The small GTPase Cdc42p is a master regulator of cell polarity. We analyzed Cdc42p localization using yeast mutants and found that endo-exocytic trafficking and septin-based diffusion barrier synergistically control Cdc42p polarization during asymmetric cell growth.
Abstract
Cdc42p plays a central role in asymmetric cell growth in yeast by controlling actin organization and vesicular trafficking. However, how Cdc42p is maintained specifically at the daughter cell plasma membrane during asymmetric cell growth is unclear. We have analyzed Cdc42p localization in yeast mutants defective in various stages of membrane trafficking by fluorescence microscopy and biochemical fractionation. We found that two separate exocytic pathways mediate Cdc42p delivery to the daughter cell. Defects in one of these pathways result in Cdc42p being rerouted through the other. In particular, the pathway involving trafficking through endosomes may couple Cdc42p endocytosis from, and subsequent redelivery to, the plasma membrane to maintain Cdc42p polarization at the daughter cell. Although the endo-exocytotic coupling is necessary for Cdc42p polarization, it is not sufficient to prevent the lateral diffusion of Cdc42p along the cell cortex. A barrier function conferred by septins is required to counteract the dispersal of Cdc42p and maintain its localization in the daughter cell but has no effect on the initial polarization of Cdc42p at the presumptive budding site before symmetry breaking. Collectively, membrane trafficking and septins function synergistically to maintain the dynamic polarization of Cdc42p during asymmetric growth in yeast.
INTRODUCTION
Cell polarity is a universal theme in eukaryotes and is important for a wide range of biological processes, including cell division, directional migration, tissue organization, and organogenesis. Polarity is established through the asymmetric distribution of proteins and lipids at the plasma membrane (see McCaffrey and Macara, 2009; Nelson, 2009; Orlando and Guo, 2009). Many of the key components that control cell polarization are evolutionarily conserved. The budding yeast Saccharomyces cerevisiae reproduces by the asymmetrical growth of its daughter cell (“budding”) and provides an excellent model system for the study of the molecular mechanisms of cell polarity.
Cdc42 is a member of the Rho family of small GTPases that plays a central role in cell polarity in most eukaryotic cells (Johnson, 1999; Etienne-Manneville, 2004; Park and Bi, 2007). In budding yeast, Cdc42p is found at both the plasma membrane and internal vacuolar compartments (Ziman et al., 1993; Eitzen et al., 2001; Muller et al., 2001; Richman et al., 2002). The localization of Cdc42p at specific domains of the plasma membrane is important for coordinating the polarized organization of actin cytoskeleton and membrane trafficking during polarized cell growth (for a review, see Pruyne et al., 2004; Park and Bi, 2007). The early establishment of Cdc42p polarization (sometimes referred to as “symmetry breaking”) has been intensively studied over these years, and two mechanisms are found to be important for the localization of Cdc42p at the presumptive sites of bud emergence. One involves an adaptor-based signaling system, including Bem1p, Cdc24p (the guanine nucleotide exchange factor for Cdc42p), and the Cdc42p downstream effectors that provide a positive-feedback loop, which amplifies an initial stochastic cluster of Cdc42p signal to break symmetry (Butty et al., 2002; Irazoqui et al., 2003; Kozubowski et al., 2008; Howell et al., 2009). The other involves membrane trafficking along actin cables for the delivery of Cdc42p to the daughter cell (Wedlich-Soldner et al., 2003; Pruyne et al., 2004; Wedlich-Soldner et al., 2004; Zajac et al., 2005; Kozminski et al., 2006; Gao and Bretscher, 2009). These two interconnected systems account for the polarized localization of Cdc42p for symmetry breaking (Slaughter et al., 2009a).
At the early bud establishment stage, Cdc42p appears as a “cap” at the presumptive site of bud emergence. Once the bud appears, Cdc42p is localized at the tip of the bud, where it controls actin organization and secretory machinery for continued cell surface expanding. How Cdc42p is maintained at the daughter cell is an open question. It is possible that, like the early stages of budding, the directional transport of vesicles to the daughter cell continuously deliver Cdc42p to the bud. But the exocytic pathways that transport Cdc42p are uncharacterized. Also, the continuous delivery of Cdc42p by exocytosis may cause its diffusion along the cell cortex away from the bud. Removal of the laterally diffused Cdc42p by endocytosis may counteract this diffusion. However, whether endocytosis is sufficient to counteract the lateral dispersal is unknown.
In addition to endo-exocytic trafficking, another major factor that needs to be considered for the maintenance of Cdc42p localization is the septin barrier. After the initial establishment of Cdc42p polarity, the septins are recruited to the bud by Cdc42p and subsequently assemble into a ring between the mother and daughter cells (Barral et al., 2000; Iwase et al., 2006). It will be interesting to examine whether the septin barrier serves to prevent Cdc42p diffusion.
In this paper, we characterized the different trafficking routes that mediate the transport of Cdc42p to the daughter cell. Furthermore, We found that endo-exocytic trafficking and septins synergistically control the maintenance of Cdc42p polarization. The study of Cdc42p in the simple organism budding yeast may reveal intersecting factors and general principles underlying eukaryotic cell polarity.
RESULTS
Cdc42p remain polarized at the daughter cell plasma membrane when the exocytic routes are separately blocked
In the budding yeast, at least two distinct populations of secretory vesicles are responsible for transporting cargoes to the plasma membrane (Harsay and Bretscher, 1995; Harsay and Schekman, 2002). Named for one of the cell wall endoglucanases it carries, the first population is known as “Bgl2p vesicles.” The second population was named “invertase vesicles,” which contain cargoes such as the periplasmic enzymes invertase and acid phosphatase (Harsay and Bretscher, 1995). These two types of vesicles are delivered to the plasma membrane via different routes: the cargo of invertase vesicles is first sorted through endosomes, whereas Bgl2p vesicles are mostly originated from the trans-Golgi network (Harsay and Bretscher, 1995; Chuang and Schekman, 1996; Yuan et al., 1997; Ziman et al., 1998; Harsay and Schekman, 2002). To understand the nature of Cdc42p trafficking and ultimately the dynamics of its polarization, it is important to determine the role of these pathways on Cdc42p transport to the daughter cells.
