a specific role in myotube formation. VCAM1 (but not α4 integrin) is expressed in satellite cells and mononucleated myoblasts and it is required for their adhesion and/or fusion to myotubes (Rosen et al., 1992). By semiquantitative RT-PCR analysis we found that in both growth medium (GM) and DM culture conditions (Figures 5 and 6, respectively), VCAM1 expression levels effectively differ in the three cell types and correlate with their fusion propensity. In fact, EPDCs express relatively high VCAM1 levels, approximately twice as high as AT-MSC, whereas endothelial cells express only basal levels, in particular when cultured in DM (Figure 6, compare lanes 1, 4, and 7).
FIGURE 5:
VCAM1 is expressed in nonmuscle human cells and is positively modulated in the presence of myoblast-conditioned medium. Human cells were cultured in GM on 0.4-μm transwell filters for 48 h in the absence or presence (+) of mouse differentiating myoblasts seeded at confluence in the lower chamber. mRNA expression was evaluated by semiquantitative RT-PCR analysis. PCR products were subjected to electrophoresis in agarose gel, visualized by ethidium bromide staining (A), and quantified by densitometric computer software after normalization for β-actin (B). A representative experiment is provided in (A). Values are the mean ± standard error of the mean (SEM) of three separate experiments. *p < 0.05; **p < 0.005.
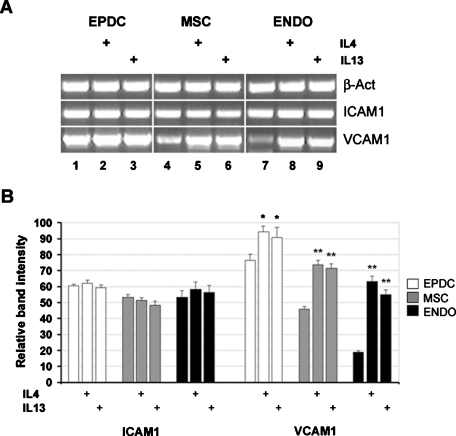
FIGURE 6:
IL-4 and IL-13 induce VCAM1 expression. Human cells were cultured for 48 h in DM in the absence or presence (+) of IL-4 or IL-13. mRNA expression was evaluated by semiquantitative RT-PCR analysis. PCR products were visualized by ethidium bromide staining on agarose gels (A) and quantified by densitometric computer software after normalization for β-actin (B). A representative experiment is provided in (A). Values are the mean ± SEM of three separate experiments. *p < 0.05; **p < 0.005.
Interestingly, in all three cell types the level of VCAM1 is significantly increased by the presence of mouse myoblast-conditioned medium (MCM) as demonstrated by coculture experiments conducted on Transwell dishes (Figure 5, lanes 2, 4, and 6). The level of VCAM1 induction is approximately twofold (100% increase) in AT-MSCs and endothelium, whereas it is weaker (approximately 20% increase) in EPDCs, probably because EPDCs express an already high basal level of VCAM1 messenger RNA (mRNA). In parallel we analyzed the expression of ICAM1, a VCAM1-related adhesion molecule at least so far not known to be involved in fusion processes. Contrarily to VCAM1, the expression level of ICAM1 is similar in all cell types tested and is not modulated by MCM (Figure 5).
These results indicate that VCAM1 expression is induced by soluble factors secreted by differentiating myoblasts. Data in the literature demonstrate that IL-4 is secreted by nascent myotubes and acts as a recruitment factor for myoblasts. The addition of IL-4, or of the common receptor ligand IL-13, to differentiating mouse myoblasts has a direct effect on myotube size, determining an increment on the number of nuclei per myotube (Horsley et al., 2003). VCAM1 expression is induced by both IL-4 and IL-13 in endothelial cells (Bochner et al., 1995). Moreover, we have evidence that treatment with IL-4 or IL-13 positively modulates VCAM1 mRNA level in human myoblasts, suggesting that the modulatory effect of those cytokines on cell fusion may be mediated at least in part by VCAM1 (Gentile and Di Rocco, unpublished data). We therefore tested whether IL-4 and IL-13 similarly induce VCAM1 expression in EPDCs and AT-MSCs. To mimic the coculture conditions, cells were cultured in the presence of IL-4 or IL-13 for 48 h in DM. Treatment with either one of the human recombinant proteins effectively increases the mRNA level of VCAM1 in all cell types tested (Figure 6). The fold induction is again reduced (but statistically significant) in EPDCs, also in this case probably because these cells express already much higher levels of VCAM1 mRNA in the absence of treatment. Similar to what was observed in the presence of MCM, ICAM1 expression is not modulated by IL-4 or IL-13 treatment.
These results indicate that the VCAM1 induction observed with MCM is mimicked by IL-4 and IL-13, which are both known to be secreted by differentiating myoblasts (Horsley et al., 2003; Jacquemin et al., 2007). It must be noted, however, that IL-4 is species-specific, so that the mouse protein does not bind to the human receptor. In fact, the addition of mouse recombinant IL-4 in the culture medium does not elicit the same VCAM1 response obtained with the human molecule. As a consequence, VCAM1 induction in human cells by mouse MCM cannot be mediated by IL-4 secreted by mouse myotubes: IL-13, or other cross-species reactive soluble factors, must be responsible for the observed effect.
