Abstract
Background
Cysteine is a glutathione precursor, but is also a homocysteine byproduct. We prospectively evaluated relationships between fasting plasma concentrations of total cysteine and total homocysteine, and subsequent myocardial infarction (MI) in women.
Methods
Among 32826 women who provided blood samples between 1989 and 1990, 239 were diagnosed with incident MI after blood collection, but before July 1998. Of these women, 144 had provided a post-fast sample. We matched controls to cases 2:1 by age, cigarette smoking status, and month and fasting status at the time of blood collection. We used conditional logistic regression to adjust for confounding.
Results
Fasting total cysteine was positively related to MI risk in matching factor-adjusted analyses [RR for highest vs. lowest quartile 3.50 (95% CI 1.44, 8.52)]. However, after controlling for conventional risk factors of MI, it was not independently associated with risk [RR for highest vs. lowest quartile 1.32 (95% CI 0.42, 4.12; P trend=0.10)]. Fasting homocysteine was positively associated with MI risk; the multivariable adjusted rate ratio (RR) for the highest versus the lowest quartile was 3.37 (95% CI 1.30, 8.70; P trend=0.014).
Conclusions
Fasting plasma concentration of total homocysteine, but not total cysteine was positively associated with MI risk.
Although substantial evidence demonstrates that plasma total homocysteine concentration (tHcy) is a predictor of coronary artery disease (CAD), recent randomized controlled trials1-5 have cast doubt on the hypothesis that homocysteine is a causal risk factor for cardiovascular events. Despite reducing plasma tHcy concentration, treatment with high dose folic acid, vitamin B12 and vitamin B6 did not reduce the incidence of the combined outcome of myocardial infarction, stroke and cardiovascular death1-3, 5. However, these trials come against the background of a vast literature that indicates that elevated tHcy concentration is associated with harm6, 7. Intracellular homocysteine is a byproduct of methionine metabolism and is either (1) re-methylated to methionine, or (2) catabolized by vitamin B6-dependent transsulfuration to cysteine8, 9 (See Figure I). Cysteine is a major precursor of glutathione (γ–glutamyl-cysteinyl-glycine; GSH)10 that protects cells from oxidative damage by removing hydrogen peroxide8. It appears that cysteine is a rate-limiting amino acid for GSH synthesis in humans11. In addition to conversion from homocysteine, intracellular cysteine also results from conversion from other amino acids12, as well as from the gamma glutamyl transferase (GGT) dependent recycling of the amino acids from extracellular glutathione13.
Figure I.
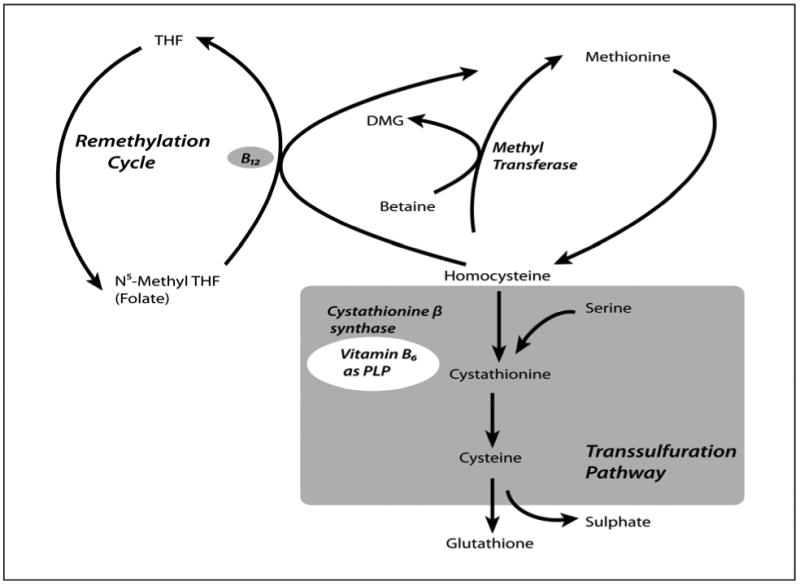
Metabolism of Homocysteine and Cysteine, including the transsulfuration pathway.