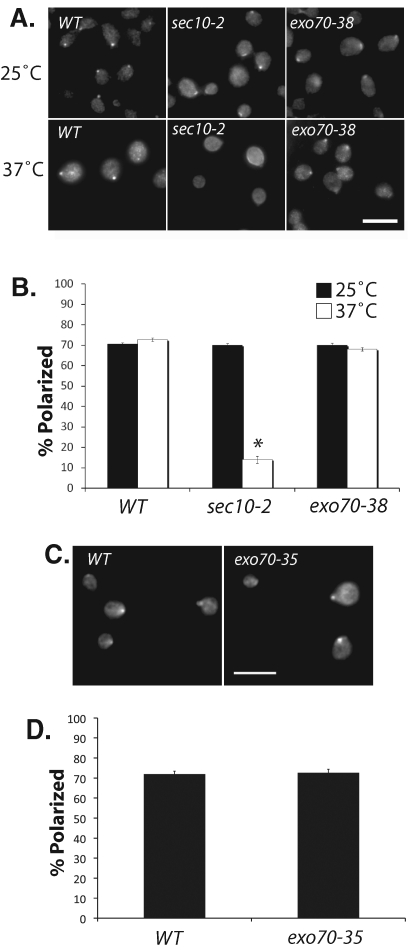
Here we examined the maintenance of Cdc42p polarization in secretory mutants defective in both exocytic routes or specifically in one of them. The exocyst is an octameric protein complex containing Sec3p, Sec5p, Sec6p, Sec8p, Sec10, Sec15p, Exo70p, and Exo84p. It mediates the tethering of secretory vesicles to the plasma membrane prior to fusion (for a recent review, see He and Guo, 2009). Mutations in most of the exocyst subunits, such as Sec5p and Sec10p, lead to a block of both classes of secretory vesicles. Mutations in the Exo70p component, however, primarily affect Bgl2p secretion (He et al., 2007). Using affinity-purified anti-Cdc42p antibodies, we performed immunofluorescence to examine the localization of endogenous Cdc42p in these mutant cells. In the sec10-2 mutant, Cdc42p became depolarized in most of the cells at the restrictive temperature (Figure 1, A and B). Similarly, Cdc42p is depolarized in the sec5-24 mutant soon after shifting to the restrictive temperature of 34ºC (Supplemental Figure 1). For the exo70 mutants, we use two different alleles. One is defective in secretion at 37ºC (exo70-38) and another at 25ºC (exo70-35), both of which are blocked in Bgl2p secretion, but not invertase secretion (He et al., 2007). The exo70-38 mutant has comparable growth defect to the sec10-2 mutant at 37ºC (He et al., 2007). Surprisingly, Cdc42p is well polarized at the daughter cell in these exo70 mutants (Figure 1, A and C, results quantified in Figure 1, B and D, respectively). The polarized localization of Cdc42p in the exo70 mutants, but not the sec10 and sec5 mutants, suggests two possibilities. First, Cdc42p may be delivered primarily via the invertase vesicles rather than the Bgl2p vesicle to the bud. Second, Cdc42p may be transported through both pathways but rerouted to the invertase pathway when the Bgl2p pathway is blocked.
FIGURE 1:
Cdc42p is depolarized in the sec10 mutant but remains polarized in the exo70 mutant cells. (A) Immunofluorescence staining of Cdc42p in wild-type (WT), sec10-2, and exo70-38 cells. Cells were grown to log phase at 25°C and shifted to 37°C for 120 min, fixed, and immunostained for Cdc42p. Bar = 10 μm. (B) Quantification of Cdc42p polarization in WT, sec10-2, and exo70-38 cells. Fifty small-budded cells were counted for each group (n = 3). Error bars represent standard error. *, p < 0.05. (C) WT and exo70-35 cells were grown to log phase at 25°C, fixed, and immunostained for Cdc42p. Bar = 10 μm. (D) Quantification of Cdc42p polarization in the WT and exo70-35 cells. Fifty cells were counted for each group (n = 3).
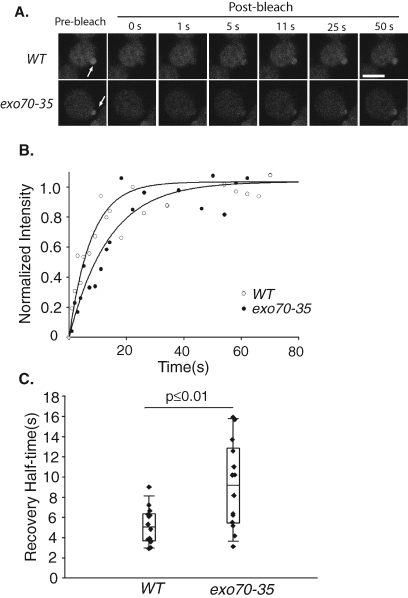
In the exo70 mutants, although Cdc42p appears polarized, the delivery of Cdc42p to the bud may be delayed because only one of the exocytic routes is functional. We therefore compared green fluorescent protein (GFP)-Cdc42p targeting to the bud tip in exo70-35 and wild-type cells using fluorescence recovery after photobleaching (FRAP). We photobleached the GFP-Cdc42p in the bud tip of small daughter cells and then measured the fluorescence recovery at various time points after the bleach (Figure 2A; representative quantification in Figure 2B). In the wild-type cells, the average half-time for GFP-Cdc42p fluorescence recovery was 5.2 s. However, the average half-time for GFP-Cdc42p fluorescence recovery was 9.1 s in the exo70-35 mutant (Figure 2C). The longer half-life for GFP-Cdc42p polarization in the exo70 mutant is consistent with its defect in one of the delivery routes.
FIGURE 2:
Targeting of Cdc42p to the bud is delayed in the exo70-35 cells. GFP-Cdc42p fluorescence at the daughter cell was bleached, and the recovery of the fluorescence signal was monitored over time. (A) Montages of FRAP of the wild-type and exo70-35 cells. The GFP-Cdc42p fluorescence recovers slower in the exo70-35 mutant cell. Arrows point to the regions of photobleaching. Scale bar represents 2 μm. (B) Fitting curves of one set of FRAP experiments on the wild-type and exo70-35 cells. The normalized fluorescence intensity is plotted over time. The curve fitting was performed using SigmaPlot as described in Materials and Methods. (C) Recovery half-times for the wild-type and exo70-35 mutant cells were plotted. Bottom and top of the box are the lower and upper quartiles, respectively. The band near the middle of the box is the median. Whiskers represent standard errors. n = 14, p ≤ 0.01.
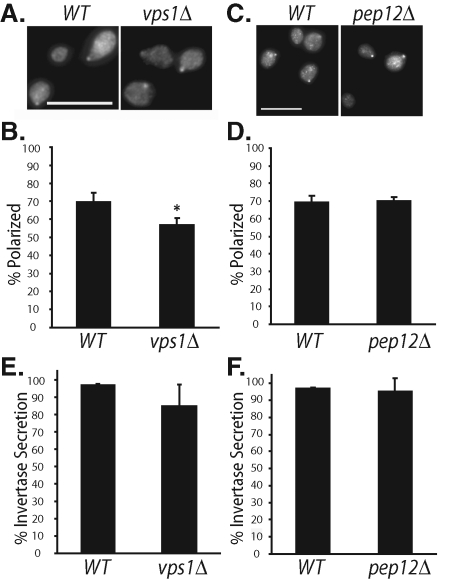
To investigate the role of invertase vesicles in Cdc42p trafficking, we used the vps1 and pep12 deletion mutants. Vps1p is a dynamin-like protein in yeast that mediates the generation of vesicles from the Golgi for subsequent delivery of vesicles to prevacuolar compartments (PVC; equivalent to late endosomes) or vacuole (Nothwehr et al., 1995; Bryant and Stevens, 1998). Pep12p (a.k.a. Vps6p) is a t-SNARE protein involved in the fusion of Golgi or early endosomal vesicles to PVCs (Becherer et al., 1996; Bryant and Stevens, 1998, Gerrard et al., 2000). It was demonstrated that deletion of either VPS1 or PEP12 blocks the biogenesis of invertase vesicles, and invertase is rerouted to the Bgl2p pathway for exocytosis (Gurunathan et al. 2002; Harsay and Schekman, 2002). We found that Cdc42p polarization was slightly disturbed in vps1Δ cells and normal in pep12Δ cells (Figure 3, A and C; quantified in Figure 3, B and D). We have also examined the GFP-tagged Cdc42p and internal FM 4-64 labeling of vacuoles in the same cells. In the pep12Δ cells, whereas the vacuoles had abnormal morphologies (often appearing as a single, enlarged vacuole) consistent with its previous categorization as a class D vps mutant (Conibear and Stevens, 1998), GFP-Cdc42p remains polarized at the plasma membrane similar to the wild-type cells (Supplemental Figure 2).