IL-4 and IL-13 positively modulate myotube recruitment of EPDCs and AT-MSCs but not endothelial cells
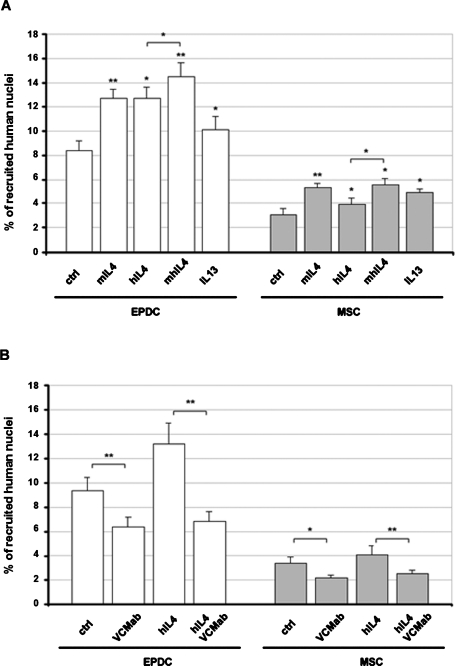
It has been reported that fusion of mouse BM-MSCs to differentiating skeletal muscle cells is increased in the presence of exogenously added IL-4 (Schulze et al., 2005). To test whether the same applies to human cells, we performed coculture experiments in the presence of IL-4 or IL-13. The addition of either one of the two cytokines did not significantly affect the fusion with myotubes of endothelial cells (unpublished data). In contrast, treatment with human IL-4 or with the cross-species reactive mouse IL-13, determined an increment of the number of fusion events for both EPDCs and AT-MSCs (Figure 7A). In particular, IL-4 increased the percentage of fusion by approximately 35% and 15% in EPDCs and AT-MSCs, respectively, whereas IL-13 raised the percentage by 20% in EPDCs and 30% in AT-MSCs (Figure 7A). Interestingly, treatment with mouse recombinant IL-4 also significantly enhanced the fusion rate to similar (in the case of EPDCs) or even higher (in the case of AT-MSCs) levels when compared to the human protein. Moreover, if the two proteins (human and mouse IL-4) are added together, their effect is additive. Because the mouse protein may only act on mouse cells, its effect on human cell recruitment has to be indirect. These results suggest that additional mechanisms, probably acting also on recruiting myotubes and not only directly on recruited cells, may mediate the effects of IL-4 on myotube growth.
FIGURE 7:
Recruitment of EPDCs and MSCs inside myotubes is stimulated by IL-4 and IL-13 while it is inhibited by VCAM1 blocking antibody. Human cells were cocultured with differentiating primary mouse myoblasts in presence of the indicated molecules for 2 d in DM. hIL-4: human recombinant IL-4; mIL-4: mouse recombinant IL-4; mhIL-4: mouse plus human IL-4; VCMab: specific anti–human VCAM1 antibody. The percentage of human nuclei inside myotubes was determined as the ratio between human lamin A/C-labeled nuclei inside TnT-labeled myotubes and the total number of human nuclei. Values are the mean ± SEM of three separate experiments. *p < 0.05; **p < 0.005.
Blockade of VCAM1 function inhibits recruitment of EPDCs and AT-MSCs inside myotubes and abrogates the stimulatory effect of IL-4
To test whether VCAM1 is necessary for the fusion of nonmuscle cells to skeletal muscle, cocultures of human cells with murine primary myoblasts were carried out in the presence of a blocking anti-VCAM1 antibody that specifically recognizes the human but not the mouse protein. As expected, and contrary to what happens with an antibody directed toward the mouse protein, the use of a human-specific antibody did not change the fusion index as well as the average myonuclear number of mouse muscle cells. The number of human nuclei recruited by mouse myotubes, however, was considerably decreased by approximately 30% for EPDCs and AT-MSCs (Figure 7B). In addition, with both cell types, blocking of VCAM1 function almost totally quenched the stimulatory effect of IL-4 (Figure 7B). The recruitment of endothelial cells seemed to be negatively affected as well, however, due probably to the extremely low number of fusion events. Results obtained were never statistically significant (unpublished data). All together these results show that, at least in the case of EPDCs and MSCs, VCAM1 is involved in the fusion of nonmuscle cells with skeletal myotubes. More importantly, they suggest that the increment of VCAM1 expression possibly mediates the positive effects of IL-4 on cell recruitment inside multinucleated myotubes.
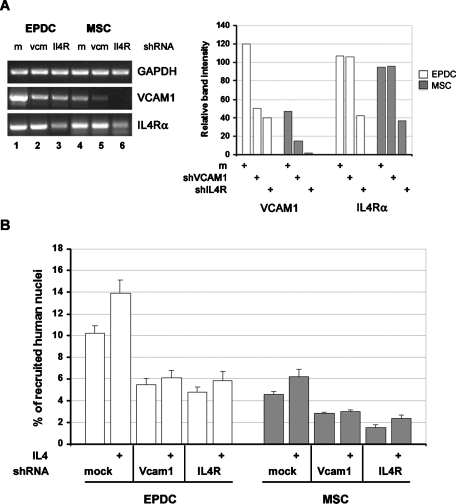
Knockdown of VCAM1 and IL-4Rα expression confirms their positive and connected role in the fusion process
To exclude possible unspecific effects of the anti-VCAM1 antibody, we performed coculture experiments with EPDCs and AT-MSCs in which VCAM1 expression was knocked down by the use of specific short hairpin RNA (shRNA). As an additional control, parallel experiments were also performed with cells where the expression of IL-4 receptor α (IL-4Rα), which mediates both IL-4 and IL-13 signaling, had been suppressed. The inhibition of RNA transcription by shRNA was evaluated by RT-PCR (Figure 8A). VCAM1 and IL-4Rα expression were reduced by approximately 65% by their specific shRNAs in both cell types (Figure 8A). In addition, whereas, as expected, IL-4Rα expression was not affected by VCAM1 suppression, VCAM1 expression was robustly reduced in IL-4Rα knockout cells, indicating that even basal levels of VCAM1 mRNA require IL-4Rα signaling (Figure 8A, lanes 3 and 6). Such reduction was also nicely confirmed by immunostaining for VCAM1 on silenced cells (Supplemental Figure 1). Consistent with the results obtained with the neutralizing anti-VCAM1 antibody, suppression of VCAM1 expression in human nonmuscle cells inhibits their rate of fusion with mouse myotubes roughly by 50% and abrogates the inductive effect of IL-4 addition (Figure 8B). Moreover, similar results were obtained when IL-4Rα expression was suppressed. All together these results not only confirm the role of VCAM1 and IL-4R signaling in the fusion process, but also strengthen the new concept that VCAM1 is a mediator of IL-4/IL-13 fusion-promoting effect.