THF is tetrahydrofolate and TMG is trimetylglycine
The one prospective study that has evaluated the relationship between total cysteine (tCys) and risk of cardiovascular disease hospitalizations and cardiovascular death found no significant relationship14. A few cross-sectional and retrospective studies have compared plasma tCys concentration between patients with established CAD to controls and found conflicting results15-17. We hypothesize that while plasma homocysteine concentration is positively associated with increased risk of myocardial infarction (MI), plasma total cysteine is likely to be inversely associated with risk of MI. We previously studied the total homocysteine: MI relationship18, but did not consider total homocysteine in the fasting state. Using prospectively collected samples in a nested case control design within the Nurses' Health Study cohort, we seek to answer two primary questions: 1) What is the relationship between plasma concentration of total homocysteine, in the fasting state, and subsequent risk of myocardial infarction? and 2) What is the relationship between plasma concentration of total cysteine, in the fasting state, and subsequent risk of myocardial infarction?
Methods
Participants
The Nurses' Health Study cohort was established in 1976 when 121,700 female registered nurses, 30–55 years of age, completed a mailed questionnaire to study the relationship between diet and lifestyle and subsequent disease. We continue to follow the cohort continues every 2 years by questionnaire to update exposure status and to identify cases of newly diagnosed disease. Data have been collected on many coronary artery disease risk factors, including height, weight, cigarette smoking, alcohol use, physical activity, age at menopause, post-menopausal hormone use, diagnosis of hypertension and diabetes mellitus, history of aspirin use, history of hypercholesterolemia, and parental family history of myocardial infarction. BMI was calculated by dividing the most recent weight before blood collection by the square of height reported in 1976. We calculated total alcohol intake per day as the sum of the alcohol content contributed from beer, wine, and liquor, assuming 12.8 g of ethanol for 360 mL (12 oz) of beer, 11.0 g for 120 mL (4 oz) of wine, and 14.0 g for 45 mL (1.5 oz) of liquor.
From 1989 through 1990, blood samples were collected from 32,826 cohort members (27% of the original cohort) who were then 43–69 years of age. Participants who provided blood samples were similar to those who did not. Details regarding the blood collection methods have been published previously19. Briefly, each woman arranged to have her blood drawn and then shipped, via overnight courier with an ice pack, to our laboratory, where it was processed and separated into plasma, red blood cells, and white blood cell components. Within 24-36 hours of being drawn, we received 97% of the samples in our laboratory. Since collection, samples have been archived at -130 °C or colder in continuously monitored liquid nitrogen freezers. As of 1998, follow-up of women who provided blood samples was over 99% complete.
We included as cases, women who provided a blood sample after fasting 10 hours or more, reported no myocardial infarction before blood collection, and who were diagnosed with myocardial infarction after blood collection but before June 1, 1998, the end of follow-up for this analysis. Overall, 144 cases of myocardial infarction (21 fatal) with adequate plasma were identified. For all cases of myocardial infarction, we requested hospital records (we could not obtain records for three cases, but all cases were retained for analysis). Myocardial infarction was classified as confirmed if symptoms met the criteria of the World Health Organization (typical symptoms and either diagnostic electrocardiographic changes or elevated cardiac enzymes).
The median (10th to 90th percentiles) time from blood collection to diagnosis was 48 months (16, 91). Where possible, two control subjects, with no diagnosis of myocardial infarction at the time of the case's diagnosis, were selected at random, matched to the case subject by age (±2 years), cigarette smoking status (current, past, and never smoker), and month of blood collection. Most (81% percent) of case-control matches were exact; the most relaxed match was within ±3 years of age, and within ±6 months of blood collection. We analyzed the matched sets together. The Committee on the Use of Human Subjects in Research at the Brigham and Women's Hospital approved the study. All subjects gave their informed consent.
Laboratory Analysis
Plasma concentrations of tHcy and tCys were measured with high performance liquid chromatography using fluorescence detection at the Jean Mayer United States Department of Agriculture Human Nutrition Research Center on Aging at Tufts University20. These assays were performed in three batches in 1994, 1996 and in 1998. Matched case and control samples were handled identically and measured within the same batch. We interspersed replicate plasma samples, labeled to preclude their identification by the laboratory, to assess laboratory precision. The intra-assay coefficients of variation for plasma tHcy and tCys, were 7.5% and 5.4% respectively.