FIGURE 3:
Polarized localization of Cdc42p in vps1Δ and pep12Δ cells. (A) The WT and vps1Δ cells were grown to log phase, fixed, and immunostained for Cdc42p. (B) Quantification of Cdc42p polarization in WT and vps1Δ cells. Fifty small-budded cells were counted for each group (n = 3). Error bars represent standard errors. *, p < 0.05. (C) The WT and pep12Δ cells were immunostained for Cdc42p. (D) Quantification of Cdc42p polarization in WT and pep12Δ cells. Fifty small-budded cells were counted for each group (n = 3). (E) Percentage of invertase secretion in WT and vps1Δ cells (n = 3). (F) Percentage of invertase secretion in WT and pep12Δ cells (n = 3). Error bars represent standard errors.
Because cargoes of invertase vesicles can be rerouted to the Bgl2p pathway (Gurunathan et al. 2002; Harsay and Schekman, 2002), Cdc42p may still be able to reach the plasma membrane. We examined the secretion of invertase in these cells and found that it was also slightly affected in vps1Δ cells and normal in pep12Δ cells (Figure 3, E and F). The invertase secretion data are consistent with the rerouting model, in which it was demonstrated previously by density gradients that the invertase peak fractions were shifted to the lighter density vesicles carrying Bgl2p in vps mutants (Gurunathan et al. 2002; Harsay and Schekman, 2002).
Together, the analyses of the exo70 mutants versus the vps1Δ and pep12Δ mutants suggest that Cdc42p can be rerouted between each of the exocytic routes. It would be ideal to test the localization of Cdc42p in exo70 vps1Δ or exo70 pep12Δ double mutant cells, in which both pathways are inhibited. However, exo70 mutants are synthetic lethal with vps1Δ or pep12Δ (He et al., 2007). On the other hand, the synthetic lethality suggests the vital role of both pathways in exocytosis and polarized cell growth.
Detection of Cdc42p in both classes of exocytic vesicles
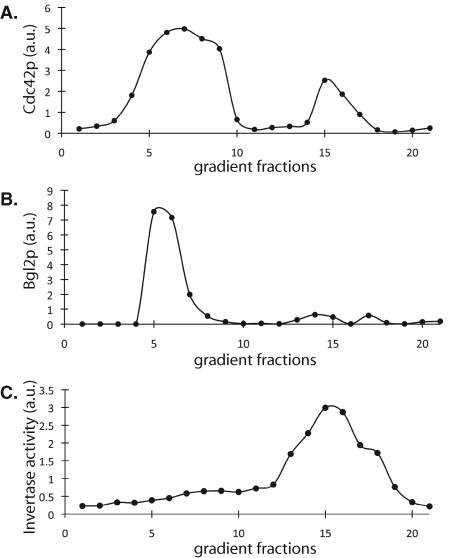
Invertase vesicles and Bgl2p vesicles have different densities (Harsay and Bretscher, 1995). Thus we examined the distribution of Cdc42p in these vesicles using density gradients. Secretory vesicle preparations from sec10-2, a mutant with defects in both Bgl2p and invertase secretion (He et al., 2007), were subjected to Percoll density gradients. The collected fractions were tested for invertase enzymatic activity and analyzed by Western blot for the distributions of Bgl2p and Cdc42p. Two major peaks in the HA-Cdc42p blot were observed (Figure 4A), one around fractions 5-9, which comigrate with the lighter Bgl2 vesicles (Figure 4B), and the other around fractions 14-17, which correspond to invertase activity (Figure 4C). The amount of Cdc42p in the lighter fractions was greater than that in the denser fractions. It is known that the majority of the exocytic vesicles are the Bgl2p-containing light-density vesicles, and the invertase vesicles constitute a small percentage of the total vesicles (Harsay and Bretscher, 1995). Therefore, we cannot infer from these results that the majority of Cdc42p are carried via the Bgl2p vesicles. The cofractionation of Cdc42p with both invertase activity and Bgl2p indicates that both vesicle populations are involved in the transport of Cdc42p to the plasma membrane. This result is also consistent with the fluorescence microscopy data above (Figures 1–3).
FIGURE 4:
Analysis of Cdc42p association with different classes of secretory vesicles by Percoll density gradients. The sec10-2 mutant cells, which have block in both Bgl2p and invertase secretion, were used to analyze Cdc42p association with vesicles. A sec10-2 mutant strain with a copy of HA-Cdc42p integrated at its URA3 locus on the chromosome was used for membrane preparation as described in Materials and Methods. The secretory vesicle preparation was then subjected to Percoll density gradients. The collected fractions were analyzed by Western blot to measure the distributions of HA-Cdc42p (A) and Bgl2p (B) or tested for invertase activity (C). Two major peaks in the HA-Cdc42p blot were observed: one around fractions 5–9, which corresponds to the lighter Bgl2p vesicles, and the other around fractions 14–17, which corresponds to denser invertase vesicles.
The coupling of endocytosis and exocytosis during Cdc42p polarization
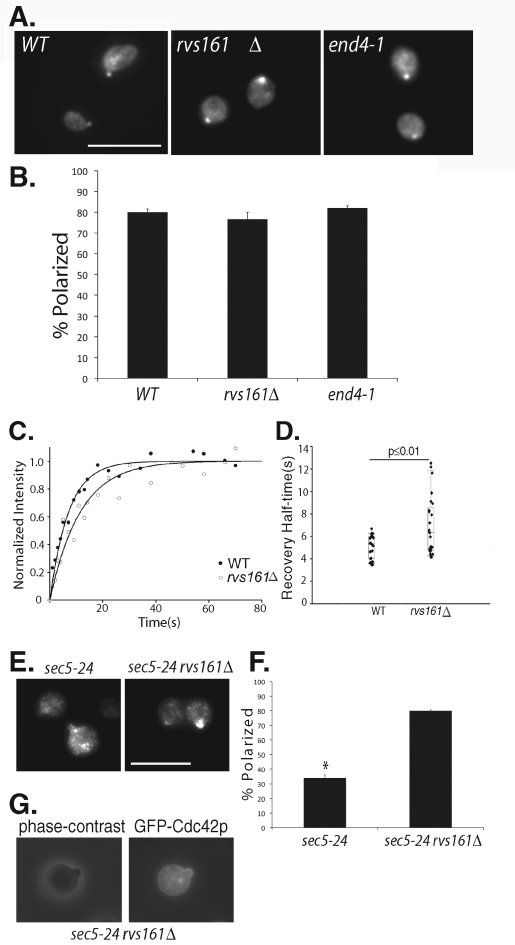
The association of Cdc42p with the invertase vesicles indicates that endosomal compartments are involved in Cdc42p trafficking. As such, it is likely that the Cdc42p on the plasma membrane is endocytosed as cargo into these endosomal compartments before being recycled back to the bud through another round of exocytosis. Indeed, previous studies using the F-actin disrupting drug latrunculin and Arp2/3 mutants have implicated endocytosis in Cdc42p polarization (Irazoqui et al., 2005; Marco et al., 2007). Also, for protein cargoes such as the v-SNARE protein Snc1p and the cell wall stress sensor Wsc1p, endocytosis was shown to be important for counteracting the lateral diffusion along the plasma membrane to maintain their polarized localization (Valdez-Taubas and Pelham, 2003; Piao et al., 2007). Here we directly examined the localization of Cdc42p in endocytosis mutants. Rvs161p is a yeast amphiphysin homolog implicated in the internalization step of endocytosis (Lombardi and Riezman, 2001). End4p (a.k.a. Sla2p) is a transmembrane protein that links actin to clathrin coats for endocytosis (Raths et al., 1993; Henry et al., 2002; Kaksonen et al., 2003). Surprisingly, we found that neither end4-1 nor rvs161Δ had an effect on Cdc42p polarization (Figure 5A, results quantified in Figure 5B). This is different from the observation on the transmembrane cargo Snc1p, in which endocytosis is sufficient to counteract its lateral dispersal (Valdez-Taubas and Pelham, 2003). The difference between Cdc42p and Snc1p is likely caused by their different diffusion rates, which is explicated later in the Discussion.