FIGURE 8:
mRNA silencing of VCAM1 or IL-4Rα inhibits fusion of EPDCs and MSCs with myotubes. Human cells were infected with lentiviral vectors encoding for specific shRNA against VCAM1 or IL-4Rα. mRNA expression was evaluated by semiquantitative RT-PCR analysis and normalized for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (A). Cells were then cocultured with differentiating primary mouse myoblasts in the absence or presence of IL-4 for 2 d in DM. The percentage of human nuclei inside myotubes was determined as the ratio between human lamin A/C-labeled nuclei inside TnT-labeled myotubes and the total number of human nuclei (B). Vcm: VCAM1; Il4R: IL4Rα. Values are the mean ± SEM of three separate experiments. Values of p between each test sample compared to its own control were all < 0.005.
Differential expression of fusion-related genes
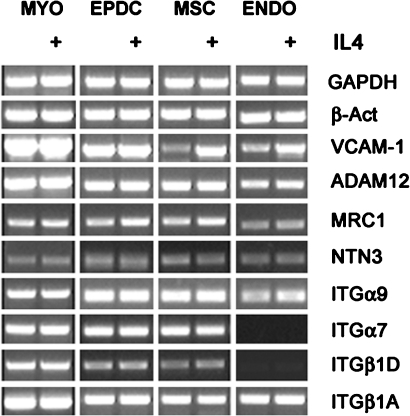
One possibility for the extremely low fusion competence of endothelial cells could be that they fail to express some fusion-related genes when compared to fusion-competent cells such as EPDCs and MSCs. To verify such a hypothesis, we conducted a mini-screening by RT-PCR for the expression of various genes known to be involved in the fusion process and at the same time tested whether they are modulated by IL-4. Those genes include the metalloprotease ADAM12, the mannose receptor (MRC1), Netrin3 (NTN3), the integrin chains α9 (ITGα9) and the isoform A and D of integrin β1 (ITGβ1A and ITGβ1D) (Schwander et al., 2003; Kang et al., 2004; Jansen and Pavlath, 2006, 2008; Quach et al., 2009). In addition, we included integrin α7 (ITGα7), which, although it has never been directly reported to be involved in fusion, represents the tissue-specific partner subunit of ITGβ1D (Mayer, 2003). We excluded from the screening molecules 1) those molecules that could act nonautonomously, such as soluble factors, because in a coculture system they could be provided by muscle cells, as well as 2) fusion molecules already known to be expressed in endothelial cells. Interestingly, with the exception of VCAM1, all the tested molecules resulted to be expressed at comparable levels in EPDCs and AT-MSCs (Figure 9). The expression of ITGβ1D and ITGα7, however, was practically undetectable in endothelial cells both in the presence and absence of IL-4. Moreover, apart from a mild inductive effect on MRC1 of approximately 20%, confirmed by normalized densitometry data (unpublished data), none of the analyzed genes seemed to be affected by treatment with IL-4. Thus, the inability to express some fusion-related genes may effectively explain the reduced fusion propensity of endothelial cells.
FIGURE 9:
Expression of fusion-related genes in human nonmuscle cells. Human cells were cultured for 48 h in DM in the absence or presence (+) of IL-4. mRNA expression was evaluated by semiquantitative RT-PCR analysis. Human myoblasts (MYO) were used as positive control. ADAM12: disintegrin and metalloproteinase domain-containing protein 12; MRC1: mannose receptor isoform 1; NTN3: Netrin3.
Dissecting the pathway leading to VCAM1 induction by IL-4 and IL-13
In hematopoietic cells, IL-4 signals can use two different receptor types: the Type I receptor, which is composed of the IL-4Rα and the common γ-chain (γC) subunits, or the Type II receptor, which is composed of the IL-4Rα and IL-13Rα1 subunits. In nonhematopoietic cells, including endothelial cells, fibroblasts, smooth muscle cells, and mesenchymal cells, the γC is not expressed and IL-4 and IL-13 signal exclusively through the common Type II receptor. Both Type I and Type II receptor complexes signal through the Jak/STAT cascade (Jiang et al., 2000). A second mechanism of signal transduction activated by IL-4 and IL-13 leads to the insulin receptor substrate family and the downstream phosphoinositide 3-kinase (PI3K). IL-4 is also known to initiate activation of MEK subfamilies, including ERK1/2, in bronchial smooth muscle and epithelial cells (Kelly-Welch et al., 2003). Finally, more recent data identify P38MAPK as an additional mediator of IL-4R signaling (Canfield et al., 2005; Jang et al., 2009).