Creatinine was measured using a modified Jaffe method. The intra-assay coefficient of variation was 12.8%. We used a modified version of the Cockcroft-Gault formula to estimate creatinine clearance21, 22. This formula is based on fat-free body mass and has the advantage of attenuating the overestimation of creatinine clearance (CrCl) in obese individuals with the Cockcroft-Gault equation while providing similar results in average-weight women22. The formula for women is (146 - age) × [(0.287 × weight) + (9.74 × height2)]/(60 × creatinine), with age in years, weight in kilograms, height in meters, and creatinine in mg/dl; the units for CrCl are ml/min. This formula has been validated compared with measured CrCl22.
Total cholesterol was measured enzymatically23 with an intra-assay coefficient of variation of 1.7%. High-density lipoprotein cholesterol (HDL) was measured using Hitachi 911 analyzer24, with an intra-assay coefficient of variation of 2.5%. C-reactive protein (CRP) was determined with a high sensitivity immunoturbidimetric assay on a Hitachi 911 analyzer, with an intra-assay coefficient of variation of 1.4%.
The laboratory personnel that conducted the assays had no knowledge of the case–control status of the samples.
Statistical Analysis
We estimated adjusted Spearman's correlation coefficients of tHcy and tCys to the other covariates among cases and controls separately, after first calculating residuals from linear regression with loge(tHcy), tCys, loge(HDL-to-total cholesterol ratio),loge(C-reactive protein), loge(BMI), loge(physical activity), and alcohol as outcome variables and age (modeled as quartiles), and batch as covariates. Log-transformation was performed where appropriate, to improve the normality of the residuals.
Conditional logistic regression was used to estimate odds ratios, and 95% confidence intervals (CI), between quartiles of the exposure variable (tHcy and tCys), and risk of MI. These odds ratios were taken as estimates25 of rate ratios (RR). We additionally controlled for potential confounders that were not part of the matching scheme. Indicator functions were used to model the categorical covariates, including menopausal status (post-menopausal and unknown-menopausal status versus pre-menopausal), current post-menopausal hormone use at blood collection (use within the previous 3 months), history of diabetes mellitus, hypertension, parental history of myocardial infarction prior to age 60, hypercholesterolemia, and aspirin use (use for greater than 14 days per month, use for 1-14 days per month, versus no use). To control more appropriately for confounding and other co-variation by continuous variables (alcohol use, BMI, CrCl, plasma CRP, total-to-HDL cholesterol), we used natural cubic splines26, with four degrees of freedom, to smooth the relationships with the log-odds of myocardial infarction. Physical activity in metabolic equivalents (MET) – hours per week in 1988 was entered into the regression models as indicator functions of the quarters, since natural cubic splines was sub-optimal for this variable. We estimated the following models: i) Control for matching factors only, including age (as a linear function); ii) Control for conventional non-biomarker risk factors for MI (considered the definitive model). As a form of sensitivity analysis, we also estimated models with plasma-derived covariates: iii) Model ii plus CrCl, total-to-HDL cholesterol ratio, and CRP; and iv) additionally controls for plasma concentrations of tCys (model with homocysteine) or tHcy (model with cysteine). We used medians to impute the few missing values in the covariates physical activity, CRP, creatinine, and total-to-HDL cholesterol ratio (all less than 4%).
We conducted tests for trend by modeling the hormone level as a linear continuous covariate and calculating a Wald statistic25. All P values are two-sided, and when less than 0.05, is statistically significant. The software used for statistical analysis were SAS release 9.127 and S-plus version 828.
Funding Sources
This study was supported by Public Health Service grant HL34594 from the National Institutes of Health, Department of Health and Human Services, as well as a grant from Merck. The corresponding author was also supported by the American Heart Association award 0475016N. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of this paper and its final contents.
Results
Table I shows the distribution of risk factors for myocardial infarction at the time of blood sampling among case women and matched controls. The median age (10th, 90th percentiles) at the time of blood collection was 63 (52, 68).
Table I.