FIGURE 5:
Cdc42p polarization in endocytosis mutants. (A) The wild-type, rvs161Δ, and end4-1 cells were grown to log phase, incubated at 37°C for 90 min, fixed, and immunostained for Cdc42p. Cdc42p is polarized in the endocytosis mutants. Scale bar = 10 μm. (B) Quantification of Cdc42p polarization in wild-type, rvs161Δ, and end4-1 cells. Fifty cells were counted for each group (n = 3). Error bars represent standard error. (C) FRAP curves of GFP-Cdc42p in wild-type and rvs161Δ cells. The normalized fluorescence intensity is plotted over time using SigmaPlot. (D) Recovery half-times for GFP-Cdc42p in wild-type and rvs161Δ cells. Bottom and top of the box are the lower and upper quartiles, respectively. The band near the middle of the box is the median. Whiskers represent standard errors. n = 25, p ≤ 0.01. (E) The sec5-24 and sec5-24 rvs161Δ cells were grown to early log phase, shifted to 35°C for 90 min, fixed, and immunostained for Cdc42p. Although mostly depolarized in the sec5-24 mutant, Cdc42p is well polarized in the sec5-24 rvs161Δ double mutant cells. (F) Quantification of Cdc42p polarization in the sec5-24 and sec5-24 rvs161Δ double mutant cells. Fifty cells were counted for each group (n = 3). Error bars represent standard error. *, p ≤ 0.01. (G) The sec5-24 rvs161Δ cells were transformed with GFP-Cdc42, grown to early log phase, and shifted to 35°C for 90 min. GFP-Cdc42p was then observed using fluorescence microscopy. GFP-Cdc42p is enriched at daughter cell plasma membrane.
Although the endocytosis mutants showed no difference in static Cdc42p localization in small-budded cells, we suspected that a block of Cdc42p recycling from the plasma membrane might affect the kinetics of Cdc42p polarization. Therefore, we performed FRAP experiments to compare GFP-Cdc42p localization in wild-type and rvs161Δ cells. We detected a longer half-life for GFP-Cdc42p fluorescence recovery at the bud tip in the rvs161Δ cells (6.9 s) versus the wild-type cells (5.1 s) (Figure 5, C and D). The data suggest that defects in endocytosis affect Cdc42p dynamics, likely by impinging on its recycling from the plasma membrane.
To better study the coupling of endocytosis and exocytosis in Cdc42p polarization, we constructed a sec5-24 rvs161Δ double mutant strain. We chose the sec5-24 allele because it is partially defective in exocytosis at 34°C or 35°C. Rather than a complete blockage, a small amount of Cdc42p can still be delivered to the bud. In the sec5-24 rvs161Δ double mutant growing at 25°C, endocytosis is already inhibited due to the rvs161 deletion. A partial block in exocytosis can then be introduced by a temperature shift to 35°C. As shown in Figure 5, E and F, whereas Cdc42p was partially depolarized in the sec5-24 mutant, it became well polarized in the sec5-24 rvs161Δ double mutant cells. This polarized localization pattern of Cdc42p was also observed using GFP-Cdc42p expressed in the sec5-24 rvs161Δ cells; GFP-Cdc42p is enriched at the daughter cell plasma membrane (Figure 5G). This result suggests that, in a system where the delivery of Cdc42p is partially affected, inhibition of endocytosis helps to preserve Cdc42p polarization, likely by preventing its continuous removal. The data further demonstrate a coupling of endocytosis and exocytosis during Cdc42p polarization.
The septin ring is required for Cdc42p polarization
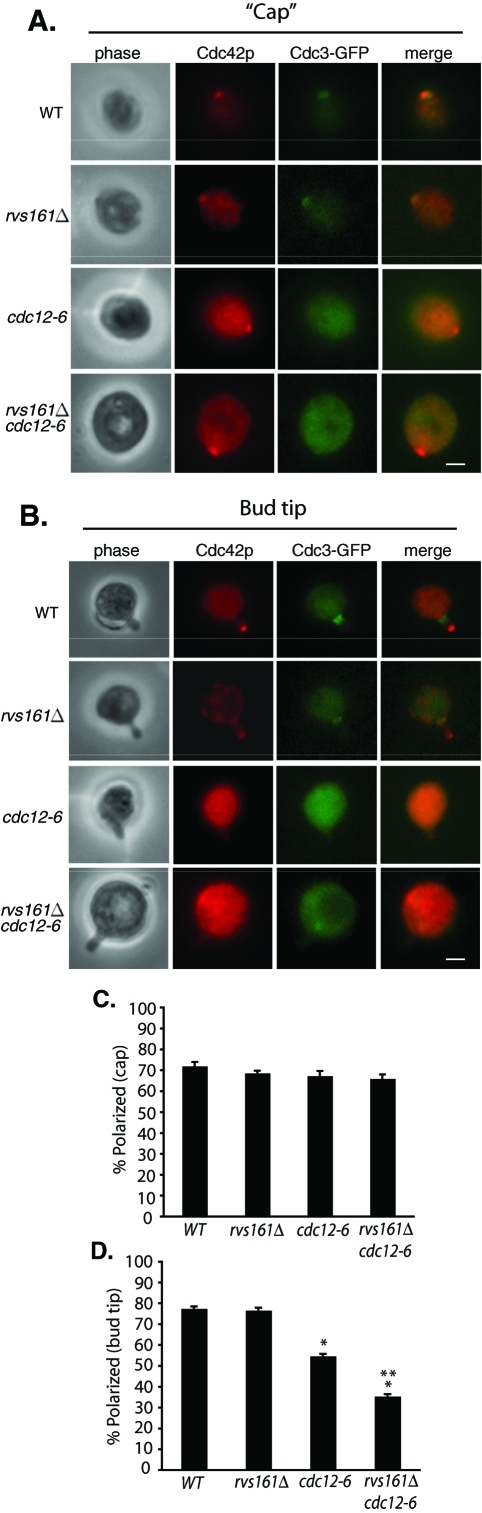
The observation that Cdc42p remains polarized in rvs161 and end4 mutants suggests that endocytosis is not sufficient to restrict Cdc42p from diffusion. What maintains Cdc42p at the bud in the endocytosis mutants? Septins are filamentous GTPases that form a diffusion barrier around the bud neck (Barral et al., 2000). It is possible that the apparent Cdc42p polarization in endocytosis mutants results from the diffusion barrier imposed by the septins. Therefore, we examined Cdc42p localization in the cdc12-6 mutant defective in Cdc12p, a component of septins. Cells were grown to early log phase at 25°C and shifted to 37°C for 90 min. In the cdc12-6 and cdc12-6 rvs161Δ mutants, we did not detect significant changes in the polarized localization of Cdc42p at the presumptive budding site (the Cdc42p “caps”) (Figure 6, A and C). However, in cells that had already generated small buds, Cdc42p became depolarized from the bud tips in some of the cdc12-6 mutant cells. In the cdc12-6 rvs161Δ double mutants, most of the cells lost Cdc42p from the bud tip (Figure 6, B and D). These results suggest that the septin diffusion barrier is important for the maintenance of Cdc42p polarization in the daughter cells after symmetry breaking but does not affect the early Cdc42p clustering during the establishment of cell polarity. We have also examined the Cdc42p localization in cells released from G0 phase synchronization. After temperature shifted to 37°C for 90 min, most of the cells generated small buds. We observed a similar decrease in Cdc42p polarization in cdc12-6 and the rvs161Δ cdc12-6 double mutants as we saw in small-budded cells of asynchronous culture (Supplemental Figure 3).