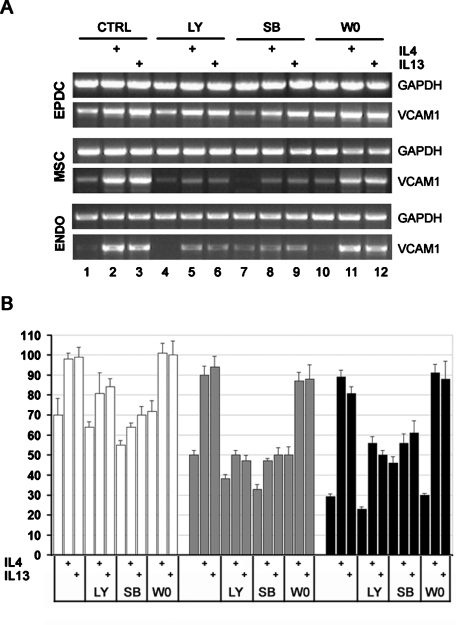
To understand which of these pathways mediates VCAM1 induction by IL-4 and IL-13 in different cell types, we cultured EPDCs, MSCs, and endothelial cells with IL-4 or IL-13 in the presence of specific inhibitors and then evaluated VCAM1 mRNA levels by semiquantitative RT-PCR. Induction of VCAM1 by either IL-4 or IL-13 is blocked by inhibitors of either the PI3K or p38MAPK pathways (LY294002 and SB203580, respectively; Figure 10). The effect is weak but consistent in EPDCs, where VCAM1 has higher basal levels, and more pronounced in MSCs and endothelial cells, as is evidenced by normalized densitometric data (Figure 10B). It has been suggested, but not conclusively demonstrated, that in endothelial cells, stimulation of VCAM1 expression by IL-4/IL-13 may be mediated by the activation of Jak/2Stat6 (Palmer-Crocker et al., 1996). We therefore tested the effect of a potent Jak2 inhibitor, W0894, on VCAM1 induction. Surprisingly, both basal and induced VCAM1 mRNA levels were unaffected by treatment with W0894 in all cell types tested (Figure 10), suggesting that the activation of Jak2 is not required for IL-4R–mediated VCAM1 induction. Finally, VCAM1 induction was unchanged by treatment with the ERK1/2 pathway inhibitor PD98059 in all cell type tested (unpublished data).
FIGURE 10:
VCAM1 induction by IL-4/IL-13 requires the PI3K and P38MAPK pathways. EPDCs, MSCs, and endothelial cells were cultured with IL-4 or IL-13 in the presence of specific pathway inhibitors. VCAM1 mRNA expression levels were evaluated by semiquantitative RT-PCR. LY: LY294002 for PI3K; SB: SB202190 for P38 MAPK; W0: W0894 for Jak2. Values are the mean ± SEM of three separate experiments. P values between each test sample compared to its own control were all < 0.005.
DISCUSSION
Cells able to fuse with pre-existing muscle fibers, possibly genetically engineered, may provide a potent means for delivering proteins to diseased skeletal muscle tissues. This study identifies the mesothelium-derived EPDCs as a new cell type able to efficiently fuse with skeletal myotubes. Taking advantage of the formation of hybrid myotubes by human nonmuscle and murine myogenic cells, we estimated that the percentage of epicardial nuclei inside myotubes after 48 h of coculture in DM reaches an average of 10% of total nuclei input. This percentage is particularly high, considering that with MSCs obtained from the same or other sources it never goes beyond 5% in our culture conditions.
Several adult cell types have been reported to be able to spontaneously fuse with myotubes. These include primary fibroblasts from different locations (Breton et al., 1995; Salvatori et al., 1995), BM-MSCs (Shi et al., 2004; Lee et al., 2005; Schulze et al., 2005), AT-MSCs (Di Rocco et al., 2006), pericytes (Dellavalle et al., 2007; Kirillova et al., 2007), and other BM-derived cells (Sacco et al., 2005). The fusion efficiency varies with cell types. Relatively high fusion efficiency, however, is observed with adherent cells such as fibroblast and stromal cells, whereas nonadherent hematopoietic cells and monocytes are refractory to fusion (Shi et al., 2004) or fuse with an extremely low efficiency (Sacco et al., 2005).
In this regard, it is interesting to note that, as only recently emphasized, MSCs, fibroblasts, and pericytes share many phenotypical markers (Covas et al., 2008) and are probably considered variations of a unique phenotype (Bianco et al., 2008; da Silva Meirelles et al., 2008; Haniffa et al., 2009). In this context, the ability to assume a mesenchymal phenotype and differentiate toward a smooth muscle lineage can in part explain the propensity of EPDCs to fuse with myotubes.