Distribution of covariates in Myocardial Infarction case and matched control participants
| Covariate | Cases, N=144 | Controls, N=258 |
|---|---|---|
| Median (10th to 90th percentiles); Number (%)* | Median (10th to 90th percentiles); Number (%)* | |
| Plasma total Cysteine (nmol/ml) | 254.3 (206.0, 346.0) | 249.5 (187.2, 318.0) |
| Plasma total Homocysteine (nmol/ml) | 11.0 (7.2,16.0) | 9.9 (6.9, 14.8) |
| Age at blood draw (years) | 63 (53, 68) | 63 (52, 68) |
| Current smokers * | 45 (31.3) | 78 (30.2) |
| Body Mass Index (kg/m2) | 25.9 (20.5, 35.4) | 24.3 (20.4, 31.3) |
| Waist to Hip Ratio | 0.81 (0.73, 0.89) | 0.78 (0.71, 0.88) |
| Average physical activity (MET-hours per week) | 7.8 (0.2, 37.2) | 9.0 (0.9, 34.2) |
| Mean alcohol consumption (grams/day) | 1.3 (0, 16.2) | 3.6 (0, 22.3) |
| Parental history of Myocardial Infarction * | 56 (38.9) | 58 (22.5) |
| History of Hypertension * | 86 (59.7) | 73 (28.3) |
| History of Diabetes Mellitus * | 31 (21.5) | 15 (5.8) |
| History of Hypercholesterolemia * | 17 (11.8) | 27 (10.5) |
| Use of Aspirin in 1988 * | ||
| None | 44 (30.6) | 81 (31.4) |
| 1-14 days per month | 55 (38.2) | 119 (46.1) |
| >14 days per month | 40 (27.8) | 51 (19.8) |
| Post-menopausal * | 128 (89.0) | 224 (86.8) |
| Use of female hormones in the three months prior to blood collection | 45 (31.0) | 94 (36.4) |
| Plasma total cholesterol (mg/dL) | 240 (180, 292) | 229 (181, 279) |
| Plasma HDL cholesterol (mg/dL) | 50.7 (35.5, 71.1) | 57.4 (41.7, 85.3) |
| Ratio of plasma total to HDL cholesterol | 4.45 (3.30, 6.93) | 3.96 (2.62, 5.66) |
| Plasma C reactive protein (mg/L) | 3.6 (0.6, 15.6) | 2.5 (0.5, 9.6) |
| Plasma Creatinine (mg/dl) | 0.73 (0.57,1.03) | 0.72 (0.58, 0.88) |
| Estimated Creatinine Clearance (ml/min) | 88.4 (59.8, 121.4) | 87.9 (67.4,116.3) |
Median age (range; 10th and 90th percentiles) of diagnosis was 66.9 (55.4,73.7). Median time from blood sample to diagnosis was 4.0 years (1.25, 7.6). Waist-hip ratio was available for 101 cases and 178 controls. Information was available for the other covariates in over 95% of study participants.
Number (%)
After adjusting for age and analysis batch, fasting plasma concentrations of tHcy correlated directly with concentrations of plasma tCys, and inversely with physical activity, among control women. In addition to its correlation with tHcy, fasting plasma concentrations of tCys correlated directly with body mass index, and inversely with alcohol consumption, among control women (Table II). After adjusting for age and analysis batch, fasting plasma concentrations of tHcy correlated directly with concentrations of plasma tCys, and inversely with physical activity, among control women. Plasma tHcy correlated inversely with creatinine clearance among cases. In addition to its correlation with tHcy, fasting plasma concentrations of tCys correlated directly with body mass index, and inversely with alcohol consumption among control women (Table II).
Table II.