FIGURE 6:
Septins regulate Cdc42p polarization in small-budded cells but not during bud emergence. Wild-type, rvs161Δ, cdc12-6, and rvs161Δ cdc12-6 cells expressing Cdc3-GFP were grown to early log phase, shifted to 37°C for 90 min, and then immunostained for Cdc42p. (A) For cells during early bud emergence, Cdc42p remained polarized at the presumptive budding sites in both the wild-type and mutant cells. Scale bar represents 2 μm. (B) For cells that had generated small buds, Cdc42p was depolarized in some of the cdc12-6 mutant, and diffused in most of the rvs161Δ cdc12-6 double mutant cells. Scale bar represents 2 μm. Cdc42p polarization was quantified in cells during early bud emergence (C) and small-budded stage (D). Groups of 50 cells were counted (n = 3) for the wild-type and each mutant. Error bars represent standard error. *, p < 0.01 vs. WT cells. **, p < 0.01 between cdc12-6 and cdc12-6 rvs161Δ cells.
DISCUSSION
A major step in morphogenesis is the asymmetric distribution of a specific set of proteins at the cell cortex. Cdc42p is such a protein and is concomitantly a determinant of cell polarity. Studying the establishment and maintenance of Cdc42p localization is important to our understanding of the mechanisms of cell polarization. Previous studies using yeast as a model system have provided important insights into the early establishment of cell polarity (sometimes referred as “symmetry breaking”). Here biochemical and microscopic analyses of yeast mutants have allowed us to study factors that cooperatively maintain Cdc42p polarization after symmetry breaking.
We found that Cdc42p is transported along at least two exocytic routes in yeast (the “Bgl2p” and “invertase” pathways) (see model in Figure 7). Blocking either one of them does not inhibit Cdc42p transport, but rather results in the rerouting of Cdc42p to the other pathway. The invertase pathway involves trafficking through endosomal compartments, which may also mediate the recycling of proteins, such as the general amino acid permease (Gap1p) (Roberg et al., 1997) and iron transporters (Strochlic et al., 2007), to the cell surface in response to a changing environment. Our finding that some Cdc42p is delivered through endosomes is consistent with the previous observation that a portion of Cdc42p was localized in vacuoles, the endosomal compartments of yeast (Ziman et al., 1993; Eitzen et al., 2001; Muller et al., 2001; Richman et al., 2002). In mammalian cells, intermediate compartments such as recycling endosomes have been well characterized not only for their recycling of transmembrane receptors (e.g., growth factors receptors) and metal transporters (e.g., transferrin receptors), but also for proteins that are critical to the generation of epithelial asymmetry such as E-cadherin (Desclozeaux et al., 2008; Orlando and Guo, 2009) and integrins important for directional cell migration (Bretscher, 1996; Li et al., 2005; Caswell and Norman, 2006; Jović et al., 2007; Balasubramanian et al., 2010). Here endosomal trafficking serves to couple endocytosis of Cdc42p from, and subsequent exocytosis back to, the plasma membrane. Thus this recycling mechanism seems to be an evolutionarily conserved strategy in eukaryotic cell polarization.
FIGURE 7:
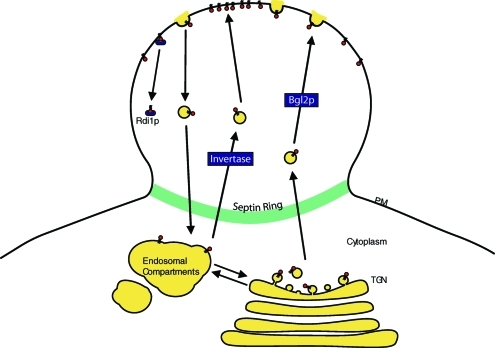
Schematic representation of the factors that regulate Cdc42p polarization in the daughter cell plasma membrane. Cdc42p is trafficked to the tip of the daughter cell through both the Bgl2p and invertase pathways. Once at the bud tip, Cdc42p may diffuse laterally along the plasma membrane. Endocytosis internalizes Cdc42p from the plasma membrane. The internalized Cdc42p can be carried as cargo back to the daughter cell through the endosomal compartments along the invertase pathway. Cdc42p can also be recycled from the daughter cell membrane by Rdi1p. The septin ring acts as a diffusion barrier to prevent Cdc42p from diffusing out of the daughter cell. PM, plasma membrane; TGN, trans-Golgi network. Cdc42p is indicated as small red circles with lipid tails.
Because many cargoes associated with invertase vesicles may originate from the plasma membrane through internalization, we examined the role of endocytosis at the plasma membrane in Cdc42p polarization. Our FRAP analyses of Cdc42p polarization in endocytosis mutants, and the use of the sec5-24 rvs161Δ double mutant, demonstrate that endocytosis is indeed tightly coupled to exocytosis in regulating the dynamics of Cdc42p polarization, which further underscores the importance of endosomal trafficking in Cdc42p recycling. Surprisingly, however, our microscopy study clearly shows that Cdc42p remains well polarized at the bud tip in yeast mutants that are defective in different steps of endocytosis (end4-1 and rvs161Δ). This is in contrast to the scenario of transmembrane cargoes such as Snc1p and Wsc1p, in which blocking endocytosis is sufficient to cause depolarization (Valdez-Taubas and Pelham, 2003; Piao et al., 2007). Therefore, although Cdc42p and these transmembrane proteins are all initially delivered in a polarized manner to the bud, their diffusion from the bud membrane is different. Based on previous studies, Cdc42p has a diffusion constant of 0.036 μm2/s, whereas Snc1p has a diffusion constant of 0.0025 μm2/s (Valdez-Taubas and Pelham, 2003; Marco et al., 2007). The more than 10-fold faster diffusion rate of Cdc42p clearly calls for mechanisms that counteract lateral dispersion in addition to endocytosis. Cdc42p differs from Snc1p and Wsc1p in its mode of membrane association. Instead of being incorporated into the lipid bilayers via a transmembrane domain, Cdc42p is prenylated at a C-terminal cysteine residue for its membrane attachment (Johnson, 1999). It was recently clearly demonstrated that Rdi1p, which extracts Cdc42p from the membrane, mediates the fast recycling of Cdc42p during early bud emergence (Slaughter et al., 2009b). However, Rdi1p alone is probably not sufficient for the maintenance of Cdc42p at the daughter cell because Cdc42p is still polarized to the bud in rdi1Δ cells (Irazoqui et al., 2005; Guo lab, unpublished data). Thus additional factors may exist that work together with endocytosis and Rdi1p to maintain Cdc42p polarization after bud formation.
A cortical barrier can serve to maintain polarity, following the premise that a protein is initially positioned within the barrier by delivery, clustering, and/or local amplification. A major event after bud emergence is the assembly of septin rings between the mother and nascent daughter cells. We speculate that the septins may carry out this barrier function after the establishment of Cdc42p polarity. A previous time-lapse microscopy study demonstrated that septins first appear at the presumptive budding site, and ring assembly takes place minutes after; the polarized recruitment of septins depends on Cdc42p, but not Rsr1p, a ras family small GTPase involved in bud site-selection (Iwase et al., 2006). Here we found that septins are important for the maintenance of Cdc42p in small daughter cells but not in cells undergoing early bud emergence. These data support a role of the septins in the maintenance of Cdc42p localization. In the cdc12-6 mutant, some cells lost Cdc42p polarization at the bud tip. Further disruption of RVS161 in the septin mutant led to diffusion of Cdc42p. This result suggests that both recycling and the cortical barrier work together to maintain Cdc42p polarization. It is possible that the speed of Cdc42p diffusion along the plasma membrane is faster than the actions of endocytosis; as such, a septin-based barrier becomes necessary for the maintenance of the polarity. The involvement of a barrier function also suggests the existence of a positive feedback loop between Cdc42p and septins: although Cdc42p is needed for the polarized recruitment of septin, the formation of the septin ring in turn helps to restrict the diffusion of Cdc42p from the daughter cell. Future quantitative studies in live cells may reveal the dynamic interplays between the septin-based diffusion restriction and endocytosis-based recycling of Cdc42p during asymmetric cell growth.