Former studies from Helen Blau’s laboratory have analyzed gene expression in heterokaryons of muscle and nonmuscle cells generated by addition of polyethylene glycol (PEG). These studies revealed significant similarities between heterokaryons reprogramming and muscle differentiation and showed that, although cells from nonmesodermal lineages reprogram at lower efficiencies than do fibroblasts, in all cases nuclear reprogramming involves the activation of several skeletal muscle differentiation genes (Blau et al., 1985; Pomerantz et al., 2009). An extensive analysis of nuclear reprogramming with spontaneously fused hybrid myotubes, including early and late skeletal markers, has never been reported, however. Moreover, whereas PEG-induced fusion is independent from the degree of differentiation, spontaneous fusion may occur only at a specific myoblast differentiation stage. Gene expression analysis of cocultured cells revealed that EPDC (as well as endothelium and MSC) nuclei inside myotubes are reprogrammed to express human skeletal muscle specific genes and that such reprogramming involves a wide array of muscle markers downstream of MyoD. Early genes, such as the satellite cells marker Pax7 and the activated myoblast marker Myf5, were never detected. This was possibly because non-muscle cells fuse with multinucleated myotubes, which no longer express early genes but are unable to fuse with mononucleated myoblasts (Sacco et al., 2005), as also suggested by the fact that hybrid myotubes always contains at least three mouse nuclei. To our knowledge, our studies for the first time also demonstrated that (although with reduced efficiency compared to other cells) endothelial cells can fuse with myotubes and undergo nuclear reprogramming toward the muscle phenotype. It seems therefore that, although fusion efficiency may vary extensively, as soon as nuclei enter a myotube, transcription of muscle-specific genes is enabled, as indicated by the expression of human muscle proteins and in analogy to what was observed with PEG-induced heterokaryons.
The molecular cues that direct muscle cell fusion are not completely understood, although several molecules that facilitate fusion have been defined recently (Jansen and Pavlath, 2008). The formation of multinucleated myotubes is a two-step process that begins with fusion between individual mononucleated cells (first step), and is then followed by myotube–myotube and myoblast–myotube fusion (second step). Although probably several molecules are involved and required for both steps, emerging data assign to secreted cytokines, such as IL-4, a specific role in the second step, that is the recruitment of additional nuclei to already multinucleated myotubes. As previously mentioned, our data indicate that fusion of human nonmuscle cells occurs with multinucleated myotubes and not with myoblasts. This fact would imply that molecules known to modulate the secondary fusion events could also affect the recruitment of nonmyogenic cells. It has been reported that fusion of mouse BM-MSCs to differentiating skeletal muscle cells is also regulated by IL-4 (Schulze et al., 2005). To test whether the same applies to different types of human cells as well, coculture experiments were performed in the presence of IL-4 or with the alternative IL-4 receptor (IL-4R) ligand, IL-13. Results show that, whereas the fusion efficiency of endothelial cells was not significantly affected by cytokine treatment, both IL-4 and IL-13 determined an increment in the fusion/recruitment efficiency of both EPDCs and AT-MSCs, although to a reduced extent compared to the increment of 200% previously reported for mouse BM-MSCs.
IL-4R target genes, which mediate the IL-4/IL-13 effect on muscle fusion, have not been identified. VCAM1 is an adhesion molecule that has been reported to be involved in myotube formation (Rosen et al., 1992). We observed that VCAM1 expression levels in different human cells correlate with their fusion propensity, and we showed that the inhibition of VCAM1 function hampers the fusion process between nonmuscle cells and skeletal myotubes. Additionally, we found that in human EPDCs, MSCs, and endothelial cells, VCAM1 levels are modulated by soluble factors secreted by differentiating myoblasts and that this effect is mimicked by treatment with IL-4 or IL-13. These results suggest that VCAM1 is required for the fusion between myotubes and nonmuscle cells and, more importantly, that the fusion rate increase obtained upon IL-4 or IL-13 treatment is mediated at least in part by VCAM1 induction. The finding that a VCAM1 blocking antibody, as well as VCAM1 gene silencing, abrogate the stimulatory effects of IL-4 on myotube cell recruitment also corroborates such a hypothesis.
The use of the non-cross-reactive murine and human forms of IL-4 in our two-species hybrid system revealed that IL-4 exerts a dual effect on the recruitment of nuclei inside the myotube: a direct one on the target cell, in part possibly mediated by VCAM1 induction, and an indirect one the mechanism of which remains unknown. As reported in the literature and as also observed in our cultures with murine IL-4, the presence of IL-4 during myoblasts differentiation shifts the balance between small and big myotubes, determining an increase in the average number of nuclei per myotubes. It is therefore possible that bigger myotubes have a higher recruitment ability, that is, are more prone to fusion than are small ones.
In endothelial cells, VCAM1 mediates the adhesion of a variety of cells, including lymphocytes and monocytes. Although endothelial cells may strongly up-regulate VCAM1 expression in the presence of skeletal myoblasts, they never acquire a fusion competence compared to the one of EPDCs or AT-MSCs. In fact, treatment with cytokines up-regulating VCAM1 increases the number of recruited nuclei in EPDCs and AT-MSCs but does not significantly alter the number of fusing endothelial cells. Therefore, even if VCAM1 may be required, it is obviously not sufficient for the fusion process. One possibility is that endothelial cells lack part of the fusion machinery that is present in more fusion-competent cells, such as EPDCs and MSCs, and that the rare fusion events that are observed may occur through a different mechanism. Our finding that endothelial cells fail to express the fusion-related gene ITGβ1D and its muscle-specific partner ITGα7, which instead are expressed in EPDCs and MSCs, would support such a hypothesis.