Adjusted Spearman's correlation coefficients between total Homocysteine and total Cysteine and other continuous variables among case and control study participants
| Covariate | Spearman's correlation coefficient (P value) adjusted for age and batch | |||
|---|---|---|---|---|
| With total Homocysteine | With total Cysteine | |||
| Cases | Controls | Cases | Controls | |
| Plasma Total Cysteine (nmol/ml) | 0.35 (<0.001) | 0.35 (<0.001) | ||
| Estimated Creatinine Clearance (ml/min) | -0.32 (<0.001) | -0.10 (0.10) | -0.10 (0.22) | -0.10 (0.11) |
| BMI (kg/m2) | 0.001 (0.99) | 0.09 (0.14) | 0.12 (0.16) | 0.20 (0.001) |
| Average physical activity (MET-hours per week) | 0.002 (0.98) | -0.15 (0.01) | -0.05 (0.59) | -0.06 (0.32) |
| Mean alcohol consumption (grams/day) | 0.09 (0.30) | 0.11 (0.07) | 0.01 (0.93) | -0.16 (0.01) |
| Plasma C-reactive Protein (mg/L) | 0.08 (0.35) | 0.002 (0.98) | 0.15 (0.07) | 0.11 (0.07) |
| Plasma total:HDL cholesterol ratio | 0.04 (0.60) | 0.005 (0.94) | 0.12 (0.16) | 0.04 (0.50) |
Fasting concentrations of tHcy were significantly positively associated with subsequent risk of MI, both controlling for matching factors only [RR (95%CI) for top vs. bottom quartile 2.89 (1.44, 5.80), p value for linear trend 0.001], or after adjusting for conventional risk factors [RR (95%CI) for top vs. bottom quartile 3.37 (1.30, 8.70), p value for linear trend 0.014]. The shape and magnitude of the relationship changed little with addition of plasma covariates to the regression model (model iii). There was some augmentation of the relationship when we additionally adjusted for plasma tCys (Table III). Fasting tCys was positively associated with risk of MI in a model that adjusted for matching factors only [RR (95%CI) for top vs. bottom quartile 3.50 (1.44, 8.52), p value for linear trend <0.001]. However, there was no clear relationship seen after adjustment for conventional MI risk factors [RR (95%CI) for top vs. bottom quartile 1.32 (0.42, 4.12), p value for linear trend 0.10]. Additional adjustment for plasma covariates resulted in further attenuation of the relationship (Table III).
Table III.
Rate ratio (RR) of Myocardial Infarction by quartile of fasting plasma total Homocysteine and total Cysteine, in the Nurses' Health Study.
| Quartiles | P value for linear trend | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Total Homocysteine, (nmol/mL) | <8.2 (median 7.2) |
8.2-9.8 (median 9.3) |
9.9-12.1 (median 11.1) |
≥12.2 (median 13.8) |
|
| No. of cases/No. of controls | 27/69 | 30/63 | 38/61 | 50/67 | |
| Matching factor adjusted OR (95% CI) | 1.0 (referent) | 1.47 (0.74, 2.94) | 2.06 (1.06, 3.98) | 2.89 (1.44, 5.80) | 0.001 |
| Adjusted OR* (95%CI) | 1.0 (referent) | 1.63 (0.65, 4.06) | 1.84 (0.74, 4.60) | 3.37 (1.30, 8.70) | 0.014 |
| Adjusted OR† (95%CI) | 1.0 (referent) | 1.58 (0.55, 4.54) | 1.88 (0.67, 5.27) | 3.83 (1.22, 12.03) | 0.04 |
| Adjusted OR‡ (95%CI) | 1.0 (referent) | 1.91 (0. 64, 5.69) | 2.25 (0.75, 6.73) | 4.81 (1.41, 16.38) | 0.05 |
| Total Cysteine (nmol/mL) | <218 (median 194) |
218-248 (median 235) |
249-284 (median 265) |
≥285 (median 311) |
|
| No. of cases/No. of controls | 29/66 | 36/62 | 31/66 | 49/66 | |
| Matching factor adjusted OR (95% CI) | 1.0 (referent) | 1.42 (0.73, 2.78) | 1.36 (0.63, 2.93) | 3.50 (1.44, 8.52) | <0.001 |
| Adjusted OR* (95%CI) | 1.0 (referent) | 0.85 (0.36, 2.02) | 0.54 (0.20, 1.47) | 1.32 (0.42, 4.12) | 0.10 |
| Adjusted OR† (95%CI) | 1.0 (referent) | 0.67 (0.24, 1.86) | 0.34 (0.10, 1.14) | 0.62 (0.15, 2.53) | 0.60 |
| Adjusted OR§ (95%CI) | 1.0 (referent) | 0.56 (0.19, 1.67) | 0.27 (0.07, 0.98) | 0.39 (0.09, 1.79) | 0.96 |
Conditional logistic regression model controlling for matching factors (age, cigarette smoking status, and month of blood collection) in addition to conventional (non-biomarker) cardiovascular risk factors [history of diabetes mellitus, history of hypertension, parental history of MI before age 60, menopausal status (postmenopausal vs. pre-menopausal vs. uncertain), current post-menopausal hormone use (use of female hormones in the three months prior to blood draw), history of hypercholesterolemia, history of aspirin use (> 14 days per month vs 1-14 days per month vs no aspirin use),physical activity in MET-hours (quartiles), body mass index (natural cubic splines with 4 degrees of freedom), alcohol intake in grams per day (natural cubic splines with 4 degrees of freedom)] and a linear function of age in years.