In summary, we demonstrate that endo-exocytic coupling and septins synergistically contribute to the maintenance of Cdc42p at the daughter cell membrane (Figure 7). First, the delivery of Cdc42p involves at least two routes of exocytic trafficking to the cell surface. The route involving endosomal trafficking serves as a mechanism that couples the removal of Cdc42p from the cell surface with its subsequent redelivery to the daughter cell membrane. As the endo-exocytotic pathways are not sufficient for maintaining Cdc42p at the daughter cell, a diffusion barrier imposed by septins helps to further restrict the dispersal of Cdc42p along the plasma membrane. These factors may work with Rdi1p (Slaughter et al., 2009b) to synergistically maintain Cdc42p polarization during asymmetrical growth of the yeast cell. Collectively, the multiple inputs to Cdc42p may fine-tune the spatial and temporal distribution of this master regulator of cell morphogenesis for polarized cell growth and possibly effective adaptation to the changing environments.
These regulatory mechanisms on small GTPases may be evolutionarily conserved in mammalian cells (Palamidessi et al., 2008; Misaki et al., 2010). Studies using budding yeast will continue to present important conceptual frameworks for future studies of cell morphogenesis in higher eukaryotic systems.
MATERIALS AND METHODS
Strains and growth conditions
The genotypes of the yeast strains used in this project are listed in Table 1. Standard methods were used for yeast media, growth, and genetic manipulations (Guthrie and Fink, 1991). For the generation of deletion mutants, primers targeting the promoter and terminator regions of RVS161, VPS1, and PEP12 were used to amplify the DNA fragments containing KanMX as well as the flanking sequences of RVS161, VPS1, and PEP12 from deletion strains generated in the S. cerevisiae Genome Deletion Project. The obtained PCR products were transformed into the wild-type, sec5-24, or cdc12-6 yeast cells to disrupt the RVS161, VPS1, or PEP12 genes from the chromosome, and cells were selected by growing on plates containing 100 μg/ml G418. Expression of GFP-tagged Cdc42p in yeast was performed by integrating GFP-Cdc42 into the ura3-52 locus (YIp211-GFP-CDC42, URA3, cut with StuI for integration). To observe septins by fluorescence microscopy, a plasmid containing the GFP-tagged septin subunit CDC3 (CEN, URA3) was transformed into yeast cells. The culturing and G0 synchronization of yeast cells by saturating growth were performed as previously described (Zhang et al., 2008).
Table 1:
Yeast strains and genotypes
| Strain | Genotype |
|---|---|
| NY179 | Mat a ura3-52 leu2-3, 112 |
| NY776 | Mat α ura3-52 leu2-3, 112 sec5-24 |
| NY784 | Mat α ura3-52 leu2-3, 112 sec10-2 |
| GY1132 | Mat a trp1, leu2, his3, ura3, lys2 |
| GY1201 | Mat α ura3-52 leu2-3 112 his3Δ200 trp1 exo70::HIS3 exo70-38,TRP1 CEN |
| GY2836 | Mat a ura3-52 leu2-3 112 trp1 his3Δ200 exo70::HIS3 exo70-35, TRP1 CEN |
| GY2955 | Mat α his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 vps1::KanMX |
| GY2956 | Mat α his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 pep12::KanMX |
| GY3016 | Mat a ura3-52 leu2-3, 112 rvs161::KanMX |
| GY3017 | Mat α ura3-52 leu2-3, 112 sec5-24 rvs161::KanMX |
| GY3500 | Mat α ura3-52, leu2, his3, trp1, end4-1 |
| GY3503 | Mat a leu2, ura3, cdc12-6 |
| GY3586 | Mat a leu2, ura3, cdc12-6 rvs161::KanMX |
| GY3601 | Mat α ura3-52 leu2-3 112 his3Δ200 trp1 pep12::LEU2 |
| GY3605 | Mat α ura3-52 leu2-3, 112 sec5-24, GFP-Cdc42 URA3 |
| GY3606 | Mat α ura3-52 leu2-3, 112 sec5-24 rvs161::KanMX, GFP-Cdc42 URA3 |
| GY3607 | Mat α leu2-3112 ura3-52 trp1 his3Δ200 pep12::LEU2, GFP-Cdc42 URA3 |
| GY3608 | Mat a ura3-52 leu2-3, 112, Cdc3-GFP CEN URA3 |
| GY3609 | Mat a ura3-52 leu2-3, 112 rvs161::KanMX, Cdc3-GFP CEN URA3 |
| GY3610 | Mat a leu2, ura3, cdc12-6, Cdc3-GFP CEN URA3 |
| GY3611 | Mat a leu2, ura3, cdc12-6 rvs161::KanMX, Cdc3-GFP CEN URA3 |
Immunofluorescence microscopy
Yeast strains were grown overnight at 25°C in yeast-extract peptone dextrose (YPD). The final cell density (A600) was between 0.5 and 1.0 U. The cells were then shifted to their restrictive temperatures for various times as indicated. After the shift, the cells were fixed with 4.4% formaldehyde. The cells were then transferred to isotonic media and the cell walls removed by zymolyase and β-mercapthoethanol. After attachment to 8-well microslides treated with poly-lysine (Carlson Scientific, Peotone, IL), the spheroplasts were treated with 0.5% SDS in phosphate-buffered saline (PBS) buffer containing 1 mg/ml bovine serum albumin (PBS/bovine serum albumin [BSA]) for 5 min. The cells were then washed 10 times with 50 μl per well of PBS/BSA, and nonspecific binding was blocked for 30 min in the same buffer. The incubation with polyclonal antibody against Cdc42p (diluted 1:500, kind gift from Keith Kozminski) was performed overnight at 4°C. After 10 washes with PBS/BSA, the cells were incubated for 1 h at room temperature with AlexaFluor594-conjugated goat anti–rabbit immunoglobulin (Ig)G antibody (diluted 1:1000; Molecular Probes, Eugene, OR). The cells were washed 10 times with PBS/BSA, and the slides were then mounted with Fluoro-Gel mounting media (Electron Microscopy Sciences, Hatfield, PA). The digital images were captured by a fluorescence microscope (DM IRB; Leica, Bannockburn, IL) using a 100× objective and a high-resolution charge-coupled device camera (model ORCA-ER; Hamamatsu Photonics, Bridgewater, NJ).