Finally, no conclusive data exist in the literature regarding the signaling pathways involved in VCAM1 induction by IL-4/IL-13. It has been recently reported that hypoxia-induced VCAM1 in mouse lung is significantly reduced in IL-4 knockout lungs but not in STAT6 knockout lungs, suggesting that VCAM1 induction in the lungs occurs via IL-4 but does not require a STAT6 pathway. This notion would be consistent with the fact that the VCAM1 promoter does not contain any STAT6-binding sites (Yamaji-Kegan et al., 2009). We show that VCAM1 induction in three nonhematopoietic cell types is negatively affected by PI3K and p38MAPK inhibitors but not by ERK1/2 or Jak2 inhibitors. Although our data do not exclude that Stat6 could be involved in any way, it is plausible to assume that VCAM1 induction by IL-4R Type II is mediated primarily by the PI3K and p38MAPK pathways.
MATERIALS AND METHODS
All experimental procedures complied with the Italian National Institutes of Health and were approved by the Institutional Animal Care and Use Committee. All experiments with human tissue were carried out according to the Local Ethics Committee and upon obtaining a written informed consent signature.
Epicardial cell isolation
EPDCs were obtained from atrial appendages of patients from 50 to 80 yr old undergoing cardiac surgery. The epicardial layer was separated mechanically from the biopsy, immediately placed in a fibronectin-coated dish, and covered by a glass coverslip to prevent the biopsy from floating. DMEM and M199 at a 1:1 (vol/vol) ratio supplemented with 10% fetal bovine serum (FBS), 1% penicillin–streptomycin (Gibco, Carlsbad, CA), and basic fibroblast growth factor (bFGF) at 2 ng/ml was used as culture medium. After outgrowth of the cells, the epicardium was removed and the cells were cultured for one or two passages.
MSC isolation
Subcutaneous AT biopsies of abdominal origin or atrial appendages after epicardium removal were digested with Collagenase A (Roche, Basel, Switzerland) at 2 mg/ml for 40 min at 37°C. A single-cell suspension was obtained after filtering through a 40-μm cell strainer and centrifuged. Cells were plated on an uncoated culture tissue dish in DMEM, 20% FBS, and 1% penicillin–streptomycin, 2 mM glutamine and were washed after 8 h to remove nonadherent cells. Both AT-MSCs and atrial MSCs were amplified in culture for three passages. Cell populations were checked for the presence of typical stromal antigens by fluorescence-activated cell sorting (FACS) analysis as previously described (Zuk et al., 2002).
Endometrial endothelial cells isolation
Endometrium samples obtained upon scraping were incubated in M199 medium containing 0.2% Collagenase Type II at 37°C for 2 h. After centrifugation, the pellet was resuspended in culture medium and transferred to fibronectin-coated culture dishes. Two to four hours later, the nonadherent cells were removed, and the adherent cells were cultured in a medium consisting of M199 additioned with 20% FBS, 5 U/ml heparin, 5 mg/ml endothelial cells growth supplement (BD, Franklin Lakes, NJ), and 5 ng/ml VEGF. The primary heterogeneous cell population was grown until near confluence, then endothelial cells were selected by anti–human CD31-coated microbeads (miniMACS, Miltenyi, Auburn, CA), according to the manufacturer’s instructions. Two rounds of CD31 selection were performed to obtain a purity of approximately 99% CD31+ cells, as assessed by flow cytometry.
Cocultures with murine primary myoblasts
Primary myoblasts were obtained as described (Di Rocco et al., 2006). Briefly, hind-limb muscles of 4-wk-old CD1 mice were minced by scissors, then digested with collagenase/dispase (Roche) at 2 mg/ml at 37°C. Cells were preplated on uncoated culture tissue dishes overnight and then transferred to fibronectin-coated dishes in GM consisting of DMEM, 20% FBS, 1% penicillin–streptomycin, 2 mM glutamine, and bFGF at 5 ng/ml. When myoblasts were approximately 50% confluent, 105/cm2 human cells (EPDCs, MSCs, or endothelial cells) were added and allowed to adhere overnight; then the culture was switched to DM consisting of DMEM supplemented with 2% horse serum, to induce myotube formation.
Media supplements
Recombinant human IL-4 (Sigma, St. Louis, MO) or mouse IL-4 (PreproTech, Rocky Hill, NJ) was added at 10 and 50 ng/ml, respectively. Recombinant mouse IL-13 (PreproTech) was used at 50 ng/ml. The blocking anti–human VCAM1 monoclonal antibody (clone CD106; R&D Systems, Minneapolis, MN) was used at 3 μg/ml. Three different concentrations were tested for each signaling pathway inhibitor (all from Sigma) and then used as follows: LY294002, 20 μm; SB202190, 30 μm; W0894, 2 μm; PD98059, 50 μm.
Lentivirus-mediated gene transfer
The third-generation lentiviral SIN (self-inactivating) vector pCCLsin.cPPT.hPGK.E-GFP.Wpre plasmid was a gift from Elisa Vigna (Follenzi et al., 2000). For viral transduction, cells were plated at 60% confluence in their expansion medium and were transduced the next day with approximately 10 transforming units per cell of lentiviral vector stocks in the presence of 6 μg/ml Polybrene® (Abbott Laboratories Corp., Abbott Park, IL). After incubation at 37°C from 1 h to overnight cells were trypsinized and subcultured at a 1:3 ratio and cultured for 2 more days before being used in subsequent experiments. Transduction efficiency was determined by FACS analysis and was estimated to be >90% in all cell types used.