Conditional logistic regression model controlling for factors in (*) in addition to creatinine clearance, plasma CRP concentration and plasma total to HDL cholesterol ratio, modeled with natural cubic splines with 4 degrees of freedom.
Conditional logistic regression model controlling for factors in (†) in addition to plasma total cysteine (natural cubic splines with 4 degrees of freedom).
Conditional logistic regression model controlling for factors in (†) in addition to plasma total homocysteine (natural cubic splines with 4 degrees of freedom).
Discussion
In this large prospective study, high plasma concentration of total homocysteine, but not total cysteine, was associated with increased rate of MI among predominantly post-menopausal women. Although tCys was associated with elevated risk of MI, after adjusting for matching factors only, the relationship did not persist after adjusting for conventional CAD risk factors. There was a significant positive correlation between fasting concentrations of total homocysteine and total cysteine.
The finding that plasma concentration of homocysteine is positively correlated with risk of MI is consistent with several other prospective studies29. We previously reported on this relationship in the Nurses Health study18, but that analysis did not consider the specific relationship of fasting total homocysteine to MI risk. This analysis among women who provided blood after fasting revealed a relationship further away from the null than in our previous analysis. This is likely due to the effect of reducing measurement error. Randomized controlled trials that have evaluated homocysteine lowering with folic acid, vitamin B6 and vitamin B12 found that this therapy, although successful in reducing homocysteine, did not reduce incidence of the combined outcome of myocardial infarction, stroke and cardiovascular death1-3, 5. This suggests either that, in addition to the beneficial effect of homocysteine lowering, the therapy had other effects on disease progression that are harmful, or that homocysteine in the ranges seen in the normal population is not a direct cause of cardiovascular disease. Homocysteine may be a correlate for an alternative causal mechanism that is unaffected by vitamin therapy. The latter may also be true for total cysteine concentration, but possibly for different reasons. We observed a positive correlation between fasting plasma concentration of tCys and the risk factors body mass index and C-reactive protein concentration (table II). Cysteine is an important precursor to intracellular glutathione, which is an essential cellular antioxidant (Figure I)10, 13. Animal studies suggest that GGT on cell surfaces plays a key role in providing cysteine for intracellular GSH production, by breaking down extracellular GSH13. GGT gene expression is increased during oxidative stress10, and several studies have shown that plasma concentration of GGT is positively associated with cardiovascular disease risk30. This is consistent with our finding that elevated tCys is positively associated with MI risk, but not after adjustment for BMI and other risk factors for CAD. Elevated plasma cysteine may thus be a marker of the body's attempt to increase intracellular GSH in response to increased oxidative stress.
To our knowledge, this is the first prospective study that has examined the relationship between plasma total cysteine and subsequent MI risk. Major strengths of this study are its prospective design, and control for confounding factors. In addition, we were able to reduce measurement error in tHcy and tCys as well as other plasma covariates by focusing on those women who had provided blood after fasting. However, given the observational nature of the study, we cannot rule out unmeasured confounding.
In summary, our investigation revealed that increased fasting concentration of total homocysteine, but not total cysteine, was significantly associated with increased risk of myocardial infarction in predominantly post-menopausal women. We recommend the conduct of future studies to facilitate a clearer understanding of the relationship between plasma total cysteine and the risk of chronic disease, particularly in younger women and in men.
Acknowledgments
We are thankful to the participants in the Nurses' Health Study for their continuing dedication and commitment.
Footnotes
Conflict of Interest: Apart from the funding agencies, which did not influence on the paper, there is no conflict of interest.