FRAP experiments
The dynamic localization of GFP-tagged Cdc42p in small-budded cells was analyzed by FRAP. Cells were grown to log phase in synthetic complete media (SC) at 25°C. One or two milliliters of culture was pelleted and resuspended in 20 μl fresh SC media. Slides containing agar pads were prepared with 2% agar dissolved in SC medium containing appropriate amino acids. Next, 2 μl of the suspension was dropped onto the agar pads; Corning number 1½ coverslips were placed on top of the agar pads; and a mixture of Vaseline, lanolin, and candlewax (modified form of VALAP) was used to seal in the cells and prevent drying. Bleaching and imaging was performed using a Leica TCS SL laser scanning confocal microscope using an oil-immersion 63× objective. Small buds were photobleached using an Argon laser at 488 nm, and pre- and postbleach images were collected over time. GFP-Cdc42p intensity was normalized to the intensity at final equilibrium, which is calculated as the average intensity from 60 s to 80 s. For each time point, the background intensity was subtracted and the data were normalized by dividing each data point by the average prebleached value. The fluorescence recovery over time was analyzed by SigmaPlot. Curve is fit by the equation I = a(1 − e−bt), where I is the normalized intensity. The recovery half-times were calculated from the graph by measuring the time when the cells reach half of the final intensity.
Separation of vesicles by Percoll density gradients
Vesicle fractionation was performed as previously described (Harsay and Bretscher, 1995; He et al., 2007). Briefly, the sec10-2 mutant cells carrying a copy of HA-Cdc42p at the URA3 locus on the chromosome were grown overnight in YPD media. Yeast cells (100-200 A600 U) were washed with YPD media and shifted to 37°C for 90 min in YPD media containing 0.1% glucose to induce invertase production and post-Golgi secretory accumulation. After washing in cold PBS containing 10 mM sodium azide, cells were resuspended in spheroplast medium (1.4 M sorbitol, 50 mM KPi [pH 7.5], 10 mM azide, 40 mM β-mercapthoethanol) with 0.15 mg/ml of zymolyase-100T and converted to spheroplasts during a 45-min incubation at 37°C. The spheroplasts were resuspended in 1.5 ml of lysis buffer (0.8 M sorbitol in 10 mM triethanolamine, 1 mM EDTA [pH 7.2]) containing protease inhibitors, homogenized by 20 strokes with a 2-ml Wheaton tissue grinder, and centrifuged at 500 × g for 3 min. The supernatant was spun at 10,000 × g in an Eppendorf 5415R bench top centrifuge for 10 min at 4°C, and then further centrifuged at 100,000 × g in a TLA100 rotor (Beckman Coulter, Brea, CA) for 1 h at 4°C to give the final supernatant and pellet. These pellets were fractionated on 20–55% Percoll step gradients (4 ml in TLA100.3 tubes), and 160-μl fractions were collected for density measurement, invertase quantification and Western blot analysis with anti-Sec4p and anti-Bgl2p polyclonal antibodies. Invertase assays were performed as described previously (Novick et al., 1980).
Supplementary Material
Acknowledgments
We thank Erfei Bi for helpful discussions on Cdc42p polarity and Edina Harsay for discussions on exocytic routes in yeast. We are grateful to Keith Kozminski for the anti-Cdc42p polyclonal antibody, Erfei Bi for the HA-Cdc42p and GFP-Cdc42p constructs, and Scott Emr and Chris Burd for endocytosis strains. We would also like to thank Peng Yue for his help on the FRAP analysis. This work is supported by NIH RO1 Grant GM64690 (to W.G.); the National Science Foundation of China (Grant No. 30900720), and the Tianjin Science Foundation (No. 09JCYBJC09000) (to P.W.). K.O. was supported by NIH Postdoctoral Fellowship F32GM08201.
Abbreviations used:
- BSA
bovine serum albumin
- FRAP
fluorescence recovery after photobleaching
- PBS
phosphate-buffered saline
- PM
plasma membrane
- PVC
prevacuolar compartment
- SC
synthetic complete media
- TGN
trans-Golgi network
- YP
yeast-extract peptone
- YPD
yeast-extract peptone dextrose
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-06-0484) on January 5, 2011.
REFERENCES
- Balasubramanian N, Meier JA, Scott DW, Norambuena A, White MA, Schwartz MA. RalA-exocyst complex regulates integrin-dependent membrane raft exocytosis and growth signaling. Curr Biol. 2010;20:75–79. doi: 10.1016/j.cub.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Mermall V, Mooseker MS, Snyder M. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell. 2000;5:841–851. doi: 10.1016/s1097-2765(00)80324-x. [DOI] [PubMed] [Google Scholar]
- Becherer KA, Rieder SE, Emr SD, Jones EW. Novel syntaxin homologue, Pep12p, required for the sorting of lumenal hydrolases to the lysosome-like vacuole in yeast. Mol Biol Cell. 1996;7:579–594. doi: 10.1091/mbc.7.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS. Moving membrane up to the front of migrating cells. Cell. 1996;85:465–467. doi: 10.1016/s0092-8674(00)81246-5. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Stevens TH. Vacuole biogenesis in S. cerevisiae: protein transport pathways to the yeast vacuole. Microbiol Mol Biol Rev. 1998;62:230–247. doi: 10.1128/mmbr.62.1.230-247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butty AC, Perrinjaquet N, Petit A, Jaquenoud M, Segall JE, Hofmann K, Zwahlen C, Peter M. A positive feedback loop stabilizes the guanine-nucleotide exchange factor Cdc24 at sites of polarization. EMBO J. 2002;21:1565–1576. doi: 10.1093/emboj/21.7.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic. 2006;7:14–21. doi: 10.1111/j.1600-0854.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- Chuang JS, Schekman RW. Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J Cell Biol. 1996;135:597–610. doi: 10.1083/jcb.135.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear E, Stevens T. Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim Biophys Acta. 1998;1404:211–230. doi: 10.1016/s0167-4889(98)00058-5. [DOI] [PubMed] [Google Scholar]
- Desclozeaux M, Venturato J, Wylie FG, Kay JG, Joseph SR, Le HT, Stow JL. Active Rab11 and functional recycling endosome are required for E-cadherin trafficking and lumen formation during epithelial morphogenesis. Am J Physiol Cell Physiol. 2008;295:C545–C556. doi: 10.1152/ajpcell.00097.2008. [DOI] [PubMed] [Google Scholar]
- Eitzen G, Thorngren N, Wickner W. Rho1p and Cdc42p act after Ypt7p to regulate vacuole docking. EMBO J. 2001;20:5650–5656. doi: 10.1093/emboj/20.20.5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S. Cdc42–the centre of polarity. J Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- Gao L, Bretscher A. Polarized growth in budding yeast in the absence of a localized formin. Mol Biol Cell. 2009;20:2540–2548. doi: 10.1091/mbc.E09-03-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard SR, Levi BP, Stevens TH. Pep12p is a multifunctional yeast syntaxin that controls entry of biosynthetic, endocytic and retrograde traffic into the prevacuolar compartment. Traffic. 2000;1:259–269. doi: 10.1034/j.1600-0854.2000.010308.x. [DOI] [PubMed] [Google Scholar]
- Gurunathan S, David D, Gerst JE. Dynamin and clathrin are required for the biogenesis of a distinct class of secretory vesicles in yeast. EMBO J. 2002;21:602–614. doi: 10.1093/emboj/21.4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C, Fink GR. Guide to Yeast Genetics and Molecular Biology, Methods in Enzymology, Vol. Vol. 169. San Diego, CA:: Academic Press; 1991. [PubMed] [Google Scholar]