Immunofluorescence
Antibodies were diluted (vol/vol) as follows: TBX18 (Santa Cruz Biotechnology, Santa Cruz, CA), 1:100; Epicardin (Abcam, Cambridge, MA), 1:200; Desmin (Chemicon, Billerica, MA), 1:100; human Troponin-T (Abcam), 1:400; Troponin-T (Santa Cruz), 1:500; human Lamin A/C (Santa Cruz), 1:100; VCAM1 (R&D), 1:100. Secondary antibodies were 488/594 Alexa fluor-labeled anti–mouse/–rabbit or –goat immunoglobulin G (Molecular Probes, Carlsbad, CA).
RT-PCR
Total RNA was extracted using an RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. RNA was reverse transcribed with M-MLV reverse transcriptase (Promega, Madison, WI), using random hexamers. PCRs of cocultures were performed using primers specific for human sequences, not recognizing murine transcripts. Control RT reactions and the presence of introns spanning the amplified sequences excluded the possibility of any DNA contamination.
Surgical procedures for in vivo experiments
All mice were anesthetized with 2.5% Avertin (2,2,2-tribromoethanol; Sigma-Aldrich, St. Louis, MO). A total of 1 × 105 cells, resuspended in 25 μl of PBS, were delivered into the tibialis anterior (TA) of 2-mo-old C57BL/10SnJ mdx mice (The Jackson Laboratory, Bar Harbor, ME). Age-matched mdx control animals received injections of PBS only. All injected animals received a daily injection of FK506 at 2 mg/kg as immunosuppressant from the day before transplantation to killing 1 wk after injection.
Immunohistochemistry
TA muscles were removed and fixed in 10% formalin for 48 h and then were embedded in paraffin and sectioned at 5 μm. Sections were deparaffinized, microwave-treated in a citric acid–EDTA buffer (10 mM, pH 7.8), and preincubated with PBS/10% bovine serum albumin for 1 h at room temperature to minimize nonspecific binding (all compounds from Sigma-Aldrich, St. Louis, MO). To visualize donor-derived fibers, sections were incubated with a primary rabbit polyclonal antibody against dystrophin (Abcam) for 1 h at 37°C. 594 Alexa fluor-labeled anti–rabbit was used as secondary antibody. Slides were mounted in ProLong Gold Antifade (Molecular Probes).
Gene silencing by shRNA
EPDCs and MSCs were infected with lentiviral constructs harboring the puromycin resistance gene and encoding specific shRNA for either VCAM1 or IL4-Rα (SHCLNG–NM_001078 for VCAM1 and SHCLNG-NM_000418 for IL-4Rα, MISSION® shRNA; Sigma-Aldrich). Infected cells were cultured in the presence of puromycin for at least 4 d before use. Effective gene expression inhibition was monitored by RT-PCR.
Supplementary Material
Acknowledgments
We thank Fiorella Di Nicuolo and Roberta Castellani for help with human primary endothelial cell isolation. This work was supported by the Italian Ministry of Health Rare Disease Project and by the Italian Telethon Foundation, grant GGP07106.
Abbreviations used:
- AT
adipose tissue
- bFGF
basic fibroblast growth factor
- BM
bone marrow
- DM
differentiation medium
- EMT
epithelial-to-mesenchymal transition
- EPDC
epicardium-derived cell
- GM
growth medium
- IL
interleukin
- MCM
myoblast-conditioned medium
- mRNA
messenger RNA
- MSCs
mesenchymal stromal cells
- PEG
polyethylene glycol
- shRNA
short hairpin RNA
- VCAM1
vascular cell adhesion molecule 1
- γC
γ-chain
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-06-0537) on January 5, 2011.
REFERENCES
- Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, Webster SG, Miller SC, Webster C. Plasticity of the differentiated state. Science. 1985;230:758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Bochner BS, Klunk DA, Sterbinsky SA, Coffman RL, Schleimer RP. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799–803. [PubMed] [Google Scholar]
- Breton M, Li ZL, Paulin D, Harris JA, Rieger F, Pincon-Raymond M, Garcia L. Myotube driven myogenic recruitment of cells during in vitro myogenesis. Dev Dyn. 1995;202:126–136. doi: 10.1002/aja.1002020204. [DOI] [PubMed] [Google Scholar]
- Cai CL, et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature. 2008;454:104–108. doi: 10.1038/nature06969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield S, Lee Y, Schröder A, Rothman P. Cutting edge: IL-4 induces suppressor of cytokine signaling-3 expression in B cells by a mechanism dependent on activation of p38 MAPK. J Immunol. 2005;174:2494–2498. doi: 10.4049/jimmunol.174.5.2494. [DOI] [PubMed] [Google Scholar]
- Christoffels VM, Grieskamp T, Norden J, Mommersteeg MT, Rudat C, Kispert A. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458:E8–E9. doi: 10.1038/nature07916. discussion E9–E10. [DOI] [PubMed] [Google Scholar]
- Covas DT, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Di Rocco G, Iachininoto MG, Tritarelli A, Straino S, Zacheo A, Germani A, Crea F, Capogrossi MC. Myogenic potential of adipose-tissue-derived cells. J Cell Sci. 2006;119:2945–2952. doi: 10.1242/jcs.03029. [DOI] [PubMed] [Google Scholar]
- Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- Haniffa MA, Collin MP, Buckley CD, Dazzi F. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica. 2009;94:258–263. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113:483–494. doi: 10.1016/s0092-8674(03)00319-2. [DOI] [PubMed] [Google Scholar]