References
- 1.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. Jama. 2008;299(17):2027–36. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 3.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 4.Loscalzo J. Homocysteine trials--clear outcomes for complex reasons. N Engl J Med. 2006;354(15):1629–32. doi: 10.1056/NEJMe068060. [DOI] [PubMed] [Google Scholar]
- 5.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. Jama. 2004;291(5):565–75. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 6.Nygard O, Nordrehaug JE, Refsum H, et al. Plasma homocysteine levels and mortality in patients with coronary artery disease. N Engl J Med. 1997;337(4):230–6. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- 7.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. Bmj. 2002;325(7374):1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salway JG. Metabolism at a glance. 3rd. Malden, Mass.: Blackwell Pub.; 2004. [Google Scholar]
- 9.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338(15):1042–50. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Forman HJ, Choi J. Gamma-glutamyl transpeptidase in glutathione biosynthesis. Methods Enzymol. 2005;401:468–83. doi: 10.1016/S0076-6879(05)01028-1. [DOI] [PubMed] [Google Scholar]
- 11.Rathbun WB, Murray DL. Age-related cysteine uptake as rate-limiting in glutathione synthesis and glutathione half-life in the cultured human lens. Exp Eye Res. 1991;53(2):205–12. doi: 10.1016/0014-4835(91)90075-p. [DOI] [PubMed] [Google Scholar]
- 12.Smith CM, Marks AD, Lieberman M, et al. Marks' basic medical biochemistry : a clinical approach. 2nd. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 13.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38(4):263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 14.El-Khairy L, Vollset SE, Refsum H, et al. Plasma total cysteine, mortality, and cardiovascular disease hospitalizations: the Hordaland Homocysteine Study. Clin Chem. 2003;49(6 Pt 1):895–900. doi: 10.1373/49.6.895. [DOI] [PubMed] [Google Scholar]
- 15.El-Khairy L, Ueland PM, Refsum H, et al. Plasma total cysteine as a risk factor for vascular disease: The European Concerted Action Project. Circulation. 2001;103(21):2544–9. doi: 10.1161/01.cir.103.21.2544. [DOI] [PubMed] [Google Scholar]
- 16.Ozkan Y, Ozkan E, Simsek B. Plasma total homocysteine and cysteine levels as cardiovascular risk factors in coronary heart disease. Int J Cardiol. 2002;82(3):269–77. doi: 10.1016/s0167-5273(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 17.van den Brandhof WE, Haks K, Schouten EG, et al. The relation between plasma cysteine, plasma homocysteine and coronary atherosclerosis. Atherosclerosis. 2001;157(2):403–9. doi: 10.1016/s0021-9150(00)00724-3. [DOI] [PubMed] [Google Scholar]
- 18.Shai I, Stampfer MJ, Ma J, et al. Homocysteine as a risk factor for coronary heart diseases and its association with inflammatory biomarkers, lipids and dietary factors. Atherosclerosis. 2004;177(2):375–81. doi: 10.1016/j.atherosclerosis.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Hankinson SE, Willett WC, Manson JE, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87(17):1297–302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 20.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr. 1987;422:43–52. doi: 10.1016/0378-4347(87)80438-3. [DOI] [PubMed] [Google Scholar]
- 21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.Salazar DE, Corcoran GB. Predicting creatinine clearance and renal drug clearance in obese patients from estimated fat-free body mass. Am J Med. 1988;84(6):1053–60. doi: 10.1016/0002-9343(88)90310-5. [DOI] [PubMed] [Google Scholar]
- 23.Allain CC, Poon LS, Chan CS, et al. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20(4):470–5. [PubMed] [Google Scholar]
- 24.Sugiuchi H, Uji Y, Okabe H, et al. Direct measurement of high-density lipoprotein cholesterol in serum with polyethylene glycol-modified enzymes and sulfated alpha-cyclodextrin. Clin Chem. 1995;41(5):717–23. [PubMed] [Google Scholar]
- 25.Rothman KJ, Greenland S. Modern epidemiology. 2nd. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 26.Therneau TM, Grambsch PM. Modeling survival data : extending the Cox model. New York: Springer; 2000. [Google Scholar]
- 27.SAS Institute . SAS/GRAPH Software. Cary, NC: SAS Institute; 2004. p. x.p. 95. Version 9. [Google Scholar]
- 28.S-PLUS. (Version 8) Insightful Corporation. Seattle, WA: 2007. [Google Scholar]
- 29.Homocysteine Studies Collaboration Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. Jama. 2002;288(16):2015–22. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 30.Fraser A, Harris R, Sattar N, et al. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women's Heart and Health Study and Meta-Analysis. Arterioscler Thromb Vasc Biol. 2007;27(12):2729–35. doi: 10.1161/ATVBAHA.107.152298. [DOI] [PubMed] [Google Scholar]
- 31.Wu K, Helzlsouer KJ, Comstock GW, et al. A prospective study on folate, B12, and pyridoxal 5′-phosphate (B6) and breast cancer. Cancer Epidemiol Biomarkers Prev. 1999;8(3):209–17. [PubMed] [Google Scholar]