- Harsay E, Bretscher A. Parallel secretory pathways to the cell surface in yeast. J Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay E, Schekman R. A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway. J Cell Biol. 2002;156:271–285. doi: 10.1083/jcb.200109077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Xi F, Zhang J, TerBush D, Zhang X, Guo W. Exo70p mediates the secretion of specific exocytic vesicles at early stages of the cell cycle for polarized cell growth. J Cell Biol. 2007;176:771–777. doi: 10.1083/jcb.200606134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KR, D’Hondt K, Chang J, Newpher T, Huang K, Hudson RT, Riezman H, Lemmon SK. Scd5p and clathrin function are important for cortical actin organization, endocytosis, and localization of sla2p in yeast. Mol Biol Cell. 2002;13:2607–2625. doi: 10.1091/mbc.E02-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell AS, Savage NS, Johnson SA, Bose I, Wagner AW, Zyla TR, Nijhout HF, Reed MC, Goryachev AB, Lew DJ. Singularity in polarization: rewiring yeast cells to make two buds. Cell. 2009;139:731–743. doi: 10.1016/j.cell.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irazoqui JE, Gladfelter AS, Lew DJ. Scaffold-mediated symmetry breaking by Cdc42p. Nat Cell Biol. 2003;5:1062–1070. doi: 10.1038/ncb1068. [DOI] [PubMed] [Google Scholar]
- Irazoqui JE, Howell AS, Theesfeld CL, Lew DJ. Opposing roles for actin in Cdc42p polarization. Mol Biol Cell. 2005;16:1296–1304. doi: 10.1091/mbc.E04-05-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase M, Luo J, Nagaraj S, Longtine M, Kim HB, Haarer BK, Caruso C, Tong Z, Pringle JR, Bi E. Role of a Cdc42p effector pathway in recruitment of the yeast septins to the presumptive bud site. Mol Biol Cell. 2006;17:1110–1125. doi: 10.1091/mbc.E05-08-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DI. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Biol Rev. 1999;63:54–105. doi: 10.1128/mmbr.63.1.54-105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jović M, Naslavsky N, Rapaport D, Horowitz M, Caplan S. EHD1 regulates beta1 integrin endosomal transport: effects on focal adhesions, cell spreading and migration. J Cell Sci. 2007;120:802–814. doi: 10.1242/jcs.03383. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Sun Y, Drubin DG. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115:475–487. doi: 10.1016/s0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- Kozminski KG, Alfaro G, Dighe S, Beh CT. Homologues of oxysterol-binding proteins affect Cdc42p- and Rho1p-mediated cell polarization in S. cerevisiae. Traffic. 2006;7:1224–1242. doi: 10.1111/j.1600-0854.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Kozubowski L, Saito K, Johnson JM, Howell AS, Zyla TR, Lew DJ. Symmetry-breaking polarization driven by a Cdc42p GEF-PAK complex. Curr Biol. 2008;18:1719–1726. doi: 10.1016/j.cub.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ballif BA, Powelka AM, Dai J, Gygi SP, Hsu VW. Phosphorylation of ACAP1 by Akt regulates the stimulation-dependent recycling of integrin beta1 to control cell migration. Dev Cell. 2005;9:663–673. doi: 10.1016/j.devcel.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Lombardi R, Riezman H. Rvs161p and Rvs167p, the two yeast amphi-physin homologs, function together in vivo. J Biol Chem. 2001;276:6016–6022. doi: 10.1074/jbc.M008735200. [DOI] [PubMed] [Google Scholar]
- Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–422. doi: 10.1016/j.cell.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey LM, Macara IG. Widely conserved signaling pathways in the establishment of cell polarity. Cold Spring Harb Perspect Biol. 2009;1(2):a001370. doi: 10.1101/cshperspect.a001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki R, Morimatsu M, Uemura T, Waguri S, Miyoshi E, Taniguchi N, Matsuda M, Taguchi T. Palmitoylated Ras proteins traffic through recycling endosomes to the plasma membrane during exocytosis. J Cell Biol. 2010;191:23–29. doi: 10.1083/jcb.200911143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller O, Johnson DI, Mayer A. Cdc42p functions at the docking stage of yeast vacuole membrane fusion. EMBO J. 2001;20:5657–5665. doi: 10.1093/emboj/20.20.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ. Remodeling epithelial cell organization: transitions between front-rear and apical-Basal polarity. Cold Spring Harb. Perspect Biol. 2009;1(1):a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Conibear E, Stevens TH. Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J Cell Biol. 1995;129:35–46. doi: 10.1083/jcb.129.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for posttranslational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Orlando K, Guo W. Membrane organization and dynamics in cell polarity. Cold Spring Harb Perspect Biol. 2009;1(5):a001321. doi: 10.1101/cshperspect.a001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamidessi A, Frittoli E, Garré M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Park HO, Bi E. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol Mol Biol Rev. 2007;71:48–96. doi: 10.1128/MMBR.00028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao HL, Machado IM, Payne GS. NPFXD-mediated endocytosis is required for polarity and function of a yeast cell wall stress sensor. Mol Biol Cell. 2007;18:57–65. doi: 10.1091/mbc.E06-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D, Legesse-Miller A, Gao L, Dong Y, Bretscher A. Mechanisms of polarized growth and organelle segregation in yeast. Annu Rev Cell Dev Biol. 2004;20:559–591. doi: 10.1146/annurev.cellbio.20.010403.103108. [DOI] [PubMed] [Google Scholar]
- Raths S, Rohrer J, Crausaz F, Riezman H. end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in S. cerevisiae. J Cell Biol. 1993;120:55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman TJ, Sawyer MM, Johnson DI. S. cerevisiae Cdc42p localizes to cellular membranes and clusters at sites of polarized growth. Eukaryot Cell. 2002;1:458–468. doi: 10.1128/EC.1.3.458-468.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberg KJ, Rowley N, Kaiser CA. Physiological regulation of membrane protein sorting late in the secretory pathway of S. cerevisiae. J Cell Biol. 1997;137:1469–1482. doi: 10.1083/jcb.137.7.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter BD, Smith SE, Li R. Symmetry breaking in the life cycle of the budding yeast. Cold Spring Harb Perspect Biol. 2009a;1(3):a003384. doi: 10.1101/cshperspect.a003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter BD, Das A, Schwartz JW, Rubinstein B, Li R. Dual modes of cdc42 recycling fine-tune polarized morphogenesis. Dev Cell. 2009b;17:823–835. doi: 10.1016/j.devcel.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strochlic TI, Setty TG, Sitaram A, Burd CG. Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling. J Cell Biol. 2007;177:115–125. doi: 10.1083/jcb.200609161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez-Taubas J, Pelham HR. Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr Biol. 2003;13:1636–1640. doi: 10.1016/j.cub.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Altschuler S, Wu L, Li R. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science. 2003;299:1231–1235. doi: 10.1126/science.1080944. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Wai SC, Schmidt T, Li R. Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J Cell Biol. 2004;166:889–900. doi: 10.1083/jcb.200405061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan DS, Dancis A, Klausner RD. Restriction of copper export in S. cerevisiae to a late Golgi or postGolgi compartment in the secretory pathway. J Biol Chem. 1997;272:25787–25793. doi: 10.1074/jbc.272.41.25787. [DOI] [PubMed] [Google Scholar]
- Zajac A, Sun X, Zhang J, Guo W. Cyclical regulation of the exocyst and cell polarity determinants for polarized cell growth. Mol Biol Cell. 2005;16:1500–1512. doi: 10.1091/mbc.E04-10-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol. 2008;180:145–158. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M, Chuang JS, Tsung M, Hamamoto S, Schekman R. Chs6p-dependent anterograde transport of Chs3p from the chitosome to the plasma membrane in S. cerevisiae. Mol Biol Cell. 1998;9:1565–1576. doi: 10.1091/mbc.9.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M, Preuss D, Mulholland J, O’Brien JM, Botstein D, Johnson DI. Subcellular localization of Cdc42p, a S. cerevisiae GTP-binding protein involved in the control of cell polarity. Mol Biol Cell. 1993;4:1307–1316. doi: 10.1091/mbc.4.12.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.