- Jacquemin V, Butler-Browne GS, Furling D, Mouly V. IL-13 mediates the recruitment of reserve cells for fusion during IGF-1-induced hypertrophy of human myotubes. J Cell Sci. 2007;120:670–681. doi: 10.1242/jcs.03371. [DOI] [PubMed] [Google Scholar]
- Jang YS, Kim HA, Park SR, Lee MR, Park JB, Kim PH. IL-4 stimulates mouse macrophages to express APRIL through p38MAPK and two different downstream molecules, CREB and Stat6. Cytokine. 2009;47:43–47. doi: 10.1016/j.cyto.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Jansen KM, Pavlath GK. Mannose receptor regulates myoblast motility and muscle growth. J Cell Biol. 2006;174:403–413. doi: 10.1083/jcb.200601102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen KM, Pavlath GK. Molecular control of mammalian myoblast fusion. Methods Mol Biol. 2008;475:115–133. doi: 10.1007/978-1-59745-250-2_7. [DOI] [PubMed] [Google Scholar]
- Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. J Allergy Clin Immunol. 2000;105:1063–1070. doi: 10.1067/mai.2000.107604. [DOI] [PubMed] [Google Scholar]
- Kang JS, Yi MJ, Zhang W, Feinleib JL, Cole F, Krauss RS. Netrins and neogenin promote myotube formation. J Cell Biol. 2004;167:493–504. doi: 10.1083/jcb.200405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- Kirillova I, Gussoni E, Goldhamer DJ, Yablonka-Reuveni Z. Myogenic reprogramming of retina-derived cells following their spontaneous fusion with myotubes. Dev Biol. 2007;311:449–463. doi: 10.1016/j.ydbio.2007.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kosinski PA, Kemp DM. Contribution of human bone marrow stem cells to individual skeletal myotubes followed by myogenic gene activation. Exp Cell Res. 2005;307:174–182. doi: 10.1016/j.yexcr.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Limana F, et al. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res. 2007;101:1255–1265. doi: 10.1161/CIRCRESAHA.107.150755. [DOI] [PubMed] [Google Scholar]
- Limana F, et al. Myocardial infarction induces embryonic reprogramming of epicardial c-kit(+) cells: Role of the pericardial fluid. J Mol Cell Cardiol. 2010;48:609–618. doi: 10.1016/j.yjmcc.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem. 2003;278:14587–14590. doi: 10.1074/jbc.R200022200. [DOI] [PubMed] [Google Scholar]
- Palmer-Crocker RL, Hughes CC, Pober JS. IL-4 and IL-13 activate the JAK2 tyrosine kinase and Stat6 in cultured human vascular endothelial cells through a common pathway that does not involve the gamma c chain. J Clin Invest. 1996;98:604–609. doi: 10.1172/JCI118829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz JH, Mukherjee S, Palermo AT, Blau HM. Reprogramming to a muscle fate by fusion recapitulates differentiation. J Cell Sci. 2009;122:1045–1053. doi: 10.1242/jcs.041376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach NL, Biressi S, Reichardt LF, Keller C, Rando TA. Focal adhesion kinase signaling regulates the expression of caveolin 3 and beta1 integrin, genes essential for normal myoblast fusion. Mol Biol Cell. 2009;20:3422–3435. doi: 10.1091/mbc.E09-02-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GD, Sanes JR, LaChance R, Cunningham JM, Roman J, Dean DC. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell. 1992;69:1107–1119. doi: 10.1016/0092-8674(92)90633-n. [DOI] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, LaBarge MA, Hammer MM, Kraft P, Blau HM. IGF-I increases bone marrow contribution to adult skeletal muscle and enhances the fusion of myelomonocytic precursors. J Cell Biol. 2005;171:483–492. doi: 10.1083/jcb.200506123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatori G, et al. Myogenic conversion of mammalian fibroblasts induced by differentiating muscle cells. J Cell Sci. 1995;108(Pt 8):2733–2739. doi: 10.1242/jcs.108.8.2733. [DOI] [PubMed] [Google Scholar]
- Schulze M, Belema-Bedada F, Technau A, Braun T. Mesenchymal stem cells are recruited to striated muscle by NFAT/IL-4-mediated cell fusion. Genes Dev. 2005;19:1787–1798. doi: 10.1101/gad.339305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Muller U. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4:673–685. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- Shi D, Reinecke H, Murry CE, Torok-Storb B. Myogenic fusion of human bone marrow stromal cells, but not hematopoietic cells. Blood. 2004;104:290–294. doi: 10.1182/blood-2003-03-0688. [DOI] [PubMed] [Google Scholar]
- van Tuyn J, et al. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells in vitro. Stem Cells. 2007;25:271–278. doi: 10.1634/stemcells.2006-0366. [DOI] [PubMed] [Google Scholar]
- Wada AM, Smith TK, Osler ME, Reese DE, Bader DM. Epicardial/mesothelial cell line retains vasculogenic potential of embryonic epicardium. Circ Res. 2003;92:525–531. doi: 10.1161/01.RES.0000060484.11032.0B. [DOI] [PubMed] [Google Scholar]
- Winter EM, et al. Preservation of left ventricular function and attenuation of remodeling after transplantation of human epicardium-derived cells into the infarcted mouse heart. Circulation. 2007;116:917–927. doi: 10.1161/CIRCULATIONAHA.106.668178. [DOI] [PubMed] [Google Scholar]
- Yamaji-Kegan K, Su Q, Angelini DJ, Johns RA. IL-4 is proangiogenic in the lung under hypoxic conditions. J Immunol. 2009;182:5469–5476. doi: 10.4049/jimmunol.0713347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